Wikipedia:Reference desk/Archives/Science/2012 April 5
| Science desk | ||
|---|---|---|
| < April 4 | << Mar | April | May >> | April 6 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
April 5[edit]
Types of copper[edit]
What are the types of copper? — Preceding unsigned comment added by Anto Christopher (talk • contribs) 07:51, 5 April 2012 (UTC)
- Do you mean phases? Plasmic Physics (talk) 08:02, 5 April 2012 (UTC)
- Our article copper refers to isotopes, alloys, oxidation states, ores, and many other topics that might be relevant depending on what you mean by "type". --Colapeninsula (talk) 09:16, 5 April 2012 (UTC)
- See also police ranks of the United Kingdom.--Shantavira|feed me 14:33, 5 April 2012 (UTC)
- An "expert-witness" in this morning's New York Times mentions 14 million alloys of copper, though I'm still not sure if he's speaking hyperbolically or literally. In any case, ASME and ASTM publish numerical and nomenclature standards for alloys of copper. There are catalogs full of this stuff. I only use two types of copper, "pure enough for electrical wiring" copper, which is some 99.9% copper, and "machine copper," McMaster calls it brass "Alloy 330" which is alloyed with tin, iron, nickel, and a bunch of other gunk, to make it hard enough to hold its shape when milled or worked. Nimur (talk) 15:36, 5 April 2012 (UTC)
- The exact quote is
He said it would be nearly impossible to prove that Rubedo is a unique mixture of metallic components, adding, "It may be one of the 14 million alloys that people have cooked up over the decades."
- The exact quote is
volume measurer[edit]
everyone knows the story of Archimedes' "Eureka! Eureka!" (I have it!) as he ran dripping naked through the streets of Athens, when Archimedes figured out how to determine for the King whether his "pure gold" crown was adulterated. Gold was the heaviest of the metals used, so if he put the supposedly pure Gold irregular crown in a full tub (as he just realized getting into one himself) the amount of water displaced would show the volume of the crown in terms of an exactly equivalent volume of water: the weight of displaced water is easy to measure for them, and if this was heavier (more volume, i.e. less dense crown at same weight) than that displaced by an amount of known pure gold WEIGHING the same as the crown (a simple pan balance lets you assemble such nuggets), in whatever shape or size -- then, since gold was the heaviest of the used metals, it must have been adulterated by something lighter, and the crown would be adulterated. The story continues that the crown was submerged, then pure gold weighing the same as the 'pure gold crown' was submerged, and when the former volume of water weighed more than the latter, the king's pilfering goldsmith was executed.
now my question today is really about how we measure volume today.
How do we measure the true volume of irregular objects? Is it by submersion? What about this idea: simply put it into an open container, close it, pump in x cc of air from outside, and see how much pressure goes up. Would this tell you how empty the (known volume) container was, i.e. how much volume was in it? (I think this would be true because if it's nearly full, all but 100 cc of empty space, the pressure would double. but if the 100 cc represented 1/10th of the available free space in there, the pressure would increase by far less. what do you guys think of this idea? (it depends on being able to pump an exact amount of air and to measure pressure accurately, and to have an enclosed container with known volume and no leaks. No water needed and it just takes a second! 188.6.92.6 (talk) 14:45, 5 April 2012 (UTC)
in practice what is the usual way of measuring the true volume of something today? submersion still or another technology? 188.6.92.6 (talk) 14:47, 5 April 2012 (UTC)
- The displaced liquid method is today still a good, easy, and accurate way of measuring volume of an odd-shaped object. In industry, 3D scanning, either articlulated probe type, laser type, or camera type, is gaining ground. In 3D scanning, probing the surface is used to build up the shape in a computer, which can then calculate the volume.
- Your air pressure method is not particularly good, because a) it is easier to measure liquid volume accurately than it is for pressure, b) you have to know the air volume pumped in accurately as well, as source of error, and c) the pressure of air varies in proportion to temperature, introducing yet another source of error. Raising the pressure of air increases its temperature, which will then slowly come back down, dropping the pressure, complicating the measurement still further, as you would have to wait until it stabilises.
- More often than not though, the material in the object is known, so all you have to do is weight it. If not, many objects of complex shape can be visuallised as several simple shapes joined together, so you can easily calculate the total volume.
- Ratbone124.178.37.185 (talk) 15:28, 5 April 2012 (UTC)
- isn't the temperature of air in a room pretty homogenous? I would think you could just have a simple piston-pump say, containing the exact volume of the whole container (like a giant syringe) with the containing part of the syringe/piston open to the air to freely circulate until the device is used. Meanwhile the temperature is measured. The object is placed inside, the syringe/piston is plunged, and if the container is empty, the pressure doubles. The less empty it is, the more the pressure increases over double pressure. The piston doesn't need to go all the way down, if the container is nearly full you don't have to subject the object to great pressure... I would think far harder than accurately pumping air (easy peasy with a piston) maybe you are right that measuring pressure is hard. But isn't that also easy - I mean can't you just have a membrane on the side that flexes out with pressure, or a kind of barometer attached to the whole thing (filled with water if you want) that is calibrated to 0 at room pressure and temperature and just goes up like a normal weather barometer? It seems this is also a solved problem.... how hard can measuring pressure be? 188.6.92.6 (talk) 15:35, 5 April 2012 (UTC)
- Trouble is, while you are perfoming your measurement, the room aircon cuts in or someone opens the door. So right away you've got a temperature change of 1 or 2 degrees, perhaps more. That alone will make your measurement eeror larger than even a sloppy measurement of displaced lequid volume. When you raise the prerssure of air, you inherently increase its temperature (gas laws), and the temperature will then slowly decay back to room temperature, lowwering the pressure. So, you would have to wait until it stabilises. Not a good method. Ratbone124.178.37.185 (talk) 15:42, 5 April 2012 (UTC)
- First of all, I believe you. But could you help me understand the physics more deeply. I can imagine for example a vessel (the whole container) with slots that are all open, everything is circulating freely. Let's say we can only measure the air temperature to +/- 5 degrees. (huge variation). You can have temperature measurements at various locations inside and outside. Now after placing the object inside (it might not be room temperature!) you press a button, and within 1/10th of a second the slots blast closed, a cylinder of compressed air is fully released, and you measure the new pressure. You know that the cylinder had been at x temperature roughly (you can measure it) and you know that it will cool off during decompression, but can't you calculate for this effect? Could you walk me through an actual example. I have a hen I want to see the volume of that can withstand 3x air temperature without ill affects. I put it into the machine which has enough room for the average sized hen to displace 20% of the air. I press the button, the slots close, the tank inside decompresses so that the typical 80% of the remaining space will be at 3x pressure, and we measure the difference between 3 times normal pressure at the temperature (+/-5 degrees) and what we actually get, the slots open to neatralize pressure, the doors slide open, and the hen has been volumed. (like 'weighted'). Now could you walk me through the physics of where we go wrong here? (meanwhile, air is being pumped into the pressure container again for the next hen).
