Wikipedia:Reference desk/Archives/Science/2013 May 17
| Science desk | ||
|---|---|---|
| < May 16 | << Apr | May | Jun >> | May 18 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
May 17[edit]
Cold myth[edit]
If its a myth that cold weather or being cold makes people catch a cold, then why do so many people get colds in such conditions? Clover345 (talk) 00:13, 17 May 2013 (UTC)
- You don't get automatically get a cold from cold whether, although, you are more prone to catching cold in cold weather. Plasmic Physics (talk) 01:04, 17 May 2013 (UTC)
- I don't know if I would call it a "myth" per see, more like "it's a complicated situation where the evidence is so overwhelming that there must be some kind of relationship, even if it's merely a correlation and not a causative one". Our article talks about it fairly briefly, but there are a number of links to more in-depth sources. Matt Deres (talk) 01:13, 17 May 2013 (UTC)
- See Correlation does not imply causation and Post hoc ergo propter hoc. It is true that "cold and flu season" is the winter in many places, and there are higher incidents of cold and influenza in the winter months, but there is little evidence that the cold temperatures themselves are directly to blame. The closest connection I have heard is that cold weather encourages people to spend more time indoors in close quarters with other people, which tends to exacerbate transmission of such illnesses, but that isn't something that's caused by cold temperatures per se. See this article from WebMD. --Jayron32 02:51, 17 May 2013 (UTC)
- I admit it goes against the mainstream, but I personally believe that cold weather does play a direct role. The mechanism is that when you are out in the cold, the temperature of the body surface drops, including the nasal mucous membranes. This drop reduces the efficiency of immune responses pretty dramatically, and makes it easier for viruses to take hold. There is literature supporting the existence of this process, but so far no really strong evidence that it plays a major role. Looie496 (talk) 03:15, 17 May 2013 (UTC)
- The Science desk is no place for spreading common superstitions that fly in the face of established science. You pointed out, correctly, that there's no strong evidence to support the purported mechanism. On the other hand, there's very strong evidence indicating that the flu spreads more easily when people are in close proximity in an enclosed area, and very strong evidence that people spend more time indoors during the winter. There's no need to fish around for complicated explanations when the obvious one works just fine. --Bowlhover (talk) 03:47, 17 May 2013 (UTC)
- [citation needed] Louie, please, [citation needed]. --Jayron32 03:48, 17 May 2013 (UTC)
- I admit it goes against the mainstream, but I personally believe that cold weather does play a direct role. The mechanism is that when you are out in the cold, the temperature of the body surface drops, including the nasal mucous membranes. This drop reduces the efficiency of immune responses pretty dramatically, and makes it easier for viruses to take hold. There is literature supporting the existence of this process, but so far no really strong evidence that it plays a major role. Looie496 (talk) 03:15, 17 May 2013 (UTC)
- Our article flu season discusses this a little and lists some common potential explanations.Phoenixia1177 (talk) 04:03, 17 May 2013 (UTC)
- Influenza and the common cold are unrelated diseases. HiLo48 (talk) 04:06, 17 May 2013 (UTC)
- Sorry about that, I'm a little foggy since I actually have the flu right now; anyways, I read that as flu not cold for some reason. My mistakePhoenixia1177 (talk) 04:26, 17 May 2013 (UTC)
- Flu and colds don't occur much mid-winter, at least here in Australia with mid-winter temperatures around 0 to 15 C diurnal cycle. They occur more at the beginning or end of winter. So that suggests that low temperatures affecting the immune system is not the complete answer. My own experience, which my GP has confirmed, is that there is a flu peak in February, ie summer. There has been some coverage of this in the news media in the last year or so. The Public Health authorities have surmised that it corresponds to flu peaks in Europe, carried to Australia by tourists and business travellors. In Autumn here, the weather tends to rapidly fluctuate. In the last week or so we've had daily maximums of 20 C and nice sunshine clear days, but yesterday it changed to wet and 25 C. I think this confuses the body somewhat - 25 C today seems pretty warm, but mid-summer it would fell damm cold. Probably tomorrow it will drop back to 20 C. Wickwack 121.221.228.142 (talk) 06:14, 17 May 2013 (UTC)
