User:Mr. Ibrahem/Tenapanor
 | |
| Clinical data | |
|---|---|
| Trade names | Ibsrela |
| Other names | Tenapanor hydrochloride |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | By mouth |
| Drug class | NHE3 inhibitors |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Chemical and physical data | |
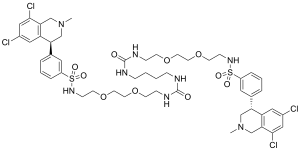
| Formula | C50H66Cl4N8O10S2 |
| Molar mass | 1145.04 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tenapanor, sold under the brand name Ibsrela, is a medication used to treat irritable bowel syndrome with constipation (IBS-C).[1] It is taken by mouth.[1]
Common side effects include diarrhea, abdominal swelling, and lightheadedness.[1] Use in pregnancy and breastfeeding is believed to be safe.[1] It acts by blocking the sodium-proton exchanger NHE3 there by decreasing the uptake of sodium by the intestines.[1]
Tenapanor was approved for medical use in the United States in 2019.[1] As of 2021; however, it is not yet in pharmacies.[2] As of 2021 it is not approved in Europe or the United Kingdom.[3]
References[edit]
- ^ a b c d e f "Tenapanor Monograph for Professionals". Drugs.com. Archived from the original on 27 January 2021. Retrieved 25 September 2021.
- ^ "Ibsrela Prices and Ibsrela Coupons - GoodRx". GoodRx. Retrieved 25 September 2021.
- ^ "Tenapanor". SPS - Specialist Pharmacy Service. 26 May 2017. Archived from the original on 23 July 2017. Retrieved 25 September 2021.