The box looks like this:
T
__S__S__S___
S T T S
S HH | T
S _HH_ S
S | | S
|txx|____|___| T
T
Thusly:
S = slots normally open until door is closed and button is pressed
T = temperature measurement device, of which there are several both inside and outside the box
xxx = cylinder pumped full of air (to the extent you would like for best results/best equation)
t = a thermometer attached to the cylinder of air.
over time if the box isn't used for long, cylinder,
which would be hotter when you first pumped it full of air,
would cool to room temperature.
It's important to know when this happens,
as after this point you will cool the box by releasing it
H = hen.
now with the proper equations, couldn't this work quite well? Also, if you know how conductive the air container is you can calculate just based on the time it was last discharged and the temperature outside (i.e. the gradient) and the materials it contains, how slowly it cools off after being pumped full. If it's like a thermos it could retain the heat quite well, and you would get the same temperature of air out as you put in (instead of colder air after the warmer container dissipated it under extra pressure). This makes a lot of assumptions, of course, but could you walk me through why the equation fails to be meaningful? 188.6.92.6 (talk) 16:09, 5 April 2012 (UTC)
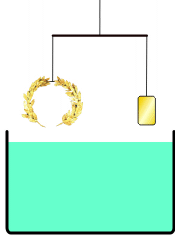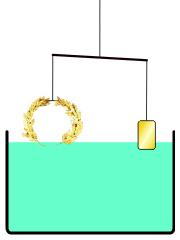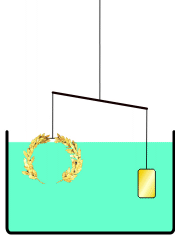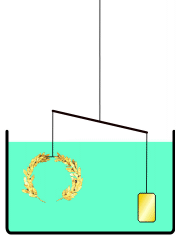
Not an answer to the question, just a comment on the story that introduced it. In the version of the story I've heard (in a podcast somewhere on this website), Archimedes did not measure the density by measuring the water displacement. Instead, he balanced the crown on a scale with a gold reference sample, and then immersed the apparatus in water.This is suggested by our article Archimedes#Archimedes' principle, see illustration reproduced here. --NorwegianBlue talk 00:03, 6 April 2012 (UTC)
- Sounds like an excessively complicated way to frighten the life out of poor chickens, should they manage to survive the overpressure. Wickwack124.178.55.81 (talk) 05:48, 6 April 2012 (UTC)
Volume measurer name ?[edit]
Is there a standard device for measuring volume, and, if so, what is it's name and do we have an article on it ? StuRat (talk) 02:12, 6 April 2012 (UTC)
- Lots of things measure volume. Graduated cylinders, volumetric flasks, syringes, beakers, pipettes. It all depends on for what purpose one is measuring volume. --Jayron32 04:49, 6 April 2012 (UTC)
- It does now, Stu! Silence denotes consent. You want to go into business with me? I have a killer business plan. 188.156.29.4 (talk) 07:30, 6 April 2012 (UTC)
- How about this: a 3D, raster scanning, laser that gives an accurate approximation? Plasmic Physics (talk) 14:32, 6 April 2012 (UTC)
- Stu, these guys are trying to get us to abandon our business plan because an insanely expensive device technically already fills this space, with lasers and precision software. We can manufacture ours in China for four-fifty if we don't mind the compressed air tank exploding and the air ducts not closing hermetically after 200 cycles, but for twenty or thirty dollars we can cut this whole "3D raster scanning laser approximation thing" which no farmer would EVER use to actually volumize a Hen, if you'll patent this with me (I think we have 90 days since I spilled the beans here) we are going to strike it rich. They say build a better mouse-trap, but you know what's better than a surface-plotting, mouse-modelling, laser-guided mousetrap? A cheap one!! Are you in or out? 188.6.92.6 (talk) 16:24, 6 April 2012 (UTC)
- Just send me a check each month, please. :-) I imagine the laser scanner would have a problem with hollow objects, while submerging in a liquid would work better, as long as there are holes to let the liquid in and air out. I wonder if water is the best option, though. It certainly is cheap, but it's tendency to soak into things like fabric might be a problem. It might also be important to rotate the object and vibrate it, to get all the air bubbles out. Then again, considering all these issues, perhaps using a gas instead of a liquid would be better. StuRat (talk) 18:18, 6 April 2012 (UTC)
- I read your user page with your philosophy. ("I believe that the movement of wealth from the poor and middle-class to the rich is a serious problem, eventually destablizing a nation, and should be countered with a heavily progressive tax system (up to 90%)") I assume, since this is quite profitable, that you would like me to just send you 10% of the money, with 90% going to the government. Of course, the government is free to spend it on whatever the current president wants, a war we disagree with for example - but doesn't actually have to take any risk on my invention. Do I have this right? So let me take on all the risk, for the chance to give civil servants who didn't do anything for it control over how 90% of it is spent. Do I have your philosophy right? If so, I look forward to sending you 10% of your money soon... 134.255.105.197 (talk) 21:45, 6 April 2012 (UTC)
- That 90% is for rich people, earning a million dollars a year or more. If you want to send me $100K a year and the gov the rest, I'm fine with that. BTW, I also believe in direct democracy, meaning we all get to vote on how the money is spent, as opposed to back room deals with corrupt politicians giving it to whoever donated the most to their campaign funds. StuRat (talk) 21:59, 6 April 2012 (UTC)
retrograde rotation of venus[edit]
Venus opposite rotation is remained mystery for duration of its discovering time till now ,and it has been refereed to some hypothetic events such as external object impact with planet which supposed it changed its rotational direction . HOw does this occure?--Akbarmohammadzade (talk) 14:54, 5 April 2012 (UTC) In fact Iwant to have any discussion ,in addition by let of Wiki site I ask you to see (http://www.gsjournal.net/Science-Journals/Research%20Papers/View/4029 )to know how i am thinking about the subject--Akbarmohammadzade (talk) 15:01, 5 April 2012 (UTC)
- If you've already written a published paper on this, then why would you ask us about it ? StuRat (talk) 17:06, 5 April 2012 (UTC)