- Low vitamin D levels at the end of winter can play a role here, the immune system doesn't work properly if you are deficient, see here. Count Iblis (talk) 14:19, 17 May 2013 (UTC)
- Flu and colds don't occur much mid-winter, at least here in Australia with mid-winter temperatures around 0 to 15 C diurnal cycle. They occur more at the beginning or end of winter. So that suggests that low temperatures affecting the immune system is not the complete answer. My own experience, which my GP has confirmed, is that there is a flu peak in February, ie summer. There has been some coverage of this in the news media in the last year or so. The Public Health authorities have surmised that it corresponds to flu peaks in Europe, carried to Australia by tourists and business travellors. In Autumn here, the weather tends to rapidly fluctuate. In the last week or so we've had daily maximums of 20 C and nice sunshine clear days, but yesterday it changed to wet and 25 C. I think this confuses the body somewhat - 25 C today seems pretty warm, but mid-summer it would fell damm cold. Probably tomorrow it will drop back to 20 C. Wickwack 121.221.228.142 (talk) 06:14, 17 May 2013 (UTC)
Up above someone said, "cold weather encourages people to spend more time indoors in close quarters with other people, which tends to exacerbate transmission of such illnesses". So in places like Phoenix, Arizona, for example, where very hot Summer weather drives people indoors in close quarters with other people, are there more colds in Summer than in Winter? 148.177.1.217 (talk) 15:24, 17 May 2013 (UTC)
- Some viruses causing common cold (Rhinovirus) require a relatively low temperature to proliferate. This may explain the connection to low ambient temperatures. Ruslik_Zero 19:41, 17 May 2013 (UTC)
Type 1a supernova with a neutron star instead of a white dwarf? (a twofer for that star)[edit]
Hi, could a neutron star accrete mass and blow apart roughly like a white dwarf is believed to in a type 1a? I suppose the nuclear combustion and light curve would be different, if it even could happen. Thanks199.33.32.40 (talk) 00:39, 17 May 2013 (UTC)
- Going by neutron star#Binary neutron stars, yes. A neutron star in a binary system can (potentially) accrete sufficient mass from its companion star to collapse into a black hole; I don't have the reference listed in the footnote handy, so I can't comment on just how violent the process would be or how exactly its appearance would be expected to differ from a Ia supernova. You might also be interested in the (plausible, but as-yet hypothetical) Thorne–Żytkow objects: red giants which have collided with neutron stars, and which may for some hundreds of years have a neutron star slowly spiralling in towards their cores. If the pair of stars are sufficiently massive, a supernova explosion and black hole may result; if the stars aren't massive enough, then you just end up with a heavier neutron star. TenOfAllTrades(talk) 02:24, 17 May 2013 (UTC)
- A Type Ia supernova occurs when accretion ignites nuclear fusion of carbon on a white dwarf. Nuclear fusion is impossible on a neutron star because there are no nuclei--the entire star is made up of closely packed neutrons, without any protons or electrons. So no, a neutron star can't undergo a Type Ia supernova. --Bowlhover (talk) 04:04, 17 May 2013 (UTC)
- Also, the bounce that happens during the collapse of a star into a neutron star is essential for converting an implosion into an explosion. No such bounces happens when a neutron star converts into a black hole. Dauto (talk) 14:22, 17 May 2013 (UTC)
Toluene[edit]
Is toluene used in consumer products anymore? 24.23.196.85 (talk) 06:26, 17 May 2013 (UTC)
- It is sold as a solvent for use in arts and crafts. Plasmic Physics (talk) 07:06, 17 May 2013 (UTC)
- If you're looking at making TNT, some printeries sell nitric acid. Plasmic Physics (talk) 07:08, 17 May 2013 (UTC)
- Let's not forget sulfuric acid, you'll need that for a catalyst. It is sold as battery acid, however, you'll need to remove impurities before you can use it. Plasmic Physics (talk) 07:22, 17 May 2013 (UTC)
- Toluene's availability as a consumer product may be more strictly regulated in your country. Plasmic Physics (talk) 07:23, 17 May 2013 (UTC)
