Wikipedia:Reference desk/Archives/Science/April 2006
April 1[edit]
is this supposed to be funny?[edit]
for some reason whenever I edit a question it duplicates the content at the bottom of the page instead of editing the origional? Are the developers having a bit of april fools fun with us?--205.188.116.74 00:14, 1 April 2006 (UTC)
- What the hell, April 1st just jumped to the middle of the page? this isn't funny--205.188.116.74 00:15, 1 April 2006 (UTC)
Soda Vending Machines[edit]
On a hot summer's day, there's nothing better than an ice cold soda from a soda vending machine. These machines obviously act like refrigerators, lowering the temperature of the drink as cold as possible without freezing it.
But what happens when the vending machine is outdoors and the weather is below freezing? Is the vending machine essentially acting as a heater to bring the soda up above freezing temperature? How is this done? Odd when you think about it, buying a nice warm soda on a cold winter's day! Am I understanding things correctly here?Loomis51 00:26, 1 April 2006 (UTC)
- not really, aren't they just simple refrigerators, with some sort of thermostat to tell them when to turn on and off? also, who leaves a vending machine outside in the winter?--205.188.116.74 00:40, 1 April 2006 (UTC)
- also you're talking about water with great amounts of sugar dissolved in it and compressed to 2 atm preasure, so it isn't exactly going to freeze at 0oC--205.188.116.74 00:41, 1 April 2006 (UTC)
- K, maybe I should point out, I'm from Canada, where the temperature drops FAR below freezing. I should also point out that it's not unusual at all here to see a soda machine outside, at say, a gas station or whatever. I undertsand that the sugar content or increased pressure may affect the freezing point, but when its -25C I don't think those factors are relevant. My best guess is that the freon (or whatever other gas) used in refrigeration is kept at a constant temperature, slightly above freezing, and air is blown over it having the effect of keeping the entire "machine" slightly above freezing. So, it a weird sense, the vending machine is actually acting as a heater. I'm just wondering if anyone has a better explanation.Loomis51 01:14, 1 April 2006 (UTC)
- I would think 2 atms worth of compressed CO2 would be more than enough to keep it from freezing regardless of temperature, I'm still not quite sure what the point of an outdoor vending machine would be, and why it would be preferable over the indoor variety, especially in a region that gets, as you said, below 248.15 kelvin outdoors--205.188.116.74 01:26, 1 April 2006 (UTC)
- You can freeze soda in an ordinary freezer, much less a Canadian winter. Usually outdoor vending machines are emptied in winter - I can't recall seeing a full one in mid-winter. Rmhermen 03:18, 1 April 2006 (UTC)
- More importantly: can a coffee machine keep coffee at the right temperature in a blast furnace? -Obli (Talk)? 00:43, 1 April 2006 (UTC)
- I've accidentally left a can of 7-Up in my parents' car once in sub-zero weather. The can froze and "exploded". - Cybergoth 04:12, 2 April 2006 (UTC)
Why are so many responses questioning the existence of outdoor vending machines? Where have you been living, under a rock? Trust me, they exist, and yes, even in sub-zero climates. As for the sugar explanation, haven't you ever heard of a popsicle? They freeze just fine in only slightly below freezing environments, and they're pretty much all sugar. Finally, as for the CO2/2atms argument, fine, I'll rephrase the question slightly: instead of a soda machine, make it a Snapple machine. No increased pressure, no carbonation. Loomis51 14:36, 2 April 2006 (UTC)
- Good question. My guess is that as soon as the temperature of the soda is 32 F or 0 C or otherwise equal to or below whatever it's supposed to be, the refrigerator shuts off. I doubt there's a PLC in there that will begin to heat up the soda at some minimum temperature. But, I would think it possible to turn a refrigerator into a heater. As I recall a refrigerator is a kind of heater in "reverse". I believe a refrigerator expels air that is hotter than the ambient temperature (look at the coils on the back of your unit) and also colder than the unit's temp (i.e. inside the actual unit). So if you reversed that...well you'd have a kind of heater. In your example the unit would be pumping out air onto the street that is colder your Canadian winter air mass and then pumping in air warmer than the inside of the unit. Check out Refrigeration cycle and Carnot heat engine and if you're still in doubt studying this should give you a definitive answer:
http://www.amazon.com/gp/product/0072402962/002-8259327-5014420?v=glance&n=283155
-Snpoj 03:26, 3 April 2006 (UTC)
A refrigerator usually is insulated, so as to keep the outside heat outside. That has the added bonus of keeping inside heat in, so if the temperature outside plunges far below what the vending machine is at, it'll take quite a while for the (relative) heat inside to leak outside. So even if there's no reversal of the refrigeration process, the machine will act like a thermos. kmccoy (talk) 01:46, 8 April 2006 (UTC)
IUPAC name of PMMA[edit]
Is the IUPAC name of polymethyl methacrylate (plexiglas) "poly(methyl 2-methylpropenoate)" or "poly(methyl 2-methylpropanoate)" or "poly(methyl 2-methylpropionate)" ? The article was recently changed. Thanks, AxelBoldt 01:02, 1 April 2006 (UTC)
- It's poly(methyl 2-methylpropenoate). The monomer, methyl 2-methylpropenoate, has a double bond, hence the -en-. —Keenan Pepper 15:22, 1 April 2006 (UTC)
- Thanks! AxelBoldt 18:18, 1 April 2006 (UTC)
Why Did The Elephants Survive?[edit]
I can see how crocodiles and hippos survived the Cretaceous metorite impact that killed the other dinosaurs, but what about elephants? They couldn't all have been in the water at the time it happened. And they were too big to be underground with the small ratty mammals from whence we evolved. Why do we still see elephants today? --Fuhghettaboutit 01:15, 1 April 2006 (UTC)
- Elephants didn't exist at the time of the dinosaurs. They're mammals, and the only mammals that possibly existed then were those "small ratty animals" that you mention that we evolved from. Just like us, believe it or not, elephants too evolved from those "small ratty mammals". Loomis51 01:24, 1 April 2006 (UTC)
Also, I think you need a little more research if you think it was a Cambrian explosion. The Cambrian Period ended hundreds of millions of years before the dinosaurs existed. You're thiking of the K-T Boundary at the end of the Cretaceous Period, probably caused by the impact of a meteor or asteroid near what is now Chicxulub in Mexico. Elephants - as Loomis points out (and hippos too for that matter) evolved far later than that impact. Grutness...wha? 01:34, 1 April 2006 (UTC)
- The Cambrian explosion was not an explosion like a bomb; it was an explosion in the number and complexity of species. Maybe the original poster was having us on a bit? A couple of people fell for it pretty good :-). --Trovatore 01:47, 1 April 2006 (UTC)
- Really sorry guys...I thought everyone would just chuckle (or not), but realize it was an april fools question--hard to imagine anyone packing that many misunderstandings (and I thought funny ones at that) into one question. The cambrian explosion...Boom!. --Fuhghettaboutit 01:57, 1 April 2006 (UTC)
- Didn't think of that. It's still March 31 in Canada. --Trovatore 01:59, 1 April 2006 (UTC)
- "crocodiles and hippos survived [the ... Explosion] that killed the other dinosaurs." fell into the category of, so ridiculous it just had to be serious (:205.188.116.74 02:02, 1 April 2006 (UTC)
- Didn't think of that. It's still March 31 in Canada. --Trovatore 01:59, 1 April 2006 (UTC)
- Really sorry guys...I thought everyone would just chuckle (or not), but realize it was an april fools question--hard to imagine anyone packing that many misunderstandings (and I thought funny ones at that) into one question. The cambrian explosion...Boom!. --Fuhghettaboutit 01:57, 1 April 2006 (UTC)
- Yeah, I thought as much, but the original question was no more crazy than a lot of the ones here, so I assumed
stupiditygood faith :) Grutness...wha? 02:01, 1 April 2006 (UTC)
- Don't feel bad. It isn't one of those questions that begin with "Everyone knows..." as in "Everyone knows that Bush hates all non-white people, so why does he hang out with Condoleeza all the time?" or "Everyone knows that God hates gays, so why do we have to have a gay guy on every television show?" or "Everyone knows that playing the lottery is the best form of investment so why don't we have a lottery for the penguins so they can get rich and buy H3's to get back and forth between the water and their breeding grounds?" --Kainaw (talk) 02:40, 1 April 2006 (UTC)
- Okay, this time I'm being serious. How does one go about punctuating an equilibrium and what are equilibriums made from that they're so hard to pierce? --Fuhghettaboutit 02:40, 1 April 2006 (UTC)
- I prefer to punctuate equilibrium like this: "Equilibrium!!!!.....?" EWS23 | (Leave me a message!) 02:46, 1 April 2006 (UTC)
- Okay, this time I'm being serious. How does one go about punctuating an equilibrium and what are equilibriums made from that they're so hard to pierce? --Fuhghettaboutit 02:40, 1 April 2006 (UTC)
Once one accepts the dual nature of light as both a particle and a wave, then its clear that the inverse multiple ratio of the retrograde rotation of the planet Venus provides the best explanation for how one goes about punctuating an equilibrium. It's elementary. Loomis51 02:55, 1 April 2006 (UTC)
- whoa! But you forgot to reverse the polarity of the neutron flow! Grutness...wha? 06:09, 1 April 2006 (UTC)
My first response was actually serious. To be honest I didn't actually take into account the "Cambrian Explosion" part because, to be honest, I'm not all that familiar with Earth's ancient past. I just answered with what I do know, which is, that elephants did not exist at the time of the dinosaurs. However, having given the matter further thought, I think the truest answer to the question is that the elephants survived underwater by using their trunks as snorkels. Loomis51 14:06, 2 April 2006 (UTC)
Now let me do my elephant impression by turning my pants pockets inside out for ears and unzipping my pants. :-) StuRat 09:23, 4 April 2006 (UTC)
Vactrains[edit]
The above question about maglev trains got me reading the Vactrain article. In the article, it says that these trains could potentially move at 4000-5000 mph. My question is about how this would affect the people in the train. Wouldn't they be subjected to rather high g forces and wouldn't that limit the number of people that could ride the train? I would think it would be at the very least uncomfortable for the elderly and small children. Dismas|(talk) 01:19, 1 April 2006 (UTC)
- 1 G is a surprisingly fast, yet mild acceleration. It's comparable to a 0-60 time of 2.8 seconds, but anyone who's experienced fast acceleration in a sports car can tell you that when properly cradled by a seat it's not any sort of violent force on the body. Night Gyr 02:00, 1 April 2006 (UTC)
- Remember that G forces and speed are only indirectly linked - the critical factor is acceleration, is how quickly do you reach your speed of 4000mph? And once you're at that speed, the passengers won't feel a thing, they can walk around as if the train was stationary (think about the last time you were on a normal train - unless you look out the window, there is no way for you to tell how fast you're going if the train's speed is constant). As for acceleration, a plane taking off subjects you to about two g's while accelerating down the runway, if you're sitting down, those kinds of forces hardly matter. It's highly unlikely a vactrain would accelerate at more than about the acceleration of a plane taking off at worst, so you would just need to be sitting down for the acceleration (which would probably last around three or four minutes), and then you can walk around as normal. Unless something went wrong, the train would have no reason to slow down during the journey, and once approaching the other end, the controller would light up the "fasten seatbelt" sign, you'd sit down for the deceleration, and that would be that. I don't really understand your comment about limited capacity - all you need is a seat with a seatbelt for every passenger, just like in an airplane - and, as you probably know all too well, you can cram a lot of people into a plane like that! :) — QuantumEleven | (talk) 09:44, 1 April 2006 (UTC)
However, slowing down at 1G (or even significant fractions thereof) would not be practical, as that would throw people out of their seats. My guess is that vactrains would a) accelerate at a lot less than 1G, and b) might use seats that swivel around mid-trip, so that the deceleration forces you back into your seat rather than out of it. But in any case, if you assume something quite mild like 0.3G, a train would take about 1040 miles to accelerate to 5000 mph. That sounds like a lot, but if you were travelling from New York to London the trip is about 3500 miles, so more than 1/3 of the trip would still be done at peak velocity. A trans-Pacific vactrain could potentially go even faster, or spend longer at vmax. --Robert Merkel
Strike Ridges?[edit]
Hi all - I'm in the process of trying to get an article up to FA standard, and therefore trying to get rid of redlinks, even if it means writing stubs on them myself :) It's a geography article (The Catlins), and one of the redlinks is in the geology section, which talks about strike ridges. I have no idea what a strike ridge is, and can't seem to find a definition of them anywhere. Anyone know anything about them? Grutness...wha? 02:06, 1 April 2006 (UTC)
- "A strike ridge is a linear, asymmetric ridge formed by the differential erosion of inclined bedrock layers. One flank of the strike ridge is a steep slope cutting across several bedrock layers (antidip or scarp slope). In contrast, the other side of the ridge is a less steep slope conforming to the slope of the underlying bedrock layer (dip slope)." [1] --Fuhghettaboutit 03:21, 1 April 2006 (UTC)
- Ex-the-LLENT! Many thanks! (Heh... I can use that for a stub article now!) Grutness...wha? 06:13, 1 April 2006 (UTC)
- Welcome --Fuhghettaboutit 07:31, 1 April 2006 (UTC)
- Ex-the-LLENT! Many thanks! (Heh... I can use that for a stub article now!) Grutness...wha? 06:13, 1 April 2006 (UTC)
HIV[edit]
I work at a pharmacy and my boss told me today that some HIV drugs cause the virus count to be undetectable and someone getting an HIV test that has it and is on these drugs will show up negative in some cases. How is it possible to spread HIV if the viral count is undetectable and you show up negative on the test?