- All the planets have a tilt to them, some more than others, and even the sun has over a 7 degree tilt. This last point was discussed recently here: Wikipedia:Reference_desk/Archives/Science/2012_March_12#Why_does_the_Sun_have_a_7.25_degree_tilt_.3F. As for the mechanisms, I suppose a moon-sized object could strike at a shallow angle, counter to the old rotation direction. However, I think it more likely it would strike at close to a right angle to the direction of rotation. Thus, Venus continued to rotate, rather than reversing direction, but the axis of this rotation was changed. This isn't necessarily an impact from a single object, either, there may have been many. This leads to a model of the early solar system with far more large objects in it than it does at present, since every surviving planet appears to have had it's rotation disturbed. StuRat (talk) 17:10, 5 April 2012 (UTC)
- It is not a peer-reviewed journal, so I suspect that the OP might not a specialist in the subject. As far as "external impact events", I don't understand why these exotic theories are needed. The explanation that makes the most sense to me is that its rotation is retrograde due to its thick atmosphere. Although the exact evolution to its current state is up for debate, with an atmosphere 92 times denser than Earth's at the surface, Venus should have had more than enough time to spin down from a conventional orbit due to tidal effects. After it had reached a near-zero rotation, it's not inconceivable that drag from the atmosphere could set it spinning in either direction, and since it seems that the dominant winds in the atmosphere are retrograde, it should be of no surprise that its rotation is retrograde as well. -RunningOnBrains(talk) 06:19, 6 April 2012 (UTC)
- Isn't the retrograde motion of the atmosphere due to the retrograde motion of the planet, not the other way around ? What do you think causes the motion of the atmosphere ? And I see how a planet would eventually stop revolving, down until it became tidally locked, but I see no way for it to start spinning again without an impact. StuRat (talk) 06:39, 6 April 2012 (UTC)
- I don't see why you would imagine that Venus's atmosphere would make a significant difference. Internal redistributions of angular momentum do nothing to affect the total angular momentum of the planet. A planet needs to interact with an external objects (e.g. other planets or the sun) in other to accomplish a net change in angular momentum. Besides which, the Earth is covered with a fluid (the oceans) significantly more massive than Venus's atmosphere. Dragons flight (talk) 19:00, 6 April 2012 (UTC)
- Yes, but the oceans are contained do basins, and so rotate with the earth and don't have a general direction of flow, while the atmosphere has a net retrograde motion in comparison to the surface. The earth's atmosphere is the number one reason for changes in the length of day. However, I see your point about external torques, my false assumption was that the jet stream and Hadley cell structure would be similar for venus in the past, but this is simply not true, because these structures only form on a rotating planet. Still, the atmosphere could have been responsible for slowing its rotation, then it would take a much less significant impact event to turn the planet retrograde.-RunningOnBrains(talk) 21:48, 7 April 2012 (UTC)
- I don't see why you would imagine that Venus's atmosphere would make a significant difference. Internal redistributions of angular momentum do nothing to affect the total angular momentum of the planet. A planet needs to interact with an external objects (e.g. other planets or the sun) in other to accomplish a net change in angular momentum. Besides which, the Earth is covered with a fluid (the oceans) significantly more massive than Venus's atmosphere. Dragons flight (talk) 19:00, 6 April 2012 (UTC)
Escaping from a black hole[edit]
It's standard to point out that even light cannot escape from a black hole. Likewise, if a cannonball is fired very fast outward toward the event horizon, the gravity will decelerate it to zero and then negative velocity. But a cannonball has no countervailing force accelerating it to offset the gravity. What if, instead of a cannonball, we have a rocket that is continuously firing its thrusters? Could a time profile of generated thrust be found that would let the rocket escape? Or would the required thrust rise to infinity as the event horizon is reached? Duoduoduo (talk) 15:36, 5 April 2012 (UTC)
- The escape velocity at any point within the event horizon of a black hole is at least the speed of light. No object with mass cannot be accelerated to (much less beyond) the speed of light, and so no rocket operating within the bounds of known physics can escape. It would fall more slowly, but still it would fall inexorably. As I understand it, though, your cannonball example is fallacious. Within the event horizon, gravity is sufficiently strong that you cannot move away from the singularity, even temporarily. So there's no launching a cannonball "up but not quite far enough"; it can only fall. — Lomn 15:54, 5 April 2012 (UTC)
- the scape velocity for outer distance from event horizon is same as first star ,the sun radius is 1/200 AU , the escape velocity from its surface is 617 Km/s .if it came to be for example any black hole ,the necessary velocity will be equal .Akbar mohammadzade IRAN — Preceding unsigned comment added by 78.38.28.3 (talk) 16:11, 5 April 2012 (UTC)
- This is not true. What will remain the same is the escape velocity from the Sun's former radius. Escape velocity is dependent on the radius from the center of gravity. So the escape velocity of a black-hole sun would be greater than the speed of light within its event horizon. -RunningOnBrains(talk) 06:46, 6 April 2012 (UTC)
- the scape velocity for outer distance from event horizon is same as first star ,the sun radius is 1/200 AU , the escape velocity from its surface is 617 Km/s .if it came to be for example any black hole ,the necessary velocity will be equal .Akbar mohammadzade IRAN — Preceding unsigned comment added by 78.38.28.3 (talk) 16:11, 5 April 2012 (UTC)
- The article escape velocity says: It is the speed needed to "break free" from a gravitational field without further propulsion. But my question involves further propulsion.