- You'll find all these useful tips in 'The Terrorist's Handbook', made infamous for being officially blacklisted by the US government for obvious reasons. That shouldnt stop you from finding a copy online. It has several versions and comes under various titles. Plasmic Physics (talk) 07:29, 17 May 2013 (UTC)
- Will the US govt blacklist you soon? HiLo48 (talk) 07:40, 17 May 2013 (UTC)
- What Plasmic left out is that one of the steps in the recipe will result in immediate vaporization of the person attempting to make the bomb. Exactly which step that is, of course is classified info. ←Baseball Bugs What's up, Doc? carrots→ 07:56, 17 May 2013 (UTC)
- Will the US govt blacklist you soon? HiLo48 (talk) 07:40, 17 May 2013 (UTC)
- Aren't you confused with nitroglycerine? Plasmic Physics (talk) 08:00, 17 May 2013 (UTC)
- No, the so-called "Terrorist's Handbook" has a step which will destroy the bomb-maker instantly. They don't want you to know that, of course. As regards nitroglycerine, it was first created by a Nobel experimenter, whose test ended tragically when he tossed a bottle of the stuff to his assistant, Dr. Klutz. ←Baseball Bugs What's up, Doc? carrots→ 08:31, 17 May 2013 (UTC)
- Aren't you confused with nitroglycerine? Plasmic Physics (talk) 08:00, 17 May 2013 (UTC)
- I wasn't recomending that particular recipe. In any case, it's not so much a step, as it is a missing step that makes it so dangerous. Plasmic Physics (talk) 09:36, 17 May 2013 (UTC)
- I'm NOT looking to make TNT, I'm concerned about possible toxicity! Just who do you think I am, dammit?! 24.23.196.85 (talk) 02:05, 18 May 2013 (UTC)
- And yes, I know what TNT is made from -- but I wouldn't try this reaction at home (even if I had a use for the final product, which I don't!) because just one slip-up could blow you to pieces if you tried it! 24.23.196.85 (talk) 02:12, 18 May 2013 (UTC)
- Well, at least you've seen the use of adding constraints to your future questions. Plasmic Physics (talk) 00:26, 19 May 2013 (UTC)
- Toluene is mainly used as an octane booster additive in petrol/gasoline (especialliy since lead-based additives were banned), and as paint stripper and paint solvent. It is used in a multitude of less important consumer products. It is the main reason why dopey street kids sniff paint and petrol, thereby making themselves even more dopey. See "Uses" in the Wikipedia article. Wickwack 121.215.70.116 (talk) 10:05, 17 May 2013 (UTC)
- Thanks! That was the ONLY helpful comment here. So, it's used in gasoline and as a paint stripper? That means I better be more careful about vapor exposure when using these. 24.23.196.85 (talk) 19:09, 18 May 2013 (UTC)
- Don't forget, it's also used as a glue solvent in arts and crafts. Plasmic Physics (talk) 00:26, 19 May 2013 (UTC)
- Yes, that too. As with the other two items, that means I should avoid getting it on unprotected skin and limit my exposure to the vapors. (Especially since I seem to have a mild allergy to toluene vapors -- even small amounts of the stuff make me want to sneeze!) 24.23.196.85 (talk) 23:16, 19 May 2013 (UTC)
- Don't forget, it's also used as a glue solvent in arts and crafts. Plasmic Physics (talk) 00:26, 19 May 2013 (UTC)
- If you have an allergy towards toluene, I suggest you avoid dill weed. It occurs naturally in it. Plasmic Physics (talk) 03:22, 20 May 2013 (UTC)
- Pure toluene isn't so bad to handle, the issue is that it's really good at solubilizing plastics and goes right through your skin. I don't know about you, but I don't really want to passively absorb polypropylene for instance. Most plastics tend to contain at least some unpolymerized monomer, and they're usually not too good for you.(+)H3N-Protein\Chemist-CO2(-) 12:38, 17 May 2013 (UTC)
- It's also a pretty good solvent to use if you're trying to do Static Light Scattering of a generic hydrophobic polymer. It's also used as a standard in a lot of light scattering instruments.. typically used to normalize raw detector counts to a known Rayleigh Ratio.(+)H3N-Protein\Chemist-CO2(-) 12:38, 17 May 2013 (UTC)
Small golden brown beetle[edit]
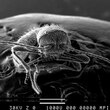
I found at home, middle of Germany, a small beetle and this was not the first one. I suspect that they live either from the wooden construction material of the house or the straw used for isolation. I made a few image and now I hope that somebody can help me name the little creature. I like the hairy bug especially that it looks like it has no eyes and a nice claw on his legs.