- Caveat: I am not expert on this topic. But it seems to stand to reason that the tests used are sensitive to a threshold level of the antibodies (or whatever it is they test for). The fact that a person's body has, say, one part per million rather than 20 parts per million (and thus is at an undetectable infection level), doesn't imply that a third party can't be infected. A tiny amount of virus enters the body and multiplies. Remember, it only takes one pregnant roach to infest an entire apartment building. Awaiting expert confirmation or chastisement. --Fuhghettaboutit 07:43, 1 April 2006 (UTC)
- Or, alternatively, you could read the AIDS and HIV test article. As far as testing goes, as well as testing for the presence of HIV, tests are usually conducted for the presence of AIDS antibodies, which the individual will have even if the virus has been reduced to undetectable levels. As far as transmission goes, while there does seem to be a connection between viral loads and infectivity, just because somebody has very low levels of virus in their blood doesn't necessarily mean that the levels are equally low in their semen or vaginal fluids. --Robert Merkel 14:15, 1 April 2006 (UTC)
One possibly more ominous reason is that the drugs may not treat HIV at all, but may only interfere with a particular testing method for HIV antibodies. Such a drug would be harmful, not helpful. Hopefully, clinical studies would show no reduction in mortality with such drugs and they would therefore not be approved for general use. StuRat 09:11, 4 April 2006 (UTC)
Eye Infections[edit]
I have been getting eye infections for the past year about every 3-5 weeks. I cant think of any reason why. I used to wear contacts, I stopped and I wear glasses now (because I thought it was because of the contacts), I dont touch my eyes, I threw away all my old eye drops, I wash my hands many times daily, and I have been to 9 different doctors and they act like its not a big deal. I told the last one, and he said "Well your eyes look perfectly healthy to me." That was a few days after I usd antibiotic eye drops. I got a convertable car about a year ago, I am not sure if its because I always drive with the top down and all the air that hits my eyes, but its driving me crazy. Any ideas or suggestions, or solutions would be appreciated.
- Put the top up when you drive. I'm not kidding. I've had nothing but eye misery when driving around in an open convertible. Keep track of your symptoms in a notepad or something. Record what your symptoms are and when and where they began. If you treat the symptoms yourself, record that and the effectiveness of the treatment. You may want to have a friend take a picture of your eyes when the problem occurs so you can show the doctor. I had a year or more of bad experiences with eye problems as a kid and by the time I got to a doctor everything was always just fine. Till my mother took me to the emergency hospital during a really bad episode. I got the right diagnosis, proper care, medications and have been able to handle every similar problem since then with no trouble. Good luck. Ande B 07:13, 1 April 2006 (UTC)
- The best solution: wear some big goggles! (Blowtorching goggles, for best effect) Tzarius 02:16, 2 April 2006 (UTC)
I got an eye infection from using moisturizing eye drops, I don't use them at all anymore. not sure if you are using them though. 24.193.235.188 07:41, 1 April 2006 (UTC)
- Yes, eye drops, such as Visine, which claim they "get the red out", actually harm your eyes by blocking the supply of blood to the eyes (which is needed to prevent infections). StuRat 08:19, 4 April 2006 (UTC)
One thought, are you sure they aren't allergic reactions to pollen, etc. ?
Note that while a few cars are designed as convertibles, and have very little air flow into the passenger compartment during top-down driving, the majority are not. They essentially just cut the roof off a car with no thought to how that will effect the airflow. This can lead to major vortices of air, dust and bacteria whenever driven with the top down. StuRat 08:23, 4 April 2006 (UTC)
Try always wearing wrap-around sunglasses to block most of the debris from hitting your eyes. StuRat 08:28, 4 April 2006 (UTC)
"I saw a pretty woman driving on the freeway with her top down. What made it even better was that she was in a convertible, too." StuRat 08:28, 4 April 2006 (UTC)
Ship's pilothouse question[edit]
The windows of a commercial vessel's pilothouse often have a circular element inset into one or more panes of the windows of the pilothouse. This image from Google shows a pilothouse with two of these round objects visible. One in the window near the depthfinder, and one in a window in the background.
http://wildlifefotofilm.de/Pilothouse.jpg
What are these called? What are they for?
--Jeff
- I believe they are spinning windows, for use in (very) bad rainfall. Think of them a like windscreen wipers except the wiper stays still and the window moves instead. The disc in the window rotates at high speed, and keeps clear of water & mist even in the worst weather conditions. --Bob Mellish 08:44, 1 April 2006 (UTC)
- The name to search for is "clear view screen" (stub article). No wipers, just centrifugal force action. Femto 16:25, 1 April 2006 (UTC)
thermal power[edit]
what is a super thermal power station?--203.101.31.27 05:55, 1 April 2006 (UTC)
im guessing heat from the earth is used to generate steam, which turns turbines... it hink they tap into the sides of volcanos, i know theyre building a new one somewhere in the world currently 24.193.235.188 07:42, 1 April 2006 (UTC)
- A quick search of Google suggests that "super thermal power" is a term used in India for large but otherwise traditional coal-fired plants. Samw 00:10, 8 April 2006 (UTC)
Circuit diagram question[edit]
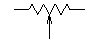
I'm attempting to construct a effect pedal for a guitar. I am having difficulty reading the circuit diagram. It indicates possibly a potentiometer by indicating a resistance and having an open arrow below it. The trouble I'm having is figuring out where the arrow connects to.
The arrows are at a certain level, and may be connected to the component on their left (or right). I have never come accross this sort of circuitry notation. (Also there is a dotted line in places, I am not sure what this means.) ---Brohan 06:12, 1 April 2006 (UTC)
- A symbol such as above, but with no connection to the wiper? Or can you find the symbol on this page. A dashed line enclosing an area of the diagram would probably mean those components are sheilded. Dashed lines as connectors would probably mean optional parts of the circuit. EricR 06:35, 1 April 2006 (UTC)
- I presume I forgot to inclue a link to what I'm talking about, [2]. After a closer look I have realized that the dotted lines are nothing special, there just are several circuit diagrams, and some of them have the dotted line. My inquiry about the potentiometer connection still stands. ---Brohan 15:43, 1 April 2006 (UTC)
- A pot has three terminals. Two of them are the opposite ends of the resistive track, and have a constant resistance between them that is the resistance marked on the pot. On a circuit diagram these terminals are shown as the two ends of a resistor. The third terminal, which is usually midway between the other two on the actual component, is connected inside to a thing called the wiper. This is represented by the arrow in the circuit diagram. When you turn or slide the pot, the wiper moves up and down the resistive track, so the resistance between the wiper and one end terminal goes up as the resistance between the wiper and the other end terminal goes down.
- The dashed line between two pots means that they are mechanically connected, or "ganged", together, so that they are both controlled by the same knob or slider. --Heron 21:09, 1 April 2006 (UTC)
- Well, there are lots of circuit diagrams at that link. Let's take the second image on the page, the first circuit diagram labelled "Basic Wah Circuit". There is just one potentiometer in the circuit, in the middle-right of the diagram, directly under the text "output". It's a 100K potentiometer, and the wiper is connected to a capacitor and then to the base of transistor Q2.
- When you say "open arrow", do you mean the downward pointing open triangles? There are five in that circuit (from left to right: on one side of a 470Ω resistor, an 82K resistor, 4.7μF capacitor, the 100K potentiometer, and a 10K resistor). They indicate a connection to the ground of the circuit, they're not symbols for a potentiometer. The 0V line (negative terminal) of the power supply would also be connected to ground. --Bob Mellish 21:23, 1 April 2006 (UTC)
- Thank you very much, I think my question has been answered. I was under the impression that the arrow was the symbol for a pot (The guy at my local electronics store said that it was). Infact the answer was staring right at me in Ground (electricity). It seems like a simpiler build now. Thank you very much for your help --Brohan 21:59, 1 April 2006 (UTC)
bermuda triangle[edit]
what is bermuda triangle exactly? why is everything sucked in? has any plane or ship escaped? how? do the people who have escaped have any story to tell? —This unsigned comment was added by Ac gokhale (talk • contribs) 10:02, April 1, 2006 (UTC).
- We have a pretty good article on the Bermuda Triangle. Short summary - all the talk about ships and planes vanishing under mysterious circumstances, supernatural forces and whatnot is rubbish, the rate of ships and planes sinking or crashing in the Bermuda Triangle is comparable to other areas of ocean, and most incidents have perfectly mundane explanations. — QuantumEleven | (talk) 09:24, 1 April 2006 (UTC)
- Lots of stuff about the bermuda triangle is myth....some planes vanished, probably crashed, over that area a long time ago and people started making up stories about it...after that a lots of disappearing cases aren't even near the bermuda triangle.... people just made up a lot of stuff.... in fact the bermuda triangle today is a busy waterway and a lots of ships pass through it everyday....even my dad's been there lots of times.... so..it means that everything doesn't get sucked in....some poor soul got a bad break a long time ago...thats it... Jayant,17 Years, India • contribs 14:34, 1 April 2006 (UTC)
- The question was kind of funny--"has any plane or ship escaped? how? do the people who have escaped have any story to tell?" Almost all planes/ships that entered the Bermuda Triangle have "escaped". They did so using thrust. The people who escaped do have stories to tell--they can tell you how wonderful their vacation was, or how blue the Bermuda Triangle's water was, or how they didn't get sucked into the ocean, or how foolish you are in thinking they would die, or... --Bowlhover 15:30, 1 April 2006 (UTC)
- Well, I sailed there in a 34-foot sailboat. The story I have to tell.. is.. well, it was pretty much like anywhere else in the Caribbean. We did run into some bad weather off Cape Lookout while heading in to Beaufort, but that was a week later and well outside the triangle. :) --BluePlatypus 23:42, 1 April 2006 (UTC)
- Wow, you're lucky to be alive! Melchoir 09:33, 2 April 2006 (UTC)
- Well, I sailed there in a 34-foot sailboat. The story I have to tell.. is.. well, it was pretty much like anywhere else in the Caribbean. We did run into some bad weather off Cape Lookout while heading in to Beaufort, but that was a week later and well outside the triangle. :) --BluePlatypus 23:42, 1 April 2006 (UTC)
- The question was kind of funny--"has any plane or ship escaped? how? do the people who have escaped have any story to tell?" Almost all planes/ships that entered the Bermuda Triangle have "escaped". They did so using thrust. The people who escaped do have stories to tell--they can tell you how wonderful their vacation was, or how blue the Bermuda Triangle's water was, or how they didn't get sucked into the ocean, or how foolish you are in thinking they would die, or... --Bowlhover 15:30, 1 April 2006 (UTC)
- It still doesn't explain the fact that when I was looking at Google Earth (this was some time ago, so the maps may have been updated), that I found a place where half of an island disappeared into a dark patch of ocean. The island? Bermuda. (Insert Twilight Zone sound effects here) --Confusing Manifestation 13:34, 2 April 2006 (UTC)
I once read a theory that beneath the seabed in some areas of the "Bermuda Triangle" is an unusually large number of pockets of gasses that occasionally get released into the atmosphere. The result is that occasionally, the atmosphere that the planes are flying through does not contain enough oxygen for the internal combustion engines of prop-planes to function properly and so they stall. Perhaps not a spooky enough explanation, but a logical theory nonetheless. Loomis51 14:23, 2 April 2006 (UTC)
That's a reference to the theory that deposits of Methane hydrate are behind some disappeareances in the Bermuda Triangle. I saw a TV documentary on it that showed a very tiny amout of methane in the air can cause airplane engines (of the type in the airplanes that went missing) to die. Also demonstated was showing how a massive release of bubbles below a ship causes it to sink because it is heavier than the foam it is now siting on. Also was proof the deposits do exist there and many other places on/under the sea floor. What is lacking is evidence that these deposits sometimes thaw out all at once (although we do know it is possible). WAS 4.250 17:34, 2 April 2006 (UTC)
- Didn't much more vessels sink in the Great Lakes? - Mgm|(talk) 11:47, 3 April 2006 (UTC)
- As a function of the water/air traffic, and the weather patterns in the area, the rate of 'disappearances' is no reason to think the Bermuda triangle is anything paranormal. Lots of travel + Finicky weather = Lots of losses. On the other hand, it could be due to the Methane hydrate in the ocean floor... that the Freemason's are harvesting to power their black helicopters and wage their secret war on the Elks!!!
"I lost my Bermuda shorts when swimming in Bermuda, does that count ?" StuRat 09:04, 4 April 2006 (UTC)
- Serves you right for doing elephant impressions. :--) JackofOz 13:13, 6 April 2006 (UTC)
marofaut peas[edit]
Hiya.im doing a project on marofat peas for my junior cert and im dead stuck.i need to know WHY they germinate faster when soaked and i need to know A.S.A.P!please help me as i cant find any relevent info. on any site!
- Try the Wikipedia article on seeds. You can find what you need in the section called "seed structure". --Bowlhover 15:42, 1 April 2006 (UTC)
I would think that sprouts, being like 90% water, would grow faster if more water is available to construct them. StuRat 08:10, 4 April 2006 (UTC)
about erp.[edit]
hi i have a project on enterprise rescurce planning (erp) and would like he softwares which come under this type of programming. —This unsigned comment was added by 210.210.63.84 (talk • contribs) .
- Have you tried to type ERP to the search box, that (among other uses) leads to Enterprise resource planning that links to List of ERP vendors and also List of ERP software packages? --Jan Smolik 16:51, 1 April 2006 (UTC)
Checking Data Transfer Integrity[edit]
I recently transferred (via ethernet cable) some files between two WinXP systems and later discovered about a third was corrupted. It may be that it was corrupted to begin with or it could be that something went wrong in the transfer (either way it doesn't matter, as the data in replaceable). I'm planning on moving about 100 GB of not-so-easily-replacable data from a WinXP system to another running Fedora Core 4. Is there are a program that will check the data integrity for me, during or after the transfer? --Username132 (talk) 15:16, 1 April 2006 (UTC)
- I'm doing exactly this (from WinXP to Fedora).
- I'm using Unison (file synchronizer).
- Unison uses the rsync algorithm.
- rsync uses MD4 for the data integrity check for each file on the source.
- (It would be marginally better to use md5sum, or better yet SHA-512, but I don't know how to fix it).
- I can use Unison as an independent check after some other program *claims* it copied all the files.
- Or I can use Unison to (efficiently) automatically detect which files are damaged or missing at the destination, and automatically re-send only those files, skipping over the good files (no matter how those good files got to the destination -- some other transfer program, or an earlier run of Unison).
- --68.0.124.33 (talk) 06:44, 19 January 2008 (UTC)
Unique Extraqpolation Method?[edit]
I devised a ethod for forecasting AIDS/HIV in the UK in 1987 and have never found it in any mathematical treatise. The method is detailed in www.AIDSCJDUK.info and I would be grateful if anyone knows whether it has been published elsewhere. Edward G. Collier MBCS CITP [email removed] —This unsigned comment was added by Edwardhfd (talk • contribs) .
[edit]
What is the most effective way for a robot (that has two sets of wheels allowing it to move forward and turn in its own footsteps, ie differential drive) to explore a rectangular area and find four objects (which will also be obstacles)? I had figured on some sort of a spiral that is changed to another sort of a spiral when a boundary is reached but I'm not sure if that will work perfectly.
- Are you trying to get us to do your engineering design project for you...? :) — QuantumEleven | (talk) 19:38, 1 April 2006 (UTC)
I'm not trying to get you to solve it for me, it still has to be programmed after I do it.