- By analogy with escape from Earth: An unthrusted cannonball, propelled only by its initial explosion to just less than the escape velocity, would not quite make it out; but if you add some more thrust continuously, couldn't you make it out at a slow speed? Duoduoduo (talk) 16:11, 5 April 2012 (UTC)
- No. Within the event horizon, gravity is constantly drawing you in at a rate greater than the speed of light. Your hypothetical rocket, therefore, would have to have, at some point, a velocity also greater than the speed of light in order to escape. Per known physics, this requires infinite energy and so cannot be done. Alternately, consider light: it is always traveling at the speed of light (the black hole does not slow it down), and yet is unable to escape. How, then, can a rocket that can never reach the speed of light ever escape? Rather, the geometry of spacetime is sufficiently altered that no escape is ever possible. The images at Black_hole#Event_horizon illustrate this. This is also my point above about the problem of the cannonball analogy: no paths within a black hole's event horizon exist that move closer to (or even remain the same distance from) the event horizon; all possible futures move strictly towards the singularity. — Lomn 16:23, 5 April 2012 (UTC)
- It's not very accurate. Gravity doesn't draw you in at a rate. It accelerates you. As you said, ordinarily you don't need to accelerate to the escape velocity to escape from something, so the argument that "you would have to accelerate to the speed of light to escape, therefore you can't escape" makes no sense and people shouldn't use it. However, it is true that you can't escape from a black hole by any means. -- BenRG (talk) 16:58, 5 April 2012 (UTC)
- My understanding is that accelerating (in any direction) while within an event horizon actually causes an object to fall faster. --Tardis (talk) 14:03, 6 April 2012 (UTC)
Inside the event horizon, "inward" and "outward" are no longer spatial directions, but they are timelike, i.e. like future and past. That's what the Schwarzschild metric is saying. Icek (talk) 20:07, 5 April 2012 (UTC)
- I can see where the OP's confusion comes from. One thing that is often glossed over in a casual discussion of escape velocity is that it is only the velocity at which you will escape the planet's gravity. It is not a velocity you must achieve to escape the planet's gravity. Indeed, one could envision an imaginary spacecraft with near-infinite fuel that could escape Earth's gravity at walking speed; all they would have to do is adjust their thrust slowly downward as they moved away.
- Another thing that is glossed over is that escape velocity is dependent on the distance from the center of gravity of the object you are escaping from (see the equation at the top of the escape velocity page), but the number that is most often cited is the escape velocity at the surface. The further you get from, say, Earth, the lower your escape velocity will be.
- In regards to your original point though, you can't think about these cases classically, because black holes are, by definition, non-classical objects. I'm not really knowledgeable on the in-depth mathematical details of black holes, so I presume Icek knows what he's talking about, but I know enough to know that even in a thought exercise where your spacecraft somehow wasn't annihilated by the ridiculous tidal forces that the concepts of acceleration, velocity, direction and distance will become non-intuitive inside the event horizon (even though, technically, physics can't say anything about that which lies past the event horizon).-RunningOnBrains(talk) 06:46, 6 April 2012 (UTC)
Body fat monitor[edit]
How do body fat monitors (example) work? How accurate are they? --SupernovaExplosion Talk 15:42, 5 April 2012 (UTC)
- I don't know much about this, only enough to tell that the relevant article is Bioelectrical impedance analysis. --NorwegianBlue talk 23:38, 5 April 2012 (UTC)
- Thanks for the link! --SupernovaExplosion Talk 04:19, 6 April 2012 (UTC)
Caramel Catastrophe![edit]
I had some delicious leftover caramel that I'd made--I put it in a plastic tupperware container (one I've regularly used in a microwave) and put it in the fridge.
Later, when I was microwaving it to drip onto ice-cream, the caramel melted through the bottom of the tupperware and burned the heck out of my hand!
I didn't leave it in for an excessive amount of time, and my friend says it has something to do with the 'heat capacity' of sugar---can any of you science-types shed some light on what that may or may not mean? Does that mean that sugar can hold a very high amount of heat without becoming a sugar vapor or some such? (please note, I'm not referring to the brand: Tupperware--I'm actively promoting the genericide of that brand)66.30.10.71 (talk) 15:51, 5 April 2012 (UTC)
- a large heat capacity means that it can hold a lot of heat energy without it's temperature increasing (feeling hot). OR, If it's hot it can lose a lot of energy (and maybe burn more stuff) before it's temperature reduces. So heat capacity is not directly responsible for melting through the bottom. Sugar does not normally vaporize. It melts and then begins to char to form caramel. If you keep heating it it will go all the way to charcoal. Most foods will never heat up enough to melt the plastic as once they reach the boiling point of water, any more energy supplied to them from the microwave will just be carried away by the steam that is formed. In the case of your caramel, no such luck. the water formed by charring reaction was too little to carry away the heat in the steam. All that energy instead raised the temperature of the caramel enough to melt the plastic. Staticd (talk) 16:26, 5 April 2012 (UTC)