--Stone (talk) 12:14, 17 May 2013 (UTC)
- You own a scanning electron microscope? --Jayron32 12:40, 17 May 2013 (UTC)
- You don't need to own one to use one, he just needs access to one or have friends in the right places. Plasmic Physics (talk) 12:56, 17 May 2013 (UTC)
- For instance, I made friends with someone who is involved with the sample preparation for a SEM at the university which I attend. That is how I managed to procure gold sputter coated glass electodes for my electrolytic station. Plasmic Physics (talk) 13:33, 17 May 2013 (UTC)
- Wow! this makes a change from the "can you get a better image" routine. Incredible images, I like the claw especially. I would suggest you send the images to Whatsthatbug? who have been excellent on the half dozen occasions I sent them stuff from the UK. Richard Avery (talk) 13:25, 17 May 2013 (UTC)
- Probably not an exact match, but here's a golden-brown eyeless beetle, living in Germany, and named after Hitler: Anophthalmus_hitleri. That one seems to be pretty rare, but the whole genus Anophthalmus is eyeless, and I don't think eyelessness is that common in beetles... SemanticMantis (talk) 15:03, 17 May 2013 (UTC)
- I looked a little bit and I think it is a Niptus hololeucus. Especially in the German text it says it lives in Timber framing houses.--Stone (talk) 16:59, 17 May 2013 (UTC)
- well, this has certainly got to be the coolest Ref Desk question ever! μηδείς (talk) 15:33, 17 May 2013 (UTC)
In the SEM images it is hard to guess where the beetle has its eyes.--Stone (talk) 17:25, 17 May 2013 (UTC)
- These are REALLY good SEM images. Well done! And yes, you can see the compound eye clearly in the first and in the last SEM image. You can even make out the ommatidia pattern. This is a small beetle species, so the total ommatidia count is rather low --Dr Dima (talk) 21:35, 17 May 2013 (UTC)
- Where are these compound eyes? Are they the bulges lateral to the antenna roots? They look hairy. μηδείς (talk) 02:28, 18 May 2013 (UTC)
- You can see the eye most clearly in the first SEM image ("front and below"), posterior to the right antennal socket (that's the one on the left side of the image). The very top of the eye is occluded by the pedicel -- the second "piece" from the base -- of the right antenna. --Dr Dima (talk) 03:25, 18 May 2013 (UTC)
- Where are these compound eyes? Are they the bulges lateral to the antenna roots? They look hairy. μηδείς (talk) 02:28, 18 May 2013 (UTC)
I think it is a Niptus hololeucus. Anybody disagree?--Stone (talk) 05:30, 18 May 2013 (UTC)
- Not an entomologer, but checking for discrepancies in things I know are often diagnostic for insects like antenna segment count I see no reason to believe it is not N. hololeucus. μηδείς (talk) 20:01, 18 May 2013 (UTC)
why silver reduces gold?[edit]
Why atomic silver reduces ionic gold? Thanks! — Preceding unsigned comment added by 134.130.94.148 (talk) 13:56, 17 May 2013 (UTC)
- See Reducing agent. Plasmic Physics (talk) 14:02, 17 May 2013 (UTC)
- Also relevant is Reactivity series and Standard electrode potential and Standard electrode potential (data page). --Jayron32 23:57, 17 May 2013 (UTC)
Sleep theory[edit]
| This discussion has been closed. Please do not modify it. |
|---|
| The following discussion has been closed. Please do not modify it. |
|