Put it, wheels and all, inside a hollow sphere touching the shere only with its wheels, as the sphere bumps things it will go around them or away from them in random ways, thus exploring the available space. WAS 4.250 01:03, 2 April 2006 (UTC)
- Like a hamster ball! Hee hee! - Cybergoth 04:19, 2 April 2006 (UTC)
Assuming you want to lay down a pattern, and not do a random search, I think a rectangular spiral starting from the outside of the rectangle would be most efficient in terms of fewest turns. When an obstacle is encountered, the robot should pass on the inside of the spiral. In the special case of an object in the center of the spiral, the robot can navigate either way around it. StuRat 08:05, 4 April 2006 (UTC)
Deoxygenated water[edit]
Hi, when people say that when water is boiled twice it is deoxygenated, ie. loses oxygen. So, if the molecule loses the oxygen bond, then don't you have oxygen gas and hydrogen gas left? But when it is boiled twice and supposedly "deoxygenated", then why are we still left with the water in the kettle? Thanks! Kilo-Lima|(talk) 21:43, 1 April 2006 (UTC)
- I assume they're talking about lowering the concentration of dissolved O2 gas, not removing the oxygen atoms from the water molecules themselves. Melchoir 22:03, 1 April 2006 (UTC)
- I boiled some water three times recently, and was left with only neutrons. They tasted horrible in my tea. --Heron 22:10, 1 April 2006 (UTC)
- They mean the air dissolved in the water. As temperature increases, the amount of gas you can dissolve in liquid decreases. As you increase the temperature, entropy becomes increasingly important to the equillibrium of free gas versus gas in solution, and the free gas state (which has higher entropy) becomes favored over the dissolved gas state. --BluePlatypus 23:02, 1 April 2006 (UTC)
- Yes, boiling must lower the oxygen dissolved in the water. I know this, because when I boiled the water in which my goldfish was swimming, it died. Must have been from a lack of oxygen, right ? :-) StuRat 07:58, 4 April 2006 (UTC)
Seriously everyone, where does someone look up this fact in Wikipedia? A cursory search of water and oxygen reveals nothing of the fact that warmer water has less disolved oxygen? One of my pet peeves is that there are lots of facts like this that defies the traditional encyclopedia organization structure. Samw 00:14, 8 April 2006 (UTC)
- Combine the power of a search engine with Wikipedia: Search for water dissolved oxygen and the first three articles are Oxygen saturation, Winkler test for dissolved oxygen, & Oxygen depletion. The first has a description of temperature and salinity affecting the amount of oxygen. -R. S. Shaw 07:02, 8 April 2006 (UTC)
April 2[edit]
Classical Physics[edit]
Greetings, Wikipedians!
I am now in my late 20s and recently an old fascination of mine has started bubbling up. I studied mathematics at the univeristy for a while (although I eventually gave it up and studied law instead) and I consider myself fairly competent in stuff like multi-variable calculus (even though I can't possibly call myself an expert). I have recently found my love for equations have started to come back to me. Everyone has to have a hobby, right? Anyway, specifically I've been fascinated with classical physics, i can spend a good chunk of a weekend (it annyos the hell out of my fianceé) studying things like this.
I never did study physics beyond high school, but I wish to pick it up again. Can anyone recommend a good book on classical physics for me? As I said, I'm fairly competent in advanced calculus, so it doesn't have to be a beginners pop-science type of a thing, but perhaps not too advanced either. I'm tempted just to pick up an annotated version of Newton's Principia, but as I understand, it's fairly archaic in it's mathematics and also fairly dense. Any recommendations?
Cheers!
Oscar
- Young's University Physics, or Benson's book by the same name seem popular to me. Both are introductory-level university texts that cover the basics of the whole field and are mostly classical, except for the obligatory chapter introducing the basics of quantum stuff. Neither of them require much more than some calculus skills. --BluePlatypus 03:28, 2 April 2006 (UTC)
- How about The Feynman Lectures on Physics? —Keenan Pepper 03:32, 2 April 2006 (UTC)
- I echo the Feynman lectures - they cover an incredibly broad range of topics, and are very well written. Admittedly, the maths doesn't get an awful lot more complex than second-year calculus, but I don't think it needs to be, either, if you're just starting out. — QuantumEleven | (talk) 09:40, 2 April 2006 (UTC)
- I recommend you give your fiancee a massage while you listen to the Feynman Lectures on tape. GangofOne 07:36, 2 April 2006 (UTC)
- Depending on your circumstances, H L Mencken's comment on physics may or may not be relevant to you and your fiancé: "It is now quite lawful for a Catholic woman to avoid pregancy by a resort to mathematics, though she is still forbidden to resort to physics and chemistry". :--) JackofOz 08:37, 2 April 2006 (UTC)
- It's not clear how the mathematical contraception works, (maybe he means the "rhythm method"?), but what I was getting at is you'll have a better life if you can get your spouse involved in your hobbies, maybe she'll enjoy the tapes so much she'll become intersested in classical physics, and more specifically, classical physiques. --GangofOne 07:47, 4 April 2006 (UTC)
- Depending on your circumstances, H L Mencken's comment on physics may or may not be relevant to you and your fiancé: "It is now quite lawful for a Catholic woman to avoid pregancy by a resort to mathematics, though she is still forbidden to resort to physics and chemistry". :--) JackofOz 08:37, 2 April 2006 (UTC)
- On a slightly separate note (I can't really recommend a good physics book, unless you're into Atmospheric Dynamics, in which case I'd recommend An Introduction to Dynamic Meteorology by J.R. Holton), have you considered becoming a Wikipedian yourself? We always need eager, well-rounded editors, and believe me when I tell you that the experience allows you to learn all sorts of interesting and eclectic information. EWS23 | (Leave me a message!) 08:43, 2 April 2006 (UTC)
I appreciate your fascination, since I share it also upon some other degree, but though classical physics remains a highly motivating subject, one must realize that the field has moved on also to some other, let be said, more esoteric fields that the masters of the old physics would have never dreamt of. Upon the coming of the New Age the philosophy of physics is also changing from reductionism to synchronicity. I'm afraid I am not helpful enough to recommend you books on classical physics (unless, of course, you have also developed an interest in Quantum Mechanics, in which case I recommend Thirty Years that Shook Physics by George Gamow), I am only able to suggest that in developing such an admirable interest in the field, you may also be interested to extend your knowledge into the more recent innovations of the area that has changed our thinking since Newton. But of course, all this is entirely supplementary and a grounding knowledge in classical physics is always good before you rush on to something else. Luthinya 09:48, 9 April 2006 (UTC)
Graphics Card/NIC combo in Fedora Core 4[edit]
I have a Cirrus Logic GD 5446 graphics card with built-in NIC. Although the graphic card part is detected and works on my Fedora Core 4 system, the network card is not recognised. When I plug in an ethernet cable connected to my modem, the green light (connection) comes on and the amber light (activity) flashes but according to Hardware Browser, it's doesn't come under 'Network devices'. Would installing a driver resolve this issue? There's one here but it doesn't specify an operating system. --Username132 (talk) 01:15, 2 April 2006 (UTC)
Is KDE installed?[edit]
It typed "yum install KDE" at the terminal in Fedora Core 4 but it didn't seem to be installing anything.
Here's how it went down;
[root@localhost numlockx-1.1]# yum install kde Setting up Install Process Setting up repositories updates-released 100% |=========================| 951 B 00:00 extras 100% |=========================| 1.1 kB 00:00 http://ftp.chg.ru/pub/Linux/fedora/core/4/i386/os/repodata/repomd.xml: [Errno 4] IOError: HTTP Error 404: Transfer-Encoding: chunked Date: Sun, 02 Apr 2006 00:46:26 GMT Content-Type: text/html; charset=iso-8859-1 Server: Apache/1.3.33 (Unix) Trying other mirror. base 100% |=========================| 1.1 kB 00:00 Reading repository metadata in from local files Parsing package install arguments No Match for argument: kde Nothing to do [root@localhost numlockx-1.1]# start kde -bash: start: command not found [root@localhost numlockx-1.1]# kde -bash: kde: command not found [root@localhost numlockx-1.1]# run kde -bash: run: command not found [root@localhost numlockx-1.1]# yum install kde Setting up Install Process Setting up repositories Reading repository metadata in from local files Parsing package install arguments No Match for argument: kde Nothing to do
How do I know if KDE is already installed? And if it is, how do I start it? If I started KDE, would it close down Gnome? My computer couldn't run two GUIs at the same time very well.
--Username132 (talk) 01:20, 2 April 2006 (UTC)
- Computers aren't smart enough to guess at what you want. Try "yum list | grep kde" to see what packages have "kde" in the name. Then, install the whole package name, such as "yum install kdebase". As you can see from the message you received, there is no packaged names "kde". --Kainaw (talk) 01:34, 2 April 2006 (UTC)
- I see, thanks :). It could have been more clear about things... how come "yum list | grep opera" doesn't produce any results, when I know "yum install opera" does something (I think Opera web browser). --Username132 (talk) 04:57, 2 April 2006 (UTC)
- Unless you've added repositories to the generic Fedora repositories, Opera is not included. Fedora only includes free, open-source programs. Opera is free now, but used to require payment (yes, there was a free preview version, but that isn't the point). Also, Opera is not open-source. Finally, depending on your version of grep, you may want to include the "-i" option to turn of case sensitivity, such as "yum list | grep -i SDL" --Kainaw (talk) 20:23, 2 April 2006 (UTC)
- Ah, thanks for the tip! --Username132 (talk) 00:33, 4 April 2006 (UTC)
chloroform[edit]
What does chloroform react with to make someone unconscious?
Scientific how does it?
- Chloroform is a general anaesthetic. According to the Wikipedia article on general anaesthetic:
- "It is now known that general anaesthetics act on the central nervous system by modifying the electrical activity of neurons at a molecular level by modifying the function of ion channels. This may occur by anaesthetic molecules binding directly to ion channels or by their disrupting the function of molecules that maintain ion channels." --Bowlhover 05:07, 2 April 2006 (UTC)
- Here's an article: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1328629
- Chloroform increases the permeability of the cell membrane to ions, which makes it harder to build up an action potential. —Keenan Pepper 14:19, 2 April 2006 (UTC)
Chloroform cannot be a true general anaesthetic, becuase if it were, it would paralyze the all the body's involuntary autonomous systems. For example, when a true general anaesthetic is used, the lungs no longer function and a breathing machine is required to keep the patient alive. From what I understand of chloroform, the subject may be "knocked out" but he or she is still able to breath...etc...Loomis51 21:44, 6 April 2006 (UTC)
Paint thinner chemical[edit]
I'm having trouble finding out the name of a chemical that is in some paint thinners and it becomes carbon monoxide when inhaled. Any guesses? KeeganB
- When fuels are burnt with insufficient amounts of oxygen, incomplete combustion can occur. Instead of the normal CO2, some CO is also produced. Some paint thinners contain (surprise!) isopropyl alcohol, also known as isopropanol, which can decompose and release CO gas. Isopropyl 05:46, 2 April 2006 (UTC)
I forgot to mention that this chemical is metabolized by the body and it becomes carbon monoxide because of the metabolization. KeeganB
- Are you sure it's carbon monoxide that it gets metabolized to? Toluene, the chemical that gives paint thinner its characteristic smell, is rather toxic; it breaks down into epoxide radicals in your body. Other than that, I have no idea what the chemical could be. Perhaps you could take a look at the packaging and look up the chemicals listed in the warnings? Isopropyl 06:29, 2 April 2006 (UTC)
- Paint thinners are generally a mixture of Hexanes (Toluene), Isopropyl, or Acetone. Really depending on the type of paint thinner you are purchasing. I don't know of any of these that would be metabolised by the body readily, more likely you're going to exhale the fumes. If any it would be isopropyl, and it's still not advisable to inhale or ingest it. This is why you wear a face mask and use paint thinners in well ventilated areas.--Tollwutig 17:53, 3 April 2006 (UTC)
SSH, PGP and CACert[edit]
I have three key-pairs: one for SSH, one for PGP and a "client certificate" from CACert. Don't all === these serve the same purpose? Is it possible to use the same key-pair for all three? —Masatran 06:52, 2 April 2006 (UTC)
- Typically, no. These (as far as I know) use different encryption algorithms, and even if you could have, say, the same private key for all of them, the public keys would differ. But why do you care? You'll certainly never interact with your own SSH key (
sshdis all that touches it), and a certificate is just a statement made and signed by the certificate authority that identifies you. Neither of those will involve you very often.
- To answer your other question: no, these are for different purposes. The SSH key is used to prove to SSH clients that your server is the same server they've used before and not an impostor. The PGP key is used by others to make messages readable only by you and by you to prove that you wrote something. The certificate is again some identification of you (perhaps your real name and a domain name of yours) signed by someone that you expect others to recognize and trust to make such identifications. --Tardis 18:47, 5 April 2006 (UTC)
--68.98.102.247 (talk) 03:33, 7 November 2013 (UTC) meetings for the hours/ of total for moeny so putt some them for the hours $______________
- There's no reason why, in a theoretical sense, you couldn't use a single public-private key pair for different applications. It's a question of the software supporting it. For example, I seem to recall that some SSH implementations allow the use of X.509 certs, and Italic textyou can extend OpenSSH to allow the use of PGP keys[3]. — Matt Crypto 19:26, 5 April 2006 (UTC)
- OK, after some research, I see that as least SSH and PGP are compatible through RSA and DSA. I thought PGP's encryption was some other variety. But I can't imagine that PGP could work together with X.509, unless what the certificate was for was the public key...? --Tardis 21:19, 5 April 2006 (UTC)
- X.509 certificates certify that a public key belongs to some entity, so there's no reason, in principle, why an OpenPGP application couldn't use a key from an X.509 certificate. It seems that PGPi supports X.509 certs[4], for example. For encrypting and/or signing messages, a key is just a key, and the differences are superficial ===
- OK, after some research, I see that as least SSH and PGP are compatible through RSA and DSA. I thought PGP's encryption was some other variety. But I can't imagine that PGP could work together with X.509, unless what the certificate was for was the public key...? --Tardis 21:19, 5 April 2006 (UTC)
--68.98.102.247 (talk) 03:33, 7 November 2013 (UTC)(file formats etc). The big issue is the PKI, i.e., how you can reliably find out someone's public key without being spoofed: web of trust (OpenPGP) vs a CA (X.509) vs, er, ad hoc verification (or none!) (SSH). — Matt Crypto 12:45, 7 April 2006 (UTC)
Satellites[edit]
Can you see Satellites in night with nakes eyes from the ground?