- I don't know, don't you make caramel in a very hot pan before the sugar 'melts' - obviously too hot for tupperware (you wouldn't put sugar in a tupperware container and put it in a microwave and heat it until it became caramel. so maybe getting it to melting temperature is equally hot, even though it was 'already caramel'. just a theory. 188.6.92.6 (talk) 16:39, 5 April 2012 (UTC)
- It's never a good idea to microwave plastic containers. Long before they actually melt, they leach toxic chemicals into the food. I suggest glass or ceramic for your microwaving needs. StuRat (talk) 17:00, 5 April 2012 (UTC)
- What about the containers in which prepared ("ready") meals or dishes are sold explicitly to be microwaved within them (some of which I save and re-use), and the plastic kitchenware specifically sold for repeated use in microwaves (which I also use)? I agree, of course, that any plastic container not clearly designated for microwave use should be avoided. {The poster formerly known as 87.81.230.195} 90.197.66.205 (talk) 17:37, 5 April 2012 (UTC)
- They might be better than foam take-home containers, which seems to melt immediately, but still I bet pretty much any plastic they sell as "microwaveable" is likely to melt and/or give off liquid chemicals and fumes before glass and ceramics designed for the microwave. One worrying sign is if tomato sauce heated in a plastic bowl turns the bowl red, and the color won't come out. This show a level of interaction between food and container which should not be allowed. StuRat (talk) 19:31, 5 April 2012 (UTC)
- I'm not sure, but if the caramelization involves enough charring, the substance would become electrically conductive and absorb a lot of energy from the microwaves. (There's a somewhat related demonstration where a beer bottle is microwaves on its own - nothing happens - then a torch flame is applied to it for a moment, and remicrowaved ... and the glass melts) Wnt (talk) 18:57, 5 April 2012 (UTC)
- The caramel could simply be heating very unevenly (it's viscous, so a local high-energy spot might not redistribute very well), leading to "that one spot" getting hot enough to melt through. I would assume that there was enough caramel total and that it is polar or ionic enough to absorb sufficient microwave energy, but if not (or it can't conduct the heat and MW energy away effectively), wherever the energy concentration is greatest just keeps hitting the plastic itself. DMacks (talk) 21:46, 5 April 2012 (UTC)
- AFAICT, the microwave energy will be absorbed by the rotational energy levels of the -OH groups in caramel, same as water. Need'nt be conductive. From there equipartition theorem calls for it's redistribution. Staticd (talk) 11:37, 6 April 2012 (UTC)
- My point is that the redistribution is slow...microwaving a frozen block of meat gives some areas that get hot enough nearly to start to cook while others are still frosty. It's unlike a mug of water, where the molecules are free to move around and allow physical redistribution of the "hotter" ones so the whole thing winds up heating more evenly. I assume that's why "defrost" is a long time of cycling of a lower energy level instead of the 100% "boil water" mode. DMacks (talk) 11:59, 6 April 2012 (UTC)
- When you add sugar (or salt) to water it elevates the boiling point. This can be above the melting point of the plastic which can be as low as 105°C. Oil also boils at high temperatures, and so microwave containers have warnings about these kinds of foods. Graeme Bartlett (talk) 22:56, 6 April 2012 (UTC)
- And thermoplastics (soft plastics) don't so much have a melting point as a wide range of temperatures over which they slowly change from solid to tacky to gel-like to runny. Anything beyond completely solid is problematic if you intend to eat the contents, as toxic chemicals will leach out at much higher rates then. StuRat (talk) 23:31, 6 April 2012 (UTC)
Instantaneous communication at a distance[edit]
Suppose I have a stick of fixed length. I assume it is absolutely incompressible. I press one end of the stick, and the other end moves (and so does an incompressible object that the far end is touching). Have I communicated instantaneously at a distance? How does this relate to the relativistic notion that (is this right?) information travels no faster than light speed? Duoduoduo (talk) 16:52, 5 April 2012 (UTC)
- Yes, that would violate the laws of physics. Thus, a perfectly incompressible solid is not possible. StuRat (talk) 16:57, 5 April 2012 (UTC)
- This is a common question on the reference desk. See WP:Reference desk/Archives/Science/2012 January 16#Information transfer Faster Than Light - DIY and WP:Reference desk/Archives/Science/2008 January 10#Communicating Faster than Light Using a Rotating Rod for starters. -- BenRG (talk) 17:16, 5 April 2012 (UTC)
- Perfect rigidity is excluded by relativity. In an object composed of matter, the atoms are held together by electrostatic forces. When every position along the object is not accelerating, the atoms are found with an equilibrium seperation, similiar to a spring. When a position along the object is accelerating, the seperation between atoms at that point compresses to a minimum relative to their acceleration. As with any process, it takes time to be compressed (however short), before the minimum is reached. Since all the atoms inside the object are free to move and compress, the whole object can compress as a cumalative effect.
- If you have a long pole, and pull very fast on it, it will stretch momentarily before returning to its normal length. If you push the pole, it will shorten. Even the motion of atoms relative to each other, is constrained by the speed of light; the distant end of the stick will only respond to a push on the near end at a rate slower than the speed of light.
