User:Physchim62/Titration error
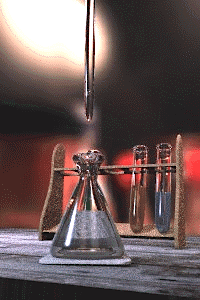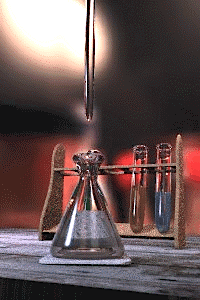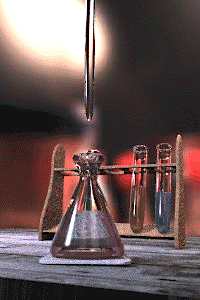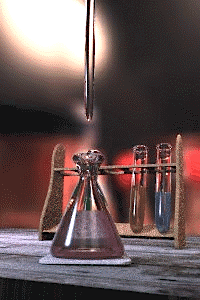
The titration error is a systematic error in the measured end-point of a titration.[1][2] It is inherent in the method, and so cannot be eliminated by performing repeated titrations on the same sample, not even by different analysts using different equipment. The titration error is usually negligible for routine titrations using standard procedures, but must be taken into account for high-precision analyses by titration and in the design of new methods.
There are two components to the titration error:
- Indicator error: the colour change of an indicator may not be exactly at the point of equivalence between the two reactants.[3]
- Last-drop error or half-drop error: the smallest volume that can be measured with a burette is equal to one drop, but it is not known what proportion of the last drop of reagent to be added was needed to reach the equivalence point.[4]
Both of these errors can be reduced by good experimental design. They can be eliminated altogether by using an interpolated endpoint, as in potentiometric or conductometric titrations, but at the expense of an increased Type A measurement uncertainty ("random error").
Volumetric analysis[edit]
Indicator error[edit]
Avoiding the indicator error[edit]
Last-drop error[edit]
Other errors in volumetric analysis[edit]
Notes and references[edit]
Notes[edit]
Bibliography[edit]
- Miller, J. C.; Miller, J. N. (1988), Statistics for Analytical Chemistry (2nd ed.), Chichester, UK: Ellis Horwood, ISBN 0-7458-0292-3.
- Vogel, Arthur I. (1939), A Textbook of Quantitative Inorganic Analysis, London: Longmans.
References[edit]
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "titration error". doi:10.1351/goldbook.T06389
- ^ International Union of Pure and Applied Chemistry (1998). Compendium of Analytical Nomenclature (definitive rules 1997, 3rd. ed.). Oxford: Blackwell Science. ISBN 0-86542-6155. p. 48.
- ^ Vogel (1939), p. 43.
- ^ Miller & Miller (1988), p. 24.