- Yes! But I've never seen one. This link tells you how to do it, as does this one. And this one tells you about the hobby. Hope this helps. Kilo-Lima|(talk) 11:25, 2 April 2006 (UTC)
- Iiridium flares, which are generated by the Iridium satellites' main mission antennas, are extremely bright (the brightest flares can reach magnitude -8, 28 times Venus's maximum brightness). Heavens Above provides predictions for iridum flares and HST/Envisat/ISS passes. Be sure to enter the latitude, longitude, and elevation of your house, not your city--the coordinates have to be very accurate. --Bowlhover 20:16, 2 April 2006 (UTC)
- They're not particularly difficult to spot - look up just after sunset or just before sunrise (the satellite above you needs to be illuminated while you need to be in darkness). If you see a star moving across the sky rapidly without blinking (if it's blinking, it's probably an airplane), you've just spotted a satellite. When I say "rapid" I don't mean rapid like a meteor (gone in about a second), but fast enough that it's easy to see it moving - it takes about a minute or two to cross the entire sky. Happy stargazing! — QuantumEleven | (talk) 21:31, 2 April 2006 (UTC)
- The ISS is particularly bright - about as bright as one of the brighter planets (say, Jupiter) I second Bowlhover's comment about Heaven's Above - it;'s a very good website if you want to find out when bright satellites will be overhead. Grutness...wha? 06:58, 3 April 2006 (UTC)
- You didn't exclude natural satellites, so I would add that the Moon is occasionally visible. StuRat 19:26, 3 April 2006 (UTC)
- A satellite in the sky often looks like an aircraft since they have a similar apparent angular motion. A commercial jet might fly 10 km high (about 30,000 feet) and travel at a speed of 1000 km per hour (about 600 mph, or 0.3 km/s). A low Earth orbit (LEO) satellite is about 30 times higher and travels about 30 times faster, so the motion looks almost the same (300 km altitude, 8 km/s). For an aircraft, you are usually seeing the lights - if it blinks, you know it is an aircraft. But you can only see a spacecraft when it is still in sunlight are you are in shadow, after sunset or before sunrise. Sometimes you can see the spacecraft enter the Earth's shadow, turning red and then fading quickly. elee 16:50, 6 April 2006 (UTC)
why is thepiratebay.org still up?[edit]
I would have thought that the RIAA/MPAA/(insert name here) would have shut it down ages ago. After all, how much closer to a blatant copyvio can you get?--Frenchman113 16:05, 2 April 2006 (UTC)
- Well, IANAL, but they do have a point that "Only torrent files are saved at the server. That means no copyrighted and/or illegal material are stored by us...". Also, it's possible the servers are in a country where the RIAA and MPAA have no power. Anyone know where the servers actually are? —Keenan Pepper 16:37, 2 April 2006 (UTC)
- Because they're not violating copyright law. They're not actually distributing the copyrighted material themselves. Merely linking to it isn't illegal. Sweden isn't the only country where this is probably the case, so the more pertinent question is perhaps why the Swedes are less prone to fold to empty legal threats? --BluePlatypus 17:27, 2 April 2006 (UTC)
- The Pirate Bay are frequently contacted by individuals and organizations requesting removal of copyrighted materials from their site. They (and their lawyers) find this extremely humorous, and post the correspondence on their site at the URI http://thepiratebay.org/legal (I think that's the right address). --WhiteDragon 19:20, 4 April 2006 (UTC)
- Almost - it's http://thepiratebay.org/legal.php. -- Marcika 21:48, 5 April 2006 (UTC)
- The Pirate Bay are frequently contacted by individuals and organizations requesting removal of copyrighted materials from their site. They (and their lawyers) find this extremely humorous, and post the correspondence on their site at the URI http://thepiratebay.org/legal (I think that's the right address). --WhiteDragon 19:20, 4 April 2006 (UTC)
Why do voltage regulators come as modules?[edit]
I was wondering why the voltage regulator on a server processor board comes as a module (VRM) instead of being soldered in place like I imagine it is on my PC motherboard. Are they prone to failure? --Username132 (talk) 14:31, 2 April 2006 (UTC)
- The answer is at voltage regulator module. --Heron 18:52, 2 April 2006 (UTC)
- So if it comes as a separate module, it must be fixed to a specific voltage? --Username132 (talk) 22:25, 2 April 2006 (UTC)
- It has a lot to do with costs. With a $5k-$30k server you're more likely to keep it a long time and upgrade processors instead of replacing the unit. If you are in the IT industry you know processor generations are about 6 months to 1 year. With a PC a system board usually only costs between $100-$300, while a server it can cost between $1000-$3000 just for the system board alone.
Now say you have a server you wish to upgrade the processors. Which is cheaper, to replace a VRM or an entire system board?--Tollwutig 16:23, 3 April 2006 (UTC)
Topic Suggestions[edit]
I need some topic ideas for my IB Extended Essay maybe you guys could help. The subject I want to do is in computer science. I have some knowledge of neural networks, genetic programing (algorithms), economics (could be relevant? modeling?) and cryptography (along with misc. computing knowledge). The essay must have an original thesis that sets out to prove somthing non-trivial (someone else is doing stuff involving computational ecomplexity). Any suggestions? (By the way: the project cannot be writing a program to do somthing (although that could be part of the essay)). Leonardo 16:17, 2 April 2006 (UTC)
Define the least intelligent worm that when let loose on the internet increases its intelligence without limit, given enough time and no serious eradication effort. The minimum computational skill set for unbounded automated learning. WAS 4.250 17:52, 2 April 2006 (UTC)
- How about building elementary logic gates with cells via DNA transcription? That's what I'm studying right now, and it's kind of interesting, I guess. Isopropyl 17:56, 2 April 2006 (UTC)
- An economics topic which interests me is ideal firm size. A program which accepts firm size and growth rates as input could calculate the ideal firm size for the maximum growth rate in several industries, study how the ideal firm size has changed in each industry over time, etc. Note that you would need to include companies which have declared bankruptcy, or you would introduce bias towards small firms. StuRat 19:16, 3 April 2006 (UTC)
- Hmmm, I think that's too economic and not computer science enough. As to isopropyl, that's an idea, but it requires more biology than I know (I guess I could try to pick it up). And as to WAS 4.250...Cool idea conceptually, but yeah that's beyond me. I wouldn't even know where to begin. I might do something with intelligent malware though. Hm...cryptovirology? Anyone have any topic ideas involving that? Leonardo 21:11, 4 April 2006 (UTC)
- An economics topic which interests me is ideal firm size. A program which accepts firm size and growth rates as input could calculate the ideal firm size for the maximum growth rate in several industries, study how the ideal firm size has changed in each industry over time, etc. Note that you would need to include companies which have declared bankruptcy, or you would introduce bias towards small firms. StuRat 19:16, 3 April 2006 (UTC)
good largescale spyware removal utility? for free?[edit]
suggestions?--172.153.199.58 17:13, 2 April 2006 (UTC)
- What do you mean by "large scale"? If you're looking for enterprise quality software you'll have to pay for it. The most popular & most frequently recommended consumer freebee apps are LavaSoft's AdAware, Spybot, and HiJackThis. Using all three provides a rather comprehensive solution. You can download these or search for others at majorgeeks.com. Also, take a class on editing the Windows registry if you use Windows. There are a number of free registry utilities but be careful if you don't know what you're doing. Registry errors can make your system nonfunctional. Ande B 22:56, 2 April 2006 (UTC)
- Adaware is a good antispyware, but what do you mean by largescale?--Frenchman113 22:59, 2 April 2006 (UTC)
- A good large scale spyware removal tool? None really get rid of all spyware as often you need to be in Safe Mode and do registry edits to get rid of the worst offenders. The best means is to prevent spyware by setting your firewall to block all traffic on port 80. Its the most effective preventative measure I have found.--Tollwutig 16:16, 3 April 2006 (UTC)
- Blocking all ports or physically disconnecting your computer from the network works even better. --Optichan 16:33, 4 April 2006 (UTC)
Integer precision[edit]
- In C++, what is the most portable way to get an integer type guaranteed to have at least n bits of precision?
- Is there any portable way to get an integer type that not only has exactly n bits of precision, but also wraps around modulo 2n on overflow? For example, an 8-bit type in which 100 + 200 == 44. —Keenan Pepper 17:14, 2 April 2006 (UTC)
- No. You will want to use sizeof to detect the size of the short an long types and then manipulate them as necessary. With 64-bit systems becoming popular, this is a real problem right now. --Kainaw (talk) 22:22, 2 April 2006 (UTC)
- For number 1, you could try GMP, which is fairly portable (certainly *nix and Windows) and has a C++ interface (it is C internally.) For number 2, that is what happens with the built-in integer types in C and C++, the problem is that the sizes of the various types (char, short int, int, long int) are not defined in the specs. -- AJR | Talk 22:36, 2 April 2006 (UTC)
- For small values of n, is it possible to use
- typedef struct { int eightBit : 8; } number;
- number a.eightBit = 257; // Stores 1 in a.eightBit Ojw 23:11, 2 April 2006 (UTC)
Okay, I didn't think #2 was possible. For #1, I was looking for something along the lines of stdint.h for C. I notice there's no <cstdint> in the standard C++ library... —Keenan Pepper 23:35, 2 April 2006 (UTC)
- <cstdint> will most likely appear in the next C++ standard update, but because of the glacial pace of the international standards process, you might have to do with <boost/cstdint.hpp> available at [[5]]. If you can limit yourself to Unix, the Single Unix Specification defines a similar <inttypes.h>, not to be confused with the C99 inttypes.h. 84.239.128.9 19:02, 3 April 2006 (UTC)
electronic marketplace[edit]
describe an electronic marketplace in which disc technology combines with the internet to provide information and services to consumers
- Do your own homework. --Bowlhover 19:27, 2 April 2006 (UTC)
- Pornography!! Tzarius 09:11, 3 April 2006 (UTC)
- Yeah I'd say pr0n is the major use of this.. also this stinks of a homework question.--Tollwutig 16:12, 3 April 2006 (UTC)
Handwashing[edit]
A friend told me that most soaps advertised as anti-bacterial actually can increase the amount of bacteria present on the hands. Is this true and why would this occur?
AO24 21:52, 2 April 2006 (UTC)
- Most likely, he was referring to the growth in super-resistant bacteria. By killing common bacteria quickly, only the resistant bacteria remains. Over time, the resistant bacteria will evolve into super-resistant bacteria that is completely immune to our common household anti-bacterial soaps, cleaners, and air sprays. All-in-all, the "anti-bacterial" craze can be seen as doing more harm than good. Not all bacteria will harm you. Many forms of bacteria help you. Killing the good ones is not a healthy thing to do. Making the bad ones resistant is not a healthy thing to do. The next time you see something that kills 99.99% of all bacteria, remember that it may only leave that 0.01% that can actually cause you harm. --Kainaw (talk) 22:11, 2 April 2006 (UTC)
- (After edit conflict)Your friend has, I think, gotten something confused, but there's a kernel of truth behind it. It's not that washing with antibacterial soap leads to more bacteria, but rather that it can lead to production of superbugs --bacteria that are resistant to normal treatment, and also kills off the helpful bacteria. It works like this: you wash with a soap containing a particular antibacterial agent. It kills 99% of the bacteria that are susceptible to it. The one percent then breed back to high numbers, but because they are the ones who survived the last treatment, they naturally have some defense to the agent you used which they pass on to their progeny. You use the same soap with the same agent again. This time, because the generation that are left are more resistant, only 70% die. They breed back to level. This cycle continues until you have bacteria that are very resistent to the agent in the soap. This is how evolution works, and is the same reason we have developed so many antibiotic resistant strains of microbes (which has been greatly increased by blanket prescribing of antibiotics where it is not necessary, as well as people's tendency to not take the full antibiotic series). --Fuhghettaboutit 22:12, 2 April 2006 (UTC)
- Not necessarily. If the "antibacterial" agent is just a disinfectant and not an antibiotic, then this shouldn't breed antibiotic resistance. People who use antibacterial soap might not wash their hands as carefully, so they don't end up killing as much bacteria on their hands as someone doing a proper hand-wash with regular detergent soap. - Cybergoth 02:13, 3 April 2006 (UTC)
- Even disinfectants can lead to resistant bacteria. Bacteria can adapt to chemicals in the environment as well as antibiotics. --Tollwutig 16:10, 3 April 2006 (UTC)
Killing off the good bacteria (which control the bad bacteria) could allow the bad bacteria to spread uncontrolled. I'm not aware of this being a problem on hands, but medicated douches can cause this very problem in the female reproductive tract. StuRat 19:05, 3 April 2006 (UTC)
April 3[edit]
Computer Technology-Measurement of the Capacity of Hard Disk Drive  [edit]
[edit]
Someone has just asked me about the capacity of my PC's hard disk drive.I have tried checking all manuals that were delivered with my PC but I could not find this information.Maybe it's because I do not know where to look for this information and also that I do not know how the capacity of a hard disk is measured.Internet research about my PC brought out this, "Storage :40/80/120/160/200/GB ATA/100 hard disk drive".This confused me even further.Is my answer found in any of the figures quoted above or I have to look elswhere?
- If you're on a Windows XP PC, click "Start" Then "My Computer" then "Local Disk (C:)" the details in the lower left will tell you the storage of your hard disk drive ("Total Size"). If you did your research right then that number will be around 40/80/120/160/200/GB. See Gigabyte for how the storage capacity of a hard disk is measured. -Snpoj 01:36, 3 April 2006 (UTC)
- If you're using Linux, run fdisk as superuser and type "p" when it asks you for a command. Also, try checking the sides of your computer. Some computers have general system information printed there. --Bowlhover 04:59, 3 April 2006 (UTC)
- If you don't have root privileges, try "df -h" though you may have to do some simple addition to get the answer. --Bth 09:26, 3 April 2006 (UTC)
- Note that "df -h" is only useful for finding out the disk size when:
- (a) There is no disk space that is not part of a partition, and
- (b) All of the partitions are mounted. --Bowlhover 04:21, 4 April 2006 (UTC)
- Note though that you can type fdisk -l to get information about the partitions without starting the fdisk program interactively. (There's a similar option to the DOS fdisk program, fdisk /status I think.) – b_jonas 14:18, 4 April 2006 (UTC)
just a note, 40/80/120/160/200/GB means 40 or 80 or 120 or 160 or 200 gigabytes. your computer could be near any of these values. if you are still having difficulty, ask your local 14 year old. good luck
- Another note, harddisk manufacturers use a different standard from most OS's to calculate size, and thus there will almost certainly not be a round number displayed. Manufacturer uses XX * 10^9 as a standard for gigabytes, while Windows uses XX * 2^30 for gigabyte. The first is about 6% smaller then the latter. SanderJK 09:30, 3 April 2006 (UTC)
Try the above suggestions first.. But the most reliable place to find your drive size is on the sticker on your hard drive itself. It's probably deep inside your computer though so you might not want to open it up to look. Also when you first start up your computer the drive size might be incorporated into the name of your hard drive in the POST start up screen. It's also difficult to notice or recognise. Again, try the above suggestions first :) —Pengo 10:11, 10 April 2006 (UTC)
UFO sightings[edit]
Yesterday and the day before yesterday, I spotted 3 UFOs. The first two were patches of dim, white light (by the way, there were thin clouds when I saw this), about 1 degree large, moving quickly across the sky. "Quickly", in this case, means "faster than the high-altitude airplanes that leave visible trails, about the speed of the airplanes that don't leave trails and whose blinking lights can be seen, but faster than satellites". The third UFO was very bright, certainly much brighter than the magnitude -5 iridium flare I saw, and it travelled from the triangle in Leo to the constellation Cancer in little less than a second. It simply vanished after reaching Cancer. (Note: I couldn't see any of Cancer's stars, but the bright Saturn marked its position).