- This is the behaviour of matter: no matter how rigid something is, nothing is pefectly rigid, and every thing can compress and stretch. Plasmic Physics (talk) 23:10, 5 April 2012 (UTC)
- Thanks, Plasmic Physics. You say "Perfect rigidity is excluded by relativity". I thought it was excluded by the physics of the small, not by relativity. Is it not possible to conceptualize an (empirically non-existent) physics of the small involving perfect rigidity? If so, It seemed to me that this kind of instantaneous communication at a distance would be possible, and would not violate relativity because nothing physical is moving at the speed of light. Is it really relativity that precludes perfect rigidity? Duoduoduo (talk) 20:27, 7 April 2012 (UTC)
Follow-up[edit]
These two questions may be identical, but I had actually been thinking of something similar for a while -- say a hypothetical "see-saw" was constructed that spanned several light years through deep space. The object is a single piece and rotates along a hinge (like a see-saw). When one end is raised, the other is lowered. Assuming the far end is raised with great speed, when would those at the near end begin to perceive a lowering? My initial feeling is that, since the atoms and molecules of the "see-saw" cannot be accelerated beyond the speed of light, the other end would not be affected until such a time as the displacement of the atoms "rippled" through to the other side. Probably an objective observer (viewing the entire object from the side) would see a slow warp in the object until the "ripple" reached the terminal point and "straightened out" the object. Is this an accurate description? Evanh2008 (talk) (contribs) 02:23, 8 April 2012 (UTC)
- Sure, but it wouldn't be any less odd or strange to the observer than watching a flexible bar bend and then straighten out. Just on a really huge scale. --Jayron32 02:29, 8 April 2012 (UTC)
Have they ever tried multi-stage balloons?[edit]
They launched a 1.35 million m³ balloon before, for the world altitude record (~50 km). Why not tie a barely inflated small balloon to it, so that by the time the carrier balloon bursts (or stops rising?), it's payload has expanded enough to be buoyant? Sagittarian Milky Way (talk) 18:10, 5 April 2012 (UTC)
- The expansion won't change the buoyancy. It is displacing a greater volume of air, but that air is reduced in density by the same amount (the pressure inside and outside the balloon need to be equal). The buoyancy is determined by the number of molecules of lifting gas and the difference in molecular weight between the lifting gas and air. The number of molecules of gas in a given volume at a given temperature and pressure will be the same, regardless of what the gas is, so one molecule of helium will always displace one molecule of air. --Tango (talk) 18:50, 5 April 2012 (UTC)
- (ec)I don't understand. Why have a second balloon rather than allowing the carrier balloon to vent lift gas so it doesn't burst? Wnt (talk) 18:50, 5 April 2012 (UTC)
- If you vent your lifting gas, you won't have the lift any more. Balloons don't usually have much more lift than is needed to counter their weight, so there isn't much you can vent before you start falling again (and you might as well have just not had that bit to start with). --Tango (talk) 22:17, 5 April 2012 (UTC)
how about this one guys?[edit]
why not just shoot a giant artillery round up that is itself a piece of artilley, timed so that when it reaches its apex, right before it starts falling again, it shoots its payload? Oh, and guess what the payload is... right, another smaller round of artillery and so on, right down to a miniature little gun that fires a little pobe into space? All the heavy artillery can parachute down for another fun go just the same. Why is this better than just one giant piece of artillery firing straight into space? Because whatever it fires doesn't have to undergo such a huge explosion, just enough to make a nice arc, and the next one likewise, and so on and on. See http://en.wikipedia.org/wiki/Schwerer_Gustav - it weighs 1300 tonnes - let's trim it down to 700 tonnes without a lot of track and equipment and guiding stuff, etc, and fires a 7-ton shell 23 miles. I suppose at a 45 degree angle. If instead it shot straight up, surely it could reach 10 miles straight up, no? Then we repeat: the 7-ton (7000 kg) projectile is itself a piece of artilly that, at the same ratio, can fire a 70 kg piece another 10 miles up in the air. We are at 20 miles. The 70 kg piece itself is able to fire 0.7 kg i.e. 700 grams, which ought to be enough to launch a 7-gram projectile another 10 miles (especially due to much lower air resistance), getting close to reaching low-earth orbit http://en.wikipedia.org/wiki/Low_Earth_orbit . Admittedly we need to trim bit more to really reach 10 stages of matruschka artillery, i.e. the 100 miles that's required for low-earth orbit, but I think we can actually do better than being able to launch only a projnectile that is 1/100th of the piece that is firing it, since all we need is an explosive chamber (no wory about reloading it on the spot), a mechanism to keep it upright right before firing (for example it could deploy its chute and fire up through a whole in the center whenever it is hoizontal (parallel to the ground) according to simple level device that doesn't weigh much. Sure, it would lose a bit of altitude after deploying chute, but you don't have to worry about timing and trajectory and all that stuff...very simple. Basically like those handheld artillery pipe things from vietnam era warcraft (don't remember what they'e called). Just a pipe you put explosive in and a shell, aim and ignite. So, what's wrong with this way of getting to space repeatedly, always retrieving the larger and larger pieces of 'artillery' as they fall with a gps tracker? 79.122.54.224 (talk) 18:57, 5 April 2012 (UTC)
- Sounds like a multi-stage rocket, only less efficient and more complex. I always thought a rocket launched from a balloon or balloons would be the best combo. StuRat (talk) 19:26, 5 April 2012 (UTC)
- A multistage rocket would be much gentler on the payload. You've specified that the payload is a space probe, and delicate electronic devices like a space probe tend to not fare so well when subjected to explosive forces. Red Act (talk) 19:53, 5 April 2012 (UTC)
- See Gerald Bull, the HARP Project, and the supergun. All the parts are out there somewhere, dunno about the drawings though. I think the missile was designed to fire while still in the gun barrel, but not positive on that. Franamax (talk) 19:33, 6 April 2012 (UTC)
how about THIS one guys[edit]
- I don't get multistage rockets, if you're not jettisoning a LARGE amount of your weight at each stage (as with my artillery) then isn't the following easiest: fill a giant balloon in ana aerodynamic shape with jet fuel. Put a small hole on the bottom with the ignition, the pressure from the balloon and gravity keeps the ignition fed and the balloon (being rubbery) contracts by itself. So you go from a huge balloon filled with jet fuel to a tiny one. Why waste all this metal on 'stages' and so on? It would be better if we could suspend the belloon, of course, so it doesn't have to stay rigid but that's tough. I guess you could have a long pole that you hang the aerodynamic balloon on, that siphons from the top and has ignition at the bottom (past the bottom of the balloon, that way the rocket-shaped balloon is suspended the whole time, but this adds an unnecessary pole to the whole thing that weighs exta. illustration 1:
Diagrams of proposed space launch vehicles
|
|---|
ILLUSTRATION 1 (NOT IMPORTANT) P P = PAYLOAD H H (for hook) = TOP OF POLE, SUSPENDING IT ALL B+B , B = BALLOON, + = SIPHON | = POLE, HERE UNDER COMPRESSION, NOT TENSION X = FIRE BB|BB BB|BB BB|BB BB|BB BB|BB BB|BB BB|BB BB|BB BB|BB BB|BB BB|BB | | | | X XXXX XXXXXXX basically, why have multiple stages instead of just jet fuel and an elastic balloon filled with it and in the shape of a rocket? Another option (bette I think) for aerodynamics is if you can find some very light metal that can withstand continuous jet-fuel burning, then you put the ignition a good distance ABOVE the aerodynmaic balloon, so that it's igniting straight down but doesn't reach the balloon of jet fuel, which feeds up through the a tiny line that is way off to the side out of the way of the flames, like this