Hi guys, I'm an MCB graduate student. I've never really edited Wikipedia. At first I thought I'd edit the Sleep page just to throw this simple sleep hypothesis/theory out there and let you guys run with it, but that didn't seem like the right place to post it; then I saw the "talk," page, but that warned that it wasn't the place either. In any case, I've got more than enough work to do so feel free to take a critical look at this, get it to anyone who might be interested, and take it from there: Sleep Theory I think the brain needs 1) "mental" energy to initiate sleep, 2) enough experience during the day to have something to do (process) during sleep, and obviously 3) environmental conditions conducive to initiate/maintain sleep: (OMG, this looks terrible posting from MS Word to here...I apologize in advance. Here's a slightly better version on the blog I'm making (which looks awful as well!) that's slightly better: http://johnfial.wordpress.com/sleep-theory/ 1. Initiation. PFC (Pre-Frontal Cortex) is likely initiating that “sleepy” feeling. It does this based on biochemical energy reserves (brain, liver? Unsure...) based on last ~48hrs of physical activity levels. PFC probably un-couples (or “unlocks”) the brain from major skeletal muscular nerves (keeping the physical body relatively motionless), and allows the rest of the brain to: a. REM: Unrestricted neural activity, maintenance of neural networks, strengthening of networks used throughout the day – especially those most-depleted of energy (high neurotransmitter/reuptake use, for instance, in neural networks associated with learning a new “riff” on the guitar) b. NON-REM: Light neural activity? Probably heavy metabolic maintenance / physiological preparation pathways. (A singer's/public speaker's brain, for instance, might “run simulations” using vocal physiological pathways during NON-REM phases. Just a hunch – I have no evidence to support this idea.) i. Reproductive pathways ii. Immune system pathways/support 2. Was there enough neural use during the day for the brain to “process” during sleep? a. Learning b. Skill acquisition/improvement c. Variation of mental/physical activities 3. Environmental factors (Both Initiation & Maintenance) a. Light & circadian rhythm b. Sound i. & vibration? Research: are there any sleep issues correlated to areas with excess ULF noise? c. Smell (try going to sleep with heavy smoke in the room!) d. Temperature e. Nutrients required to maintain metabolic (& physiological) pathways? i. Nutrients: All else being equal (physical activity + mental experiences), macro-nutrient deficiencies likely cause the biggest sleep challenges 1. Amino-acid or fatty-acid deficiency? Sleep is DONE. Wake up! Go find some food! 2. Micro-nutrient deficiency? Possibly responsible for smaller-scale issues. a. REMEMBER that micro-nutrient “intake” (i.e. through the mouth, via food or supplements) does NOT NECESSARILY mean those nutrients get into the bloodflow or transported into the cells that need them! ii. Drugs: Any “positive” drugs during the day may have “negative” side-effects at night. 4. Maintenance: Next sleep cycle? a. Were physical activity levels in last ~48hrs enough to continue into next sleep cycle? b. Enough neural/mental activity during day to justify staying asleep another cycle? c. Do environmental conditions (external world & internal nutrients) persist? — Preceding unsigned comment added by A957835895 (talk • contribs) 21:20, 17 May 2013 (UTC) |
- Sorry, but nowhere in Wikipedia is the place for unsourced original speculation such as this. As an encyclopedia, everything here needs to be referenced from reliable secondary sources. As an science graduate (I'm assuming MCB is Molecular and Cell Biology?), you should know that hunches such as these are useless without evidence, or at least, some plan for experiments to produce that evidence. Rojomoke (talk) 21:56, 17 May 2013 (UTC)