Any ideas about what the three UFOs I saw were? Thanks. --Bowlhover 05:14, 3 April 2006 (UTC)
- If you don't know what it was, how do you know how big it was? And if you don't know how big it was, how do you estimate how fast it was? --BluePlatypus 13:39, 3 April 2006 (UTC)
- Also, the ufo guys used some device that kept photons for reaching his eye, so Bowlhover could estimate that they were farther. --DLL 18:57, 3 April 2006 (UTC)
- I don't know how big the UFOs were, or how fast they were moving. However, I do know how big they appeared to be, and how fast they appeared to move. Sorry for the confusion--when I said "faster than the high-altitude airplanes", I was refering to the apparent speed, not the absolute speed.
- I originally thought that the third UFO was a meteor, too. However, the Wikipedia article on meteors said that the British Astronomical Association defined fireballs as meteors that are magnitude -5 or brighter, implying that fireballs are rare. There was no meteor shower yesterday, I had been outside for only 3 hours when I saw the UFO, and the UFO was much brighter than magnitude -5. Could I really have been that lucky? --Bowlhover 19:43, 3 April 2006 (UTC)
- Yes. In any case, keep good records. If there were actually physical objects, then others may see them. If you don't keep records of exact time and position in the sky, etc, it's just hearsay. If there is an actual phenomenon, then over time correlations between various records will reveal a pattern. GangofOne 00:45, 4 April 2006 (UTC)
- I originally thought that the third UFO was a meteor, too. However, the Wikipedia article on meteors said that the British Astronomical Association defined fireballs as meteors that are magnitude -5 or brighter, implying that fireballs are rare. There was no meteor shower yesterday, I had been outside for only 3 hours when I saw the UFO, and the UFO was much brighter than magnitude -5. Could I really have been that lucky? --Bowlhover 19:43, 3 April 2006 (UTC)
- I agree, but whether I keep accurate records or not will not influence whether my claims are hearsay. They're hearsay no matter what. Also, I know that to many people, "UFO" is a synonym for "alien spacecraft". I don't think the UFOs I saw were alien spacecraft--they were simply objects I could not identify at the time (although I strongly suspected the third one to be a meteor--I still do). --Bowlhover 04:17, 4 April 2006 (UTC)
- In a legal sense, if you write observations down in a notebook at the time they occur, they will have more evidentiary status in a court of law or scientific dispute, as time passes, than if you say, 'in the beginning of March 2006 I saw some lights in the sky in an easterly direction', which is worthless. GangofOne 07:27, 4 April 2006 (UTC)
- I agree, but whether I keep accurate records or not will not influence whether my claims are hearsay. They're hearsay no matter what. Also, I know that to many people, "UFO" is a synonym for "alien spacecraft". I don't think the UFOs I saw were alien spacecraft--they were simply objects I could not identify at the time (although I strongly suspected the third one to be a meteor--I still do). --Bowlhover 04:17, 4 April 2006 (UTC)
- If the third object looked like a bright meteor to you, it seems likely that it was. According to the American Meteor Society FAQ, fireballs of magnitude -4 can be expected about once in every 20 hours of observing and magnitude -6 or better for every 200 hours. So maybe you DID just get lucky. It's hard to guess what the first two objects (patches of dim white light) were from the description alone. Is it possible that they could have been searchlights reflecting off the thin clouds that you mentioned were present? --DannyZ 07:14, 5 April 2006 (UTC)
Density of argon[edit]
Anyone got the density of solid argon at freezing point (84K IIRC) handy? -- Миборовский U|T|C|M|E|Chugoku Banzai! 05:36, 3 April 2006 (UTC)
- This page gives the density as 1636 kg/m^3, though doesn't state what temperature and pressure that's measured at. --Bth 07:16, 3 April 2006 (UTC)
- This has the most information I can find and gives the density of 535.6 kg/m^3 at Argon's critical point of -122.3 C Nothing I can find shows much information about Argon's solid form of -199.3C Bth reported the Density of the Gas at 0C. --Tollwutig 16:05, 3 April 2006 (UTC)
- I used to have density charts for all the phases of the various chemical elements. But alas, now they argon. StuRat 07:49, 4 April 2006 (UTC)
- That's very unhelpful of that page to give it as the "solid" density, then. Thanks for the correction, though. --Bth 08:54, 4 April 2006 (UTC)
- Kaye and Laby to the rescue! (ish) They give the density as 1656 kg/m^3 at 40K (and standard pressure if I'm reading them right, so it should be in the solid form), but that seems suspiciously similar to the gas-at-zero-celsius if Tollwutig is right. They do give the melting point of 84K that Miborovsky remembered. --Bth 12:41, 5 April 2006 (UTC)
Synesthesia/Light Organ[edit]
Hello, I recently read an article, but I cannot find it again. It pertained in some way to synaesthesia, among other things. It mentioned a composer, possibly Scriabin, and how he used a light organ, as well as how he experimented on having smells waft through the audience. The article also stated that he wanted his magnum opus to be performed at the base of a mountain. I don't believe synthesthia was the main focus of the article. It had something to do with sound I think, and I remember there were a few charts on it. There was perhaps some kind of system, and its inventor had a name that was a double entendre, its other meaning being a part of the brain, I believe. Is there an author out there who knows what I speak of? Thank you, KeeserSilver
- See Clavier à lumières. –Mysid 07:44, 3 April 2006 (UTC)
Words losing all meaning[edit]
Why is that when you repeat a word several times it eventually "loses all meaning", as in the word appears wierd and you see it differently ts hard to describe buti hope someone knows what i mean. Thanks Kingstonjr 14:49, 3 April 2006 (UTC)
- I know what you mean (try typing or writing a word forty or fifty times, or staring at a page of a book with the same word repeated over and over on the whole page - I think this was done in Microserfs) - but I don't know if there's a word for it. I couldn't tell you the cause, either. Proto||type 15:31, 3 April 2006 (UTC)
- yea i know exactly what you mean, it is hard to explain i dont know what causes that though. modesty 16:22, 3 April 2006 (UTC)
Let me propose a theory: Our minds are designed to only notice new or changing things. For example, if you look at a scene, your attention will immediately be drawn to the moving object and will virtually ignore the rest. A repeated word gets the same treatment. You should keep this in mind and avoid sentences like "I excitedly saw how excited they were due to the excitement of the current situation and the exciting day in general". A better sentence would be "I was happy when I saw how glad they were due the excitement of the current situation and the interesting day in general". StuRat 18:41, 3 April 2006 (UTC)
- I found an interesting hypothesis on some random web site regarding this: Any word in any lexicon will start to sound funny if you repeat it aloud enough times. That's because after hearing a word x times, your mind starts to realize that there's no context associated with it, and therefore it lacks the meaning(s) it would have under normal circumstances. Your mind starts to analyze the sounds that make up the word, and the actions that are being taken by your vocal cords, tongue, teeth and nasal passages to create the sounds. Makes sense, but who knows? – ClockworkSoul 00:59, 4 April 2006 (UTC)
- That "dynamic change" theory explains a lot of things, such as why we feel dizzy long after we (and the little doodad in the inner ear) have stopped spinning, and various visual illusions (especially the one with the ring of pink dots and the phantom green dot). Tzarius 08:29, 4 April 2006 (UTC)
I looked for some scientific reasearch on this a while back, and I found that the term for the phenomena is "auditory habituation", where the repeated words undergo a "verbal transformation", so you may want to use those as search keywords if you plan to do more reasearch on the subject. You could also have a look at this paper from APA Online, however you have to pay to view it unless you college/school library has a subscription. Hope this helps. --Aramգուտանգ 05:49, 5 April 2006 (UTC)
- This effect is generally called semantic satiation ("verbal transformation" is hearing other words in a repeated stimulus). – 74 19:53, 12 March 2009 (UTC)
Ancient puzzle[edit]
When I was a kid I read the following (paraphrased) puzzle in a book:
A man wants to cross a bridge that can support 10 lbs. more than he weighs. He is carrying (3) balls that weigh 5 lbs. each. How can he cross the bridge without collapsing it?
The answer was to juggle the balls as he crosses the bridge. Ostensibly he would be carrying only (2) balls at a time, as one would always be in the air. However, the force necessary to toss the third ball into the air would create additional downward force that I believe would be enough to collapse the bridge. Does it matter to the bridge that the balls are being juggled?
- hrmm, you also have to think when he catches a five pound ball (5 pound balls seem like a lot to juggle btw) its going to produce a force stronger than 5 pounds from it falling a distance of 1-2 feet or so. i think for this to even consider to be workable hes going to have to have a maximum of 1 ball on him at any given time modesty 16:26, 3 April 2006 (UTC)
- Why doesn't he just cross the bridge with two balls, go back and get the third one? User:Zoe|(talk) 16:33, 3 April 2006 (UTC)
- Because monkeys will steal the third ball if he leaves it behind?
- lol yea, and really if the bridge can only support EXACTLY 10 pounds over his weight, will it break if he steps too hard? the whole question is dumb i dont think weight limits work that way modesty 18:08, 3 April 2006 (UTC)
1) Kill all the monkeys on this side of the bridge.
2) Cross the bridge without your balls.
3) Kill all the monkeys on the other side of the bridge.
4) Go back over the bridge.
5) Toss your balls over.
6) Cross the bridge yourself and reward yourself with a nice monkey brain stew. StuRat 18:30, 3 April 2006 (UTC)
- Think of holding a single ball as accelating it at 1 g. To throw the ball the max acceleration w/o exceeding the limit of the bridge would be 2g. Say you take 1 second to throw the ball. That's 2g of acceleration for 1s, 1s for the ball to reach it's apex, 1s to fall back to your hand and 1s to decelerate the ball at 2g. Two seconds required to throw and catch gains you two seconds of flight time for the ball.
- To get across, raise the side of the bridge you are on, using rocks, dead monkeys etc., then roll across (see inclined plane.) EricR 20:03, 3 April 2006 (UTC)
- So essentially its Myth:busted on the fact that you cannot gain any legitmate force-over-time by juggling (other than the delight of onlookers). I agree that the premise is preposterous in a real world setting amyway since forces from footsteps, wind, evil monkeys, etc would combine and create a great likelihood that the bridge will fall regardless of your juggling antics. --Jmeden2000 20:36, 3 April 2006 (UTC)
- Free body diagram. Peter Grey 06:18, 7 April 2006 (UTC)
dual booting linux/windows[edit]
hey i currently have windows xp running on a 60 gig hd. i want to install linux dual boot for a number of reasons. however i read that i would have to partition this hard drive and split it between the two OS. i have been meaning to get a new hard drive anyway for additional storage and to not risk losing my old hard drive data in the process. so my questions are: | 1. if i do get a second hard drive thats say 120 gigs or so, can i give linux 60 gigs of that and windows another 60? | 2. how would i go about doing that- the red hat site lists a program called partitioner or something | 3. will i be able to access files(mp3s) stored on my current hard drive(which i guess has a windows file management system) in linux? | 4. lastly if you recently bought or know a good quality quiet hard drive that holds 100-200 gigs for an ok price give me a link:D cheers- modesty 16:32, 3 April 2006 (UTC)
- Yes.
- The installer for your chosen Linux distribution will let you partition your hard drive. By only allocating 60GB in that program, you can then choose to use the remaining space to create another partition in Windows using Disk Management under Control Panel -> Administrative utilities (I think that's the English name for it).
- Yes, but you may need to upgrade your Linux kernel to be able to read NTFS partitions. This varies between distributions, and *may* require you to re-compile a new kernel yourself. If you also want to access the Linux disk from Windows, you should use ext2 or ext3 for your Linux partition and install a file system driver for XP (such as Ext2fsd). Then, you can assign a drive letter using Disk Management. --Pidgeot (t) (c) (e) 16:55, 3 April 2006 (UTC)
- Don't tell newbies to compile their own kernels. That's just cruel.
- 1. You could do that, but I don't see why you would want to. Personally, I would format most of the new disk as something both Windows and Linux can access, like FAT.
- 3. It depends which "windows file management system" it is. If it's FAT, Linux will have no problem with it. If it's NTFS, you might have some problems. I know there's a native Linux driver for NTFS that can read files, but I think the write support is still buggy. Reverse engineering is hard.