[^]
/[ ] <--- [payload] <<-- FAR LEFT: FUEL LINE, HELD OUT AWAY FROM IGNITION FLAME
fuel--> / [_]
line / [x] <--- ignition point and can be diected by payload for correct guidance
/ x|x
/ xx|xx <-- flames licking the metal (x = flame)
/ |
/ | <- far left - fuel line
/ | <--- metal to suspend the aerodynamic balloon, now under tension, not compression
/ |
| |
| |
| + <-- good insulation on the line so it doesn't heat up too much fom the above flames
| +
| +
|__________+
| +
| +
| (F)
\ (F)
\ (FFF)
\ (FFF)
\ (FFF)
\ (FFF) <--- balloon with jet fuel (F) in it
\ (FFF) could just be canvas with rubber bands around it
\ {FFF}
\{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
{_F_}
it could also have two fuel lines for balance, thusly:
[^]
/[ ]\
/ [_] \
/ [x] \
/ x|x \
/ xx|xx \
/ | \
/ | \
/ | \
/ | \
|-+-+-+-+--|-+-+-+-+--|
\ | /
\ + /
\ + /
\ + /
\ + /
\ + /
\ + /
\ (F) /
\(F)/
(FFF)
(FFF)
(FFF)
(FFF)
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
{FFF}
(FFF)
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
{FFF}
(FFF)
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
{FFF}
(FFF)
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
(FFF)
{FFF}
{FFF}
{_F_}
|
Hang it on a long pole that siphons from the top (due to the pressure from the balloon) and near the top of pole put the ignition. Now you go all the way to space with a shrinking balloon that weighs less and less. 79.122.54.224 (talk) 21:32, 5 April 2012 (UTC)
- I haven't read your whole idea because your premise is wrong. Multistage rockets *do* jettison a large portion of their mass. Take the Saturn V rocket that took man to the moon, for instance. The first stage (excluding fuel) was 2,300 tonnes. The rest of the rocket (including the fuel for the other 2 stages) was 621 tonnes. By jettisoning the first stage and using the much smaller second stage engine, they were able to lose about 80% of their mass. --Tango (talk) 22:17, 5 April 2012 (UTC)
- The idea of keeping the fuel in a "balloon" is not that much different from keeping it in the casing of a rocket, except that a rocket will actually work flying up through the air. And when the balloon shrinks, what you're really saying is, keep the extra bits of outer shell even though much less fuel is being held by it; whereas the multistage rocket dispenses with the bits of extra shell when the fuel they enclose is burned.
- It's more fun to think of ways of having really powerful fuel, like red oxygen, variants on inverse sodium hydride and other such bizarrities. Wnt (talk) 23:11, 5 April 2012 (UTC)
- All this stuff about altitudes is beside the point. Space is an arbitrary altitude, and getting there briefly isn't very useful (but for silly people with too much money and astronomers with too little). What's useful is orbit, which allows you to stay up for a long time. Orbit is so much more than "getting up really high"; even if you build a 100km tall tower, when you stepped off it you'd drop like a stone. To be in orbit you need speed; absolutely huge speeds, in fact. The lowest elliptical orbital speed that article quotes is 6.5 km/s, which is 14500 mph. That's a massive speed, and to get to it you need to expend a massive amount of energy - that's why astronauts strap themselves to gigantic exploding bomb things and blast violently into orbit on a huge column of flames. Air launched systems (be it from balloons or aircraft like Pegasus) have some plusses from range safety and bad-weather avoidance, and some modest gain from avoiding some of the atmosphere, but you don't get to orbit without that massive speed, and you won't get a balloon going at 14500 mph. -- Finlay McWalterჷTalk 23:36, 5 April 2012 (UTC)
- It's understandably hard to get your head around the forces involved, but if you did you'd see why the balloon idea is just silly. Have you ever had a water balloon explode in your hand because you tried to throw it too hard? Now imagine that you're not throwing something, but applying almost 10,000,000 pounds (4,500,000 kg) of force to it (forgive my abuse of units, fellow scientists). There is no "elastic material" in existence that could survive such a journey.
- Your entire thought experiment is unnecessary, however. You seem to assume that we could somehow save the amount of fuel needed to get into space by cutting down on the weight of materials used to build the rocket. Using the space shuttle as an example, the fuel needed to reach orbit weighs 20 times as much as the object it is lifting! So you see, our efforts would be much better spent trying to reduce the weight of the fuel, as Wnt points out above. -RunningOnBrains(talk) 07:13, 6 April 2012 (UTC)
- Also, a balloon is going to be far less aerodynamic; it'll tend to bulge out rather than be long and thin. Rockets have fins etc for stabilisation in the atmosphere; without a rigid structure these aerodynamic features would be less useful (imagine a floppy airplane). --Colapeninsula (talk) 09:18, 6 April 2012 (UTC)
= be-all end-all[edit]
I have the be-all end-all of all fuel-containing solutions!!!!
This eliminates 100% of the enclosing container.
This is what you do.
You FREEZE the rocket fuel in a long rocket-shaped cone, and melt off the bottom as you use it!!! You can space the distance between the burning unit and the bottom of the frozen rocket fuel such that just enough heat goes up to keep melting it. Granted, you still need loads of rocket fuel, but you now have ONLY a payload at the top of the chunk of frozen rocket fuel and a drip container and burning unit at a variable distance at the bottom:
P P = payload FFF FFF FFF FFF F = frozen rocket fuel FFF FFF FFF \D/ D = drip container, dripping down to burning unit | | | = variable length somewhat insulated pneumatic aparatus for keeping the burning | just far enough to melt what you want, with a tube inside to drip from D to xx /xx\ xxxx x = blastoff fire xxxx
For a mini rocket, how can you get ANY more streamlined than having NOTHING containing the rocket fuel? Depending on how fast you get into orbit, you can keep the whole thing in a cryogenic container until just the right moment, then blast off and hopefully get into orbit before it all melts. Does this math work, guys? — Preceding unsigned comment added by 188.6.92.6 (talk) 09:34, 6 April 2012 (UTC)
- Your problem isn't maths, it is engineering. I can't see how that design could possibly work - what's holding the engine onto the frozen fuel? You are also mistaken in thinking that the container is a significant issue. Let's take the Saturn V first stage as an example. Unfueled, it weighed 131 tonnes. The fuel tank (empty) weighed only 11 tonnes. The tank is only a fairly small amount of the weight. Conventional, multi-stage rockets are very well designed and are very efficient - you're not likely to improve on them without a lot of training and experience (you would be better off trying to invent whole new ways of getting into orbit - so much work has gone into rockets that they are pretty much as good as they'll get). --Tango (talk) 12:17, 6 April 2012 (UTC)
- I only read thru your second sentence, will be back later to finish. "what's holding the engine onto the frozen fuel?" - the engine is pushing up on the frozen fuel! even if it were not the frozen fuel would rest on it of its own pendulous weight, but the thrust makes this even greater than 1g (a lot greater than one g). so the same thing holds onto it as a server (waiter/waittress) who swings a plate from below to weave past something). more later. 188.6.92.6 (talk) 14:03, 6 April 2012 (UTC)
- Tango. I've read the rest of your comment. First of all, I welcome any suggestions as to 'new ways' to get into orbit other than rocketry. Secondly, let us say that the fuel tank weighed only 11 tonnes. Now let me ask you another question: how much do you think those 11 tonnes cost? How about designing them?
