Vedaprofen
Appearance
| |
| Names | |
|---|---|
| IUPAC name
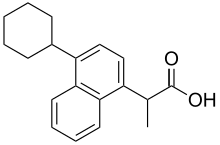
2-(4-Cyclohexyl-1-naphthyl)propanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.068.339 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H22O2 | |
| Molar mass | 282.383 g·mol−1 |
| Pharmacology | |
| QM01AE90 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Vedaprofen is a nonsteroidal anti-inflammatory drug (NSAID) used in veterinary medicine for the treatment of pain and inflammation due to musculoskeletal disorders in dogs and horses and for the treatment of pain due to horse colic.[1] It is a member of the profen drug class.
Synthesis
[edit]Vedaprofen can be synthesized beginning with 1-cyclohexylnaphthylene (left).[2][3] Chloromethylation followed by functional group interconversion provides the ester (center right). Alkylation with methyl iodide then gives vedaprofen (right).

References
[edit]- ^ "Specific Nonsteroidal Anti-inflammatory Drugs". Merck Veterinary Manual. Merck Sharp & Dohme Corp.
- ^ BE 870553
- ^ U.S. patent 4,218,473