- 4. I bought a Seagate 120G for like $50 which has been running fine for a good while now. —Keenan Pepper 17:04, 3 April 2006 (UTC)
- As a side note always install Windows first and Linux second, as the Windows installer is ALWAYS set to rewrite the boot sector of the disk. --Tollwutig 17:59, 3 April 2006 (UTC)
- thanks, i just got this computer in december and its a dell e510... dunno if its fat or nts (probably whichever is newer?) with xp media center edition, and im installing fedora core 5... this will be cool if it works out as easily as you both have made it sound... thanks again, fast replies 129.32.93.17 18:02, 3 April 2006 (UTC)
- XP uses NTFS by default unless you changed something during install. There are utilities for reading/writing NTFS under Linux, but they're all experimental and I wouldn't mess with it. Personally, I have a FAT32 partition that I use to store common files for use under both Windows and Linux, as they can both safely read/write this format. Isopropyl 18:09, 3 April 2006 (UTC)
- thanks, i just got this computer in december and its a dell e510... dunno if its fat or nts (probably whichever is newer?) with xp media center edition, and im installing fedora core 5... this will be cool if it works out as easily as you both have made it sound... thanks again, fast replies 129.32.93.17 18:02, 3 April 2006 (UTC)
- And there is no saying that you have to use just 2 partitions. You could split the drive into 3 Partitions. 30 GB for Windows XP install (NTFS), 30 GB for Fedora Core 5, and then a 60 GB FAT32 Partition for your Data. This way you won't have to compromise your Windows Security (not quite an Oxymoron)by using FAT on the OS. Really though if you move install your Programs into the Data Drive you can get by with a 10-15 GB partition for your OS installs. The advantage of this is you don't have to mount the other OS install partition, so you don't mess up something on the other OS install.--Tollwutig 18:47, 3 April 2006 (UTC)
- hrmm thx all seems pretty easy. i think the fedora 5 reads ntfs so i dont think file format will be an issue, just wondering how im going to go about splitting this up. i might just install linux to the hard drive i have now, give it 20 GB, and then get a nice 200gb hard drive and put fat32 on it... if anyone stills cares to answer, will there be any difficulties partioning t hsi hard drive seeing its already got about 35 gb of random crap (in ntfs) on it? if not ill just put linux on it tonight modesty 19:32, 3 April 2006 (UTC)
- Shouldn't have an issue as long as you can resize the current partition. Norton Partition Magic if you have it is great for this sort of thing, as you cannot change the system partition inside of Windows. If you can find a means to repartition outside of Windows it's fairly straight forward.--Tollwutig 19:48, 3 April 2006 (UTC)
- Piggybacking: is this FAT/NTFS thing the reason why on my previous machine I used to be able to modify my Windows 98 drive from within Linux, but on my newer computer with XP the whole of /mnt/windows/ is read only? --Bth 08:42, 4 April 2006 (UTC)
FEASIBILIYY REPORT[edit]
"you have a fun fair in university. Youyou have to stall for anything prepare a feasibility report on it" help me before 10_4_2006
 Please do your own homework.
Please do your own homework.- Welcome to Wikipedia. Your question appears to be a homework question. I apologize if this is a misinterpretation, but it is our aim here not to do people's homework for them, but to merely aid them in doing it themselves. Letting someone else do your homework does not help you learn nearly as much as doing it yourself. Please attempt to solve the problem or answer the question yourself first. If you need help with a specific part of your homework, feel free to tell us where you are stuck and ask for help. If you need help grasping the concept of a problem, by all means let us know. —Keenan Pepper 17:17, 3 April 2006 (UTC)
- Your question is also poorly written and impolite. Use proper English and say please, if you want our help. StuRat 18:24, 3 April 2006 (UTC)
- Probably not an English-speaker primarily, so some slack is granted. What's a "fun fair"? How about making your own wine and setting a vomitorium booth? It would be cheap to do , and very popular. GangofOne 23:19, 3 April 2006 (UTC)
- I'm pretty good at recognizing accents, even in writing. This person looks to be a native speaker to me, specifically a native speaker of British English. StuRat 07:44, 4 April 2006 (UTC)
- Yep, classic lack of grammatical articles. Superm401 - Talk 23:20, 4 April 2006 (UTC)
- THis follow up, Wikipedia:Reference_desk/Science#APOLOGY, says you're wrong, although probably correct about the type of English attempted to be spoken. GangofOne 22:03, 6 April 2006 (UTC)
- I'm not sure I believe it, though. Note that the apology is written in fairly good English. This makes me think they were just really sloppy on this post and didn't want to admit it, so made up that story about not speaking English well. StuRat 19:55, 8 April 2006 (UTC)
astronomy/cosmogeny[edit]
If the Cosmic Microwave Background Radiation is due to a single event in the history of the Universe, then why do astronomers continue to record it from every point in the sky? In other words, why has it not rushed by the Earth never to return? —Preceding unsigned comment added by Paul venter (talk • contribs)
- Perhaps not suprisingly, you can find your answer at Cosmic_microwave_background_radiation. Chapuisat 19:26, 3 April 2006 (UTC)
Afraid not - if anything, the author of that piece is also confused ....."The photons continued cooling until they reached their present 2.7 K temperature. Accordingly, the radiation from the sky we measure today comes from a spherical surface, called the surface of last scattering, from which the photons that decoupled from interaction with matter in the early universe, 13.7 billion years ago, are just now reaching observers on Earth." What an amazing coincidence! With 13.7 billion years to get here, the timing is such that they turn up just when we start looking, and even more amazingly are centred on Earth, since if Earth were not central to the "spherical surface" mentioned above, the radiation would not be isotropic. So nice try, but no cigar. User Paul venter 3 April 2006
- You're using a straw man argument. The article makes perfect sense, just not your misinterpretation of it. —Keenan Pepper 21:21, 3 April 2006 (UTC)
- Also, there is no such person as "the author of that piece". Wikipedia is collaboratively authored and edited. See Wikipedia:Introduction. —Keenan Pepper 21:23, 3 April 2006 (UTC)
If I misinterpret the article it is because it lacks logical clarity. On the other hand if you see sense in it, then please enlighten me in detail. As for whether the article has author or authors, it affects the credibility and logic by not a single iota. User:Paul venter 3 April 2006
- The universe is constantly expanding. Therefore, there are longer and longer paths for light from the big bang to take before reaching us. There is no need for an "amazing coincidence". —Keenan Pepper 00:05, 4 April 2006 (UTC)
If the 'spherical surface of last scattering' is expanding, the surface remains spherical and does not explain the isotropy or persistence of the CMBR --Paul venter 07:37, 4 April 2006 (UTC)
- The "surface of last scattering" is what happens when you look back in time to the early universe from some point inside the current universe. But from the point of view of the history of the universe, there was a particular point in time when the universe cooled down enough to become transparent (because the processes that absorbed and reemitted the photons all the time stopped). When we look back we see the light from the sphere formed by all the points that were at that stage at the right time for the photons they emitted to now be reaching us.
- But I rather suspect you're not interested in the real answer. --Bth 09:05, 4 April 2006 (UTC)
There are some difficulties here....firstly, the way I understand the theory is that it was a spherical surface and not a sphere which emitted the light. Secondly, was the emission a burst of light lasting only a very short time, a very long time or is it an ongoing process? If it were only for a short time, then the spherical shape of the emitting surface would mean that when the light does reach any point (except the geometric centre) within that sphere, its behaviour will certainly not be isotropic. And if it were a burst lasting only a short time, then consequently any observer within the sphere would only experience the light later on as a burst of radiation starting at one point in the sky and moving in the shape of a band to a point diametrically opposite - the complete cycle taking anything from seconds to billions of years, depending on where the observer is located inside the sphere.
What a strange thing to think that I am not interested in the real answer..... If you know the real answer, then do share it. --Paul venter 11:13, 4 April 2006 (UTC)
- My relpy was an attempt at explaining the real answer. But let's try another way. It is not really helpful to think of it as "a spherical surface emitting the light". The light is the light that was "emitted" when the universe became transparent -- that happened throughout the universe at the same time (in the appropriate coordinate system), about 300,000 years after the Big Bang.
- Now the important fact is that as we look out into the universe we are looking "back in time". This is not a special claim of the Big Bang theory, it's a consequence of the finite speed of light. Because it's about two and a half million light years away, we are seeing light from the nearest big galaxy, Andromeda as it was approximately 2.5 million years ago. It should be fairly obvious that there is a spherical shell of radius 2.5 million light years centred on us in which everything we see is at it was 2.5 million years ago, it's just that Andromeda's pretty much the only interesting thing in it. And further out you can have a shell of everything a billion years ago, further out still a shell of everything as it was ten billion years ago. When you get back to 13.4bn years ago, your shell contains the CMB photons because that's the time when the universe became transparent. Beyond that, you can't see because the universe wasn't transparent. That's why it's a spherical shell, it's just a consequence of the fact that we're looking back, the original emission was in all directions -- it's just that all the ones that didn't head off in the right direction for us to see them are out being part of the CMB as seen from elsewhere. The "last scattering" event (ie the universe becoming transparent) happened everywhere simultaneously, pretty much, because it was a consequence of the temperature of the universe, which was still very homogeneous at that point, dropping below the critical temperature needed for any atoms that formed to be instantly reionised -- after that the atoms that formed stuck around (until stars started forming and reionising everything). If you could follow the spot of the universe that we're in now backwards in time, you'd find that it too had been part of that process 13.4bn years ago. To a hypothetical observer 13.4bn light years away photons that were emitted then from our location now are just arriving as their CMB photons.
- Is that making any more sense?
- (Note that strictly the last scattering shell is not 13.4bn light years in radius as I've implied; when you factor in general relativity and the evolution of the universe it gets more complicated.) --Bth 12:00, 4 April 2006 (UTC)
OK..let's see whether I've got this right. About 13bn years ago (where does this figure come from?) there was a colossal expansion of a very large mass. The expansion was so rapid that after about 300,000 years the outermost edges of the expanding sphere had reached a radius of at least 13bn light-years, since 13bn years after the bang we are still receiving radiation from an event that took place at 300,000 years post big bang. This sphere of matter had at that time cooled sufficiently for internal scattering to end and to become transparent to radiation, resulting in a burst (short-lived?) of radiation which we now observe as the CMBR. This CMBR will have started arriving at Earth from 300,000 yrs post bang, continuously to the present moment, coming from spherical shells progressively further away. At some point in the future one presumes that the CMBR will cease fairly abruptly having come from the outermost shell of the big bang sphere at the moment of transparency - at which point also any radiation having come from outside the big bang sphere will become visible. I am still puzzled as to the mechanism which limits the distance to which we can observe - on the one hand we have the pre-transparency opaque barrier and on the other hand we have Hubble's Law driving matter to a speed faster than that of light. Please let me know whether I have at least the rudiments correct. --Paul venter 22:15, 4 April 2006 (UTC)
- In some sort of order:
- The 13bn years is derived from plugging the observational evidence into the theory. (Judging by some of your edits elsewhere on the 'pedia, you're unconvinced by the idea of a finite-age universe at all. I suggest you have a look at Olbers' paradox for a nice simple proof of that simply from the fact that the sky is dark at night.)
- According to the now standard version of the theory, the really rapid expansion was at the very earliest stage; see cosmic inflation.
- There wasn't an "expanding sphere" -- space itself was (and continues to this day) expanding. "Blowing up a balloon" analogies for the Big Ban only really work when you consider them from the point of view of observers embedded in the Flatland-like two dimensional surface of the balloon. The Big Bang was not in any way like a normal "explosion", it's an expansion of everything. (I think this may be the key misconception at play here?)
- No, the CMBR will not suddenly disappear in the future. We will see the earliest moments of parts of the universe currently inaccessible to us (the universe is bigger than the currently observable universe) -- every CMBR photon that arrives is from a region of the universe that until that moment was previously too far away to see. (But bear in mind that the universe was smaller at the CMB epoch.)
- The mechanism which limits the distance to which we can observe is the finite age of the universe. There's nothing to see "the other side" of the last scattering surface, everything earlier than that was opaque. An important but subtle distinction: no matter is being "driven to a speed faster than that of light", the universe itself is expanding with that matter embedded in it. (That expansion can be faster than c.)
- Any clearer for you? --Bth (not signed in) 5/4/06
A couple of points to note. Olbers' paradox : There could be any number of reasons for light from stars beyond a certain radius not being visible to us - big bang opacity and redshifted light being just two of them. By itself it certainly is not proof of a finite-age universe.
I carefully avoided use of the term 'explosion'
I also think the CMBR will never disappear, but for different reasons. However I'm confused - if the big bang bubble was of finite size at the moment of transparency, then surely at some time in the future, we will observe the CMBR from the last scattering surface. There could be no more CMBR from this particular big bang - or am I missing something?
"There's nothing to see "the other side" of the last scattering surface, everything earlier than that was opaque." I was under the impression that the opacity was a quality of the space inside the big bang bubble before transparency - did this opacity extend outside the bubble as well? Thank you for your patience --Paul venter 07:13, 6 April 2006 (UTC)
- Please explain what you mean by "Big Bang bubble". Even though you've avoided using the exact word, between that and your repeated references to "spheres" you sound an awful lot like you're falling victim to the "it's an explosion inside some pre-existing three-dimensional space" fallacy. --Bth 08:42, 6 April 2006 (UTC)
big bang bubble - My phrase, which perhaps I should have defined. Let me do so now. The bubble would include all matter resulting from the big bang/expansion. Radiation from the bubble would obviously define a much larger sphere. And you're quite right - I do think that the big bang was local and within a pre-existing infinite space in which no doubt countless other big bangs have taken place, are currently taking place, and will within the infinite future take place. Is there any good evidence to indicate that this is not the case? --Paul venter 11:56, 6 April 2006 (UTC)
Intermolecular Bonds[edit]
I can't figure out why, when I tear paper, the bonds holding the paper together on an intermolecular level break but when I put the two sheets next to one another and add pressure they don't form agian. It seems like a rather stupid question but I can't think of an explaination on a chemical (molecular) level. Thanks. -Haon 18:29, 3 April 2006 (UTC)
- Poor example as you aren't really breaking chemical bonds but physical bonds of interwoven fibers. You're just pulling the cellulose fibers apart. In order to get the paper to come back together you would have to reweave the Cellulose fibers back together. Chemically speaking, breaking bonds releases energy. Creating chemical bonds requires energy.
A good example of this is Sugar. Plants make sugar out of carbon dioxide and water using the Energy of absorbed light. (Photosynthesis) At same time all life (even plants) take the Sugar and use Oxygen to break it down into Carbon Dioxide and Water releasing the energy which was stored in it by photosynthesis. This process is called Cellular Respiration.--Tollwutig 18:39, 3 April 2006 (UTC)
- Chemically speaking, breaking bonds releases energy. Creating chemical bonds requires energy. This is wrong. If breaking bonds released energy, they would break spontaneously and molecules would not be stable.
- Plants use the energy they get from sunlight to break the strong bonds of H2O and CO2 and form sugars with weaker bonds. Animals break the weak bonds of the sugars and form the strong bonds of H2O and CO2 which releases energy.