- How much is a safely and properly designed conventional rocket in MONEY TERMS, as opposed to a device that is meant to just push some frozen fuel up into the air by burning it off, just like an inverse candle: what could be simpler than a candle?
- I am talking, amateur rocketry for example. If all you have to do is freeze the rocket fuel (or maybe even gasoline!!) in a special shape, place it onto this aparatus, and have it lift off without having to jettison anything or have any moving parts or do anything other than control how fast the fuel melts into its recepticle - which can be done with a simple feedback loop (it can be purely mechanical if you want) moving the rocket exhaust out whenever the recepticle is more than half full and retracting it again if it is less than half empty? One thing nobody has done, however, is done the calculations: does frozen fuel stay frozen long enough to make it to space? Rentry is seriously hot - how about getting to space to begin with?
- This would be like a home kit you buy for very cheap, plus your fuel costs, a form to freeze your gas into and a trajectory, put it outside with your payload on top and blastoff into low earth orbit. I can't imagine anything simpler than this, if the physics allow it. 188.6.92.6 (talk) 16:17, 6 April 2012 (UTC)
- If the fuel isn't securely attached it will just fall off. Rockets don't fly smoothly through the air, there is a lot of turbulance. You have also neglected to consider the oxidiser. There is no oxygen is space, so you have to take it with you. Rockets generally have two fuel tanks, one containing the fuel itself and one containing liquid oxygen. They have to be mixed in just the right ratio just before being fed into the engine or the rocket doesn't work. --Tango (talk) 16:24, 6 April 2012 (UTC)
- Well we can't take frozen 02. i'll have to think about this... do you want to take up my other bright idea with me if stu doesn't? 188.6.92.6 (talk) 16:47, 6 April 2012 (UTC)
- If the fuel isn't securely attached it will just fall off. Rockets don't fly smoothly through the air, there is a lot of turbulance. You have also neglected to consider the oxidiser. There is no oxygen is space, so you have to take it with you. Rockets generally have two fuel tanks, one containing the fuel itself and one containing liquid oxygen. They have to be mixed in just the right ratio just before being fed into the engine or the rocket doesn't work. --Tango (talk) 16:24, 6 April 2012 (UTC)
- This would be like a home kit you buy for very cheap, plus your fuel costs, a form to freeze your gas into and a trajectory, put it outside with your payload on top and blastoff into low earth orbit. I can't imagine anything simpler than this, if the physics allow it. 188.6.92.6 (talk) 16:17, 6 April 2012 (UTC)
- Freezing rocket fuel sounds like a poor way of creating a solid fuel rocket. Now I suppose if you can have caseless ammunition you ought to be able to design some kind of rocket made entirely out of fuel. Modeling different rates of burning, creating fuel with good tensile strength, controlling the rate at which it burns so that it somehow remains firmly pressed against a nozzle (or otherwise forms its own?) ... Some kid at Caltech should come up with such a thing sometime for a class project. Somehow I doubt it would replace sanely made rockets though. Wnt (talk) 04:31, 7 April 2012 (UTC)
- I'm sorry, but all solid rocket fuel is frozen. If you mold it first, then it was liquid before it was frozen. Your point doesn't change my proposal at all. I am "that caltec kid." 134.255.3.231 (talk) 09:00, 7 April 2012 (UTC)
- Perhaps you should read combustion chamber. In some toy rockets and fireworks, the casing is made of paper, or there is no casing at all. In powerful rockets, the operational chamber pressure is large (because it is a rocket engine). Chamber walls are necessary for structural integrity. High pressure hot gas impinging on explosive fuel or on load-bearing structure can cause structural damage, even if only a tiny gas leak impinges on a tiny portion of the structural material. As was discovered during the investigation into STS 51-L, a very small amount of exposure can cause total structural failure. You are suggesting to expose the entirety of the structural, load-bearing portion of the rocket to the internal environment of the combustion chamber. This will cause dangerous explosions. If you are indeed building small rockets, consider taking ample safety precautions. Even small toy rockets can contain large amounts of energy. Before you ever fire up your rocket, use water to test structural integrity at high pressures - well above your design pressure - because when your design fails, and the water rapidly depressurizes, it does not expand volumetrically, unlike hot gas. Remember that at high temperatures and pressures, and in the presence of oxidizer, solid steel can be more flammable than gasoline. Rocket science is difficult because the inside of a rocket motor is a very unusual environment. Many of your intuitions about material strengths, time-constants, and general behaviors of material are dangerously invalid in this chemical and physical environment. It is for this reason that rocket engineering relies so heavily on rigorous theoretical analysis and very careful experimental validation of subcomponents. Nimur (talk) 16:37, 7 April 2012 (UTC)
- I'm sorry, but all solid rocket fuel is frozen. If you mold it first, then it was liquid before it was frozen. Your point doesn't change my proposal at all. I am "that caltec kid." 134.255.3.231 (talk) 09:00, 7 April 2012 (UTC)