- The stuff about interwoven fibers was the right answer. —Keenan Pepper 21:17, 3 April 2006 (UTC)
- Some chemical reactions are exothermic (releasing energy) and others are endothermic (using energy). Those reactions that release energy, like say the detonation of dynamite, are rather unstable, but still require specific conditions for the reaction to take place. Typically, this is some combination of heat and/or pressure. StuRat 07:37, 4 April 2006 (UTC)
- "when I tear paper, the bonds holding the paper together on an intermolecular level break but when I put the two sheets next to one another and add pressure they don't form agian". If you alligned the atoms EXACTLY the way they were, the bonds WOULD form again. The problem is , when you rip paper the ripped edge has about, oh, maybe about 10^22 bonds , give or take, that you have disturbed, and the atoms exposed are vibrating (at room temperature) and maybe combine with the air, and the fibers got jumbled and distorted, so when you put them together, they no longer match up. If you take the time to match up all 10^22 joints, then the joint will be seemless. Let us know when you're done. GangofOne 23:10, 3 April 2006 (UTC)
- Actually, this sort of thing can happen. It blew my mind when I first learned of cold welding. Chemical bonding, in addition to geometric considerations, is also a component of friction. As a poster above said, there is mechanical bonding involved in holding paper fibers together, but under the right conditions (particularly high-temperature polished surfaces in a vacuum) you can get all sorts of fun things to happen like sintering. —Ben FrantzDale 02:44, 6 April 2006 (UTC)
SARS relationship to Avian Flu, H5N1[edit]
I have spent considerable time studying the situation regarding the potential pandemic due to the currently circulating H5N1 A virus. SARS was also a virus, though of a different kind, and that pandemic was tightly contained. According to WHO about 8000 people fell ill with SARS and about 800 of them died. That is a miniscule set of numbers compared to even the most optomistic estimates of what might happen in the human population should H5N1 change to become easily transmitted among people. Why is there such a huge quantitative difference? How is it that we managed to survive SARS so well and seem to be so vulnerable to H5N1?
I have been to both the WHO and CDC sites as well as Wikipedia seeking some insights but I can find nothing. Everyone treats each illness quite independently of the other.
I'd appreciate any help you can bring to bear on this question. - unsigned
- H5N1 is pretty much a standard influenza virus and follows the same pattern as other. As for its ability to spread if it mutates to be contagious between humans, it'll spread like any other influenza. While I do not know the specific information on SARS pathogenicity, it is not as easily transmitted as influenza, which is only surpassed in contagiousness by the Common Cold. Influenza is contagious 12-18 hours before symptoms are ever apparent.(not sure about SARS). This means that someone who gets Influenza in Tokyo could get on a plane, spread the virus to all other passengers and arrive in L.A. with only a slight sniffle. In the meantime now you have several hundred people who are potentially infected, and not showing symptoms.
- As a side note the mortality rate of H5N1 is over exaggerated as the reports of 80% mortality are obscured by the fact that only the most severe cases have been reported. How often is H5N1 deadly in cases where the symptoms do not warranty treatment at a medical facility, and thus go unreported?--Tollwutig 19:44, 3 April 2006 (UTC)
- SARS has low inherent transmissibility. Flu has high inherent transmissibility. It's the difference between a fire on a dry grass plain and a fire on a wet grass plain. Extensive studies looking for mild missed H5N1 cases in Cambodia found NONE. Avian adapted H5N1 kills half the humans that get it, but has near zero human to human transmissibility. If and when a human adapted strain of H5N1 evolves, no one knows what its transmissibility nor what its mortality rates will be. We have no data to guess with because this strain is acting different (more deadly, more virulant, more transmissible) than all other known strains of flu - ever. The world's best experts say they are very scared and have stocked up months worth of food and water and medicine. Dr. David Nabarro, chief avian flu coordinator for the United Nations, describes himself as "quite scared"; says avian flu has too many unanswered questions; and if the disease starts spreading to humans, borders will close, airports will shut down, and travelers everywhere will be stranded. If the worst happens, the best way to survive is to lock your doors, stay inside,and have no contact with anyone outside your home for as long as your food and water last. In the 1918 pandemic some entire villeges were wiped out while some entire cities blocked all incoming traffic of any kind and had no deaths. WAS 4.250 21:11, 6 April 2006 (UTC)
Humming and Software[edit]
Does anyone know of any software where you hum/whistle/la/etc. into a microphone and it gives you an approximate pitch (or note)? Even better would be one where it measures the duration of the note as well and records it. The reason I ask is because I find it easy (as I am sure many other people do) to invent and hum a tune, but when it comes to playing it on an instrument or writing it down, I have no clue what to do (even though I can play music reasonably well). Alternatively, can anyone give me some pointers on how I would write a basic program that could do this? Any suggestions would be greatly appreciated. 80.229.152.246 20:27, 3 April 2006 (UTC)
- While I do not know of a specific program that does what you want in one complete package with a pretty pink bow on top, it isn't really complicated to record the microphone into a wav file and then convert the wav to midi. Once in midi, the notes will quanitized to specific pitches and durations. Also, there are many composition programs that allow you to view midi files as sheet music or rolling bar graphs so you can adjust it further. --Kainaw (talk) 22:01, 3 April 2006 (UTC)
- Used to be such a thing for sale. See also Vocoder, Fourier transform GangofOne 22:55, 3 April 2006 (UTC)
- "...and then convert the wav to midi." — That's equivalent to the original problem. —Keenan Pepper 23:52, 3 April 2006 (UTC)
- Apple's GarageBand (a part of Apple's $79 iLife suite) has an instrument tuner that shows what note is being played, and that includes through a plain ol' microphone. I had some fun humming "Do Re Mi" and seeing how close I was to actually humming the correct notes. Of course, GarageBand is Mac-only, so I doubt that'd help. :P —OneofThem(talk)(contribs) 00:28, 4 April 2006 (UTC)
- just record it regularly, and then match each note to a note on the keyboard or a guitar. the main problem is that youre probably not singing in tune, but tuned to some weird quarter note or half pitch to begin with. try playing a note on the guitar or keyboard, then matching that note, and then hum from there while recording it. then you can play it back and learn it. if you do this enough your ears will get real good at figuring out which note is which. or you could try what everyone else has suggested... if the point is to get good at playing at the instrument youre probably cheating yourself if you use a program to do the work for you modesty 01:56, 4 April 2006 (UTC)
- On a side note, a clean whistling sound is very similiar to a pure sine wave, which is really trivial to find a matching frequency with by using some mathematical tricks. I wonder if somone coded a program for this already, shouldn't be hard. (I did some nasty thing like this on mIRC, and while it did work, it didn't work all that nicely) ☢ Ҡi∊ff⌇↯ 06:06, 4 April 2006 (UTC)
- Software of the type the questioner is asking for must exist, because what he's describing is also the basic mode of operation of the Singstar games. --Bth 09:38, 4 April 2006 (UTC)
- Thanks everyone for your answers. The point was not to get good at playing the instrument but to see if a computer program has already been written. I'll probably have a go myself and put it on Sourceforge if it is any good. P.S. Thanks for the sine wave tip.
80.229.152.246 18:12, 4 April 2006 (UTC)
I was facing the exact same issue when I had to write a song for a project in college. I ended up using the Transcribe!, software, which is a very compact, but extremely easy to use program. You can hum the tune into your mic, which the program will record, and then show the waveform graph. You can then select sections (e.g. single notes that you hummed) within the graph, and the program shows you nice distribution graph of the pitches it detected in that section over a piano layout (see the screnshots section, and you'll understand). I then played the notes on my MIDI keyboard to determine which were the ones I intended to hum, and wrote the notes down. It's probably not the best program out there and isn't as robust as many others, but it was so simple to use, that I ended up sticking with it. Hope this helps. --Aramգուտանգ 05:49, 5 April 2006 (UTC)
Question about the VLA[edit]
In the article Very Large Array there is aline which reads:
- There are four commonly used configurations, designated A (the largest) through D (the tightest, when all the dishes are within 600 m of the center point).
What is this difference between these configurations, by which I mean what do they allow observers to see? are the images clearer in one configuration than another, or is this done simply to change perspective of an observed object?
- Briefly, wider array configurations give a narrower field of view and higher resolution. (This is one reason for the creation of the VLBA – the Very Long Baseline Array – which links a network of radio telescomes spanning 8,000 km). Moving the dishes closer together widens the field of view so that larger objects can be observed. Different configurations of the dishes represent different compromises between field of view, resolution, and apparent brightness of radio sources.
- Here's a schedule for the different configurations over the next few years. (The VLA is operated under each configuration for approximately 4 months at a time.) Finally, here's a technical publication detailing the resolution limits of the VLA in each configuration at a range of wavelengths. TenOfAllTrades(talk) 02:03, 4 April 2006 (UTC)
- Thanks. I apreciate it.
Chemical Reaction[edit]
I am making a battery out of a lemon for school. For the project, I am using a "juicy" lemon to create electricity. I am putting a strip of copper and a strip of zinc in the lemon so the zinc and copper don't touch. I want to know how the the juice from the lemon, copper, and zinc creates a chemical reaction to produce electricity. Please explain how the chemical reaction works and how it generates electricity, but please keep it relatively simple if it's possible (I'm only in 6th grade). Thanks! Anna
- Did you see the lemon battery article first? - Cybergoth 23:09, 3 April 2006 (UTC)
April 4[edit]
Neutrinos[edit]
It has come to my attention that we are under constant attack by neutrinos. I was shocked to learn that there has been no study of the health effects of these unwelcome particles. Has there been no attempt to build a neutrino shield? I believe it is a liberal conspiracy.
- Neutrinos are naturally produced and have been bombarding Earth since it was created (at least, that's what I'm assuming by reading the article on neutrinos). I seriously doubt that they're harmful, nor that there's a "liberal conspiracy" involved. (lol) —OneofThem(talk)(contribs) 00:40, 4 April 2006 (UTC)
A neutrino shield would have to be several million miles thick. With all that material, I'd rather build a dyson sphere. Night Gyr 01:01, 4 April 2006 (UTC)
- The neutrino article says you'd need something like a half light year of lead to make an impact on neutrino bombardment. Is this a serious question? If this is a conspiracy, daylight savings time is the government's way of stealing time from you. Isopropyl 01:13, 4 April 2006 (UTC)
If neutrinos are harmful to living things, you will never know. This is because it is impossible to have a control (case) to test the effects of not being bombarded with neutrinos. Ohanian 01:32, 4 April 2006 (UTC)
Given that all terrestial life has been under neutrino bombardment since since the Earth was created, removing those neutrino could theoretically be harmful. We know too little about them to say otherwise, and have no experimental or anecdotal evidence of whether life could even exist without their presence. Consider a somewhat extreme analogy - no-one knew much about oxygen prior to the 18th century - thankfully no-one decided to shield life from it. Or, to put it simply: it ain't broke - don't try to fix it. Grutness...wha? 01:49, 4 April 2006 (UTC)
- What I read was that the neutrino cross section of a human being per time is about 1 neutrino/70 years or so. When you actally interact with one, you die. How else can you explain this amazing coincedence? GangofOne 07:18, 4 April 2006 (UTC)
- Your ideas intrigue me, and I wish to subscribe to your newsletter. Tzarius 08:40, 4 April 2006 (UTC)
I would say they are both harmful and helpful. They are one of many factors which may cause genetic mutations. Most mutations have little effect, and at most kill the cell. However, some are harmful, such as causing cancer, and quite rarely they are actually helpful, if the mutation is in a reproductive cell and is beneficial. Thus, they may actually help to drive evolution. StuRat 07:24, 4 April 2006 (UTC)
- Neutrinos do not cause genetic mutations. --BluePlatypus 12:01, 4 April 2006 (UTC)
- While the vast majority pass right through the human body (and even the planet) without reacting, a rare few do cause a reaction. That is how we can detect them. If this reaction happens to occur by a strand of DNA, a mutation can occur. StuRat 20:00, 8 April 2006 (UTC)
Bots[edit]
I'd like to do some experimenting with internet bots (e.g. Wikipedia editing bot, Bluebot). What programming language does this require? Does anyone know good links for downloading a compiler or for a good tutorial? Thanks. -Snpoj 01:29, 4 April 2006 (UTC)
- AFAIK, many Wikipedia bots use Python. Different types of bots may be programmed using different languages. For example, I've programmed a responding IRC bot in Perl because of its efficiency in string handling. –Mysid 06:18, 4 April 2006 (UTC)
Has this already happened[edit]
- The folly of basing Scientific Research on unobservables events. Ernst Mach did not believe in the theory of the atom, saying that "atom" is just a word and nothing more.
- Mach went on. If you do believe in the atom, what would happen? Well physics will generate many many results. Physicists will construct models of the atom and then they will find out that these model do not work.
- So then physicists will replace the models with newer mathematical models because mathematical terms can be stretch to fit any data. And then physicists will end up saying that "because we can't observe it, we can't measure it. So the position might be this and the energy is probably that."
- Then the physicists will be driven to the theory that the energy comes and goes more or less at random. All sorts of trash will appear in their theory. There will be "elementary particles" with strange names and anti-matter which ought to be there but isn't. These "particles" would either behaving like a wave or discrete particles depending on the observable outcome. So the laws of physics will degenerate into mumbo jumbo.
My god! Imagine living in a world where this has already happened. What an utterly weird world it must be. Ohanian 01:40, 4 April 2006 (UTC)
- I don't know what your point is, but that quote is a fake. Melchoir 01:50, 4 April 2006 (UTC)
- Holy crap! They've figured out that we're making it all up! Run! -- An evil particle physicist 01:51, 4 April 2006 (UTC)
- Particle physics ain't simple and the mathematics correlates with experimentation. There's nothing wrong with revising "models" and making analogies to help us understand physical concepts on a microscopic level. imo, our inability to interpret obscure mathematics (the one "true", non-analogous model) is why quantum mechanics must appear to our mind as "mumbo jumbo". Trust the math even if we're destined not to fully comprehend it.
- As for the subject's question, do you mean stuff like Polywater and other forms of so-called Pathological science?
- -Snpoj 01:54, 4 April 2006 (UTC)
I believe the true test of any model, such as the model of the atom, is whether it can predict things which aren't already known. I believe the models of the atom have predicted most, but not all, atomic interactions. If we look at a simpler model, a globe is a model of the Earth. While not specifically designed to measure the distance from Bombay to London, it could be used for this purpose by laying a string on the globe between the two, then scaling up the string's length to account for the scale of the globe. However, in other respects, like the density, the globe may not be correct. Thus, it is a good, but not perfect, model. I think atomic theory falls into the same category. The earliest versions, with electrons in planar, circular orbits, is the least accurate, and the current, far more complex model with electrons inhabiting waveform probability functions, is the most accurate model. StuRat 07:15, 4 April 2006 (UTC)
- And there is no such thing as a "perfect model" -- a model must, by definition, be something lesser than the ultimate truth. And for good reason, too! Not much one can do with a 1:1 map. --Fastfission 01:14, 5 April 2006 (UTC)
- One must here realize the difference between ideas and reality. Physicists, in modelling our world with theories and mathematics etc., have never really claimed that their work is how nature really is behind the scenes. Models, as they are so named, are only made to help our understanding and use of the world and materials around us, but never as a direct replacement for what actually is there. In the ancient world, for instance, unlike what was later related, many educated individuals never believed the heliocentric model to be what the world was "really" like, but they accept it anyway simply because it is often the easiest way then conceivable to interpret the observations around us. Thus it is the same with your query- models are essentially idealistic, yet no idea can truely capture reality in the sense of that- which- is. In a way physicists are creating the laws of nature- we have no idea, and can never know, whether atoms really existed "behind the scenes". But the best way to understand physical models are to think of them as the- very limited!- human versions of nature's workings as we see it. They never are, and were never meant to be, replacements of what actually is present. Sometimes we are so involved in our symbol means that we believe the symbols to be reality itself, when in fact it is only a weak manifest. Luthinya 20:07, 10 April 2006 (UTC)
multi-language support website problem[edit]
Hai,
i am programmer in php . i am creating a website with multi-language support. i.e english,tamil,sinhalese support website. and my problem is how to show the site content in different languages.(because browser doesnot support the languages how to install fonts dynamically or how to use unicode support for my website). please any one provide a solution.waiting for your reply
Thanking you, sudhakar.
- Create your website in UTF-8 and use Content negotiation to select the language automatically. Installing fonts is not your problem, it is the problem of the website user. —Masatran 03:17, 4 April 2006 (UTC)
- I think he wants to make it his problem, so the user won't have to deal with it, but can still see the page correctly. - Taxman Talk 23:33, 4 April 2006 (UTC)
- I think it would be safe to assume that the user would have the appropriate fonts for their language/script already installed on their computer. I don't think there would really be much point them using that computer otherwise. --alien2k 22:38, 11 April 2006 (UTC)
- I think he wants to make it his problem, so the user won't have to deal with it, but can still see the page correctly. - Taxman Talk 23:33, 4 April 2006 (UTC)
Does light have mass?[edit]
Does light have mass? I have only found conflicting or incomplete answers on the internet. Thanks
--Jared
- Photons (the particles that carry EM forces) have no mass, but can impart momentum -- see Compton scattering Raul654 03:46, 4 April 2006 (UTC)
- Nice explanation from the physics FAQ. EricR 04:59, 4 April 2006 (UTC)
Another planet?[edit]
If there were a planet similar to Earth in both size and solar distance, always orbiting on the opposite side of the sun, how would we be able to know it was there?
- Hmm... even if the Earth 2 were on an exactly opposite orbit from Earth at some point in time, perturbations from the other planets would affect the twins unequally, and that should eventually mess up the alignment enough to make Earth 2 directly visible from Earth. You could do a numerical simulation to figure out how long that would take; I'm not sure how to estimate it. Melchoir 04:37, 4 April 2006 (UTC)
- See Counter-Earth for more information. — Knowledge Seeker দ 04:48, 4 April 2006 (UTC)
- The orbit of earth around the sun is an ellipse. See Kepler's laws of planetary motion for why Earth 2 could never be always on the opposite side of the sun. EricR 04:56, 4 April 2006 (UTC)
- Then the other planet would have to be an ellipse. Without a symmetry-breaking influence (such as the other planets), there is nothing preventing another planet from occupying an exacly opposite orbit. Melchoir 05:26, 4 April 2006 (UTC)
- You're right of course. Looks like i also egagerated the eccentricity. EricR 05:50, 4 April 2006 (UTC)
- I think you were right to start with Eric, but I've posted my analysis as another question at the bottom of the page. DJ Clayworth 20:32, 6 April 2006 (UTC)
- Then the other planet would have to be an ellipse. Without a symmetry-breaking influence (such as the other planets), there is nothing preventing another planet from occupying an exacly opposite orbit. Melchoir 05:26, 4 April 2006 (UTC)
- My dad once theorized that there might be an identical Sun on the opposite side of the Milky Way with an identical Earth orbiting it that supports (and has) life on it, and that the only reason that neither Earth has detected the other is because we can't see through the center of the galaxy very well. :P —OneofThem(talk)(contribs) 17:29, 4 April 2006 (UTC)
- Recent work in viewing distant galaxies has used a gravitational magnifying glass sort of trick caused by the gravitational bend of clusters of nearer galaxies. Why wouldn't the clusters of stars in the center of the galaxy provide the same effect for seeing clearer images of the opposite side of the galaxy. But, to the point of this question, the opposite side of the Sun has been examined repeatedly by our satellites. I have no idea how many we've slung out into space, but many of them have seen around the Sun while looking at other planets, moons, asteroids, etc... --Kainaw (talk) 13:51, 5 April 2006 (UTC)
Tatto Help[edit]
Dear Readers, I'm in the middle of getting a tattoo but i cant find the formula for this saying. It goes,"Once you are Born, You begin to Die." Its not the actuall saying but thats what it means. There is a formula for this, the Theory starts with an "E". Please Help Thank You, Bare Skin
- Are you thinking of entropy? TenOfAllTrades(talk) 05:59, 4 April 2006 (UTC)
Cause of death[edit]
I am researching my family history and have a relative aged 19 months who died from " Tabes mesenterita" according to her detah certificat. Could anybody please advise what this is? Tired wikipedia amd medical networks but could only find that "tabes" means gegeneration - i think!
May thx
Andy
- A mailing list archive from 7 years ago contains a similar question. Maybe the words could have been Tabs Mesenterica, which means tuberculosis of the lymph glands inside the abdomen – a children's illness. (source) –Mysid 07:11, 4 April 2006 (UTC)
Your term is spelled correctly but is difficult to match to a specific modern diagnosis. Tabes is an old medical term that means wasting. It is a nonspecific term, and could have resulted from many different diseases. Mesenterica is the adjectival form of the word mesentery, which in modern medical usage refers to the tissue that connects the intestines to the rest of the abdominal cavity and contains lymph nodes and blood vessels. The interpretation might vary by the date and place of the death certificate: the term tabes was used more widely in French than English medical writing and survived in more contexts in the 20th century. In English, the term tabes disappeared from medical usage in the 20th century except as part of the term tabes dorsalis, which is a degeneration of spinal nerves caused by syphilis, not likely the cause of an infant's death.
I also suspect mesenteric may have also been used before the late 19th century in a wider sense to mean "intestinal" but I am not certain of this.
If the death certificate was in English and before the late 19th century, the diagnosis was probably not based on an autopsy or diagnostic tests, and so represents a description of the disease process. Describing wasting as a cause of an infant's death mainly implies that the cause of death was not physical trauma, acute infection or illness, or sudden cessation of breathing but a more prolonged process in which failure to thrive was the chief manifestation. If I am correct that a practicing physician might use the term mesenteric more broadly to imply "involving the intestines or abdomen", then it suggests that diarrhea or abdominal distention were the most prominent signs of the illness. There are many causes of failure to thrive with gastrointestinal manifestations that could have led to death before the 20th century: cystic fibrosis, or chronic infection such as tuberculosis. The other type of abdominal disease that could have caused a wasting death would have been an obstruction of the stomach or intestines (e.g., pyloric stenosis, duodenal atresia). I do not know whether tubercular mesenteric adenitis could cause enough enlargement to be palpable in the abdomen and identifiable by a doctor as a cause of death without an autopsy. Can you give us some more context (date and location of death)? alteripse 11:30, 4 April 2006 (UTC)
How to change the magnetic strength of the magnet bar ?[edit]
I have one magnet bar and one ion bar , one man talk me that he can make the magnetic field of this magnet,it shall be stronger or weeker by placing one ion bar with the adjustable thickness . Do you aggree this?
Location of the first observed point : Z, Location of the magnet bar : M, Location of the ion bar : I, Location of the 2nd observed point : Y
Z-------------MI-------------Y
Case (1): thickness of the ion bar is 5mm; Case (2):thickness of the ion bar is 1mm
In the case (1), we use one ion bar which is applied to the magnet bar with the thickness of about 5mm . In the case (2), we use one ion bar which is applied to the magnet bar with the thickness of about 1mm .
At the point of Z, (in the both cases) the magnetic field is the same strength.
And at the point Y in the case (1), by causing of the reluctance effect , and the thickness of the ion bar is thicker , the magnetic field shall be weeker than the point Y of the case (2) . A man talk me that he can make the magnetic field of this magnet,it shall be stronger or weeker by placing one ion bar with the adjustable thickness . I.e. that he want to talk at the point of Z: At Z in the case (1), the field is smaller than Z in the case (2). Is it right? Wiki
- Your diagram doesn't seem to display for me, because it's on your computer. You need to upload it to Wikipedia so we can see it. Try using the "Upload file" link on the left to do this.
- Changing the material and material quantity can have an effect. Some materials, like iron, conduct magnetic fields better than others, like air. Resistance to a magnetic field is called reluctance. StuRat 08:43, 4 April 2006 (UTC)
Kids, if you find an ion bar on the street, do not touch it!!! They can be very reactive, and are used to propel starships. --Zeizmic 15:18, 4 April 2006 (UTC)
- Is that kinda like an oxygen bar? --jpgordon∇∆∇∆ 15:33, 5 April 2006 (UTC)
Using the same disk space for swap space and pagefile on a dual boot computer[edit]
My situation is as follows. I have a dual boot computer with Windows XP and Ubuntu 5.10. I have told Windows to put its pagefile in a dedicated partition located at the beginning of the hard drive (I have just one). My question is: can I tell Linux to use that pagefile as a swap file in an effective way? Just telling it the standard way (swapon etc.) does not work well because apparently it tries to mount the swap space before that partition, and so it cannot see it. When it mounts that partition later, it does not know that it has to swapon that file again. So the result is no mounted swap space. I was also told (Google groups) that I could format that partition to a swap partition every time Linux starts and format it back to FAT32 every times it shuts down (and re-create the swap file), but I do not know how to do this. Can anyone give me advice, please? Any suggestion about using the same physical space for swap/virtual memory is very welcome. Cthulhu.mythos 10:16, 4 April 2006 (UTC)
- It's possible, but it's not easy to do.
- You can use the same file in both linux and windows as a swap file, but the problem is that both linux and windows require that the file is prepared in a special way before it can be used as a swap file (this is in contrary to BSD, which I think doesn't require this). For linux, this is easy to do, because you can use the mkswap utility every bootup before you turn the swap on. For windows, it's more difficult, because there's no simple way this preparation can be done. I've read about this problem a few years ago, and it said that a kludge that works is to save the first few blocks of the file after windows have prepared it, and resore that every time linux is shut down (I've never tried this myself). However, that was ages ago, for an older version of windows, and at the time when hard disk capacity was more expensive. These days, it's probably not a problem to allocate separate swap space for each OS you install. (Still, if you insist, I wouldn't recommend making a FAT partition every time linux shuts down, I'd rather use a swap file instead of a partition.)
- Yeah, I've found the document talking about this: Linux Swap Space Mini-HOWTO. – b_jonas 13:36, 4 April 2006 (UTC)
- Thanks. It does semm quite difficult, actually. But maybe I will try to do it for the sake of it. Cthulhu.mythos 12:17, 5 April 2006 (UTC)
- If you want to do it for the geek/hobbey value, knock yourself out, but otherwise, I'd say it's not worth it. hard drive space is cheap. For great justice. 00:01, 6 April 2006 (UTC)
- Thanks. It does semm quite difficult, actually. But maybe I will try to do it for the sake of it. Cthulhu.mythos 12:17, 5 April 2006 (UTC)
- One more note. Do not set a too large swap space, as that wastes the main memory. Twice the size of the memory is enough. – b_jonas 19:55, 7 April 2006 (UTC)
Heliocentric[edit]
The word Heliocentric is taken from astronomy (sun centered model. What would be the term in social meanings - when a system is centered around a person?
- A particular person, or people in general? "Anthropocentric" for the latter, not too sure for the former, it would probably depend on what makes that person so special that they have a system built around them. You might get more useful replies over at the Language refdesk, to be honest. --Bth 10:56, 4 April 2006 (UTC)
- Egocentric. --Hughcharlesparker 09:30, 5 April 2006 (UTC)
Thanks :-)
Mental illness: Medicine[edit]
I want detailed reading that would throw light on what factors could show that a person did or did not have a mental illness in the past, if there is no evidence of it in the present
Please reply, My email is (address removed to prevent e-mail spam)
THANKS!!!
- It's difficult enough if you are a mental health professional with access to the patient. Any speculation like this, while possibly fun, is simply speculation. For great justice. 23:59, 5 April 2006 (UTC)
- You need to do a search for "forensic psychiatry." To get decent texts, you should try a college library, preferably one with an affiliated medical school. Public libraries are often able to do inter-library loans for works kept in nearly any library imaginable, including foreign libraries. Ande B 05:28, 6 April 2006 (UTC)
Old names for illnesses[edit]
I was looking at the vital statistics in an old almanac a few months ago and discovered a list of the number of people who died from different diseases and ailments - among which were "Cats in the belly" and "Planet". I've hunted both online and offline and cannot find any clue as to what Planet might be, though I found some mention of cats in the belly related to problems with childbirth. Can anyone throw any light on what these ailments might be? Grutness...wha? 13:51, 4 April 2006 (UTC)
- I can help with the second one - "planet" listed as a cause of death was saying that the death of the person was caused by a planet being in the wrong place, and causing so much misfortune that the person simply keeled over. Astrology taken to an extreme, if you will. Looking at it from a modern perspective, it was an explanation given by doctors for deaths which didn't have an obvious cause, at least not to the medicine of their time. Possible 'real' causes might have included things like strokes, heart failure and other sudden-death conditions which give little warning. — QuantumEleven | (talk) 17:11, 4 April 2006 (UTC)
an interesting question...[edit]
hey guys......can someone explain me why some types of substances are insoluble(in pure water) but soluble in acid or in base?? just like zinc, calcium carbonate,zinc oxide.
- It is because the substance is insoluble in water, but reacts with acid or base to create a soluble product. For example, calcium carbonate (which is insoluble) reacts with hydrochloric acid to form calcium chloride, which is soluble: CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l) -- AJR | Talk 16:50, 4 April 2006 (UTC)
- Can you do my homework for me too, AJR? —This unsigned comment was added by 12.41.204.3 (talk • contribs) 19:47, 4 April 2006 UTC.
- No, but I believe that there are site that will, but they charge a fee. Jon513 17:11, 10 April 2006 (UTC)
John MacAdam[edit]
John MacAdam invented the hardtop roads, but I would like to know when and how. Your page on John MacAdams is small and says nothing (besides the tiny nibble I added) about hardtop roads.
someone anser me...plzzzzzzzzz
Research Goya theory?[edit]
I am trying to find information on a theory proposed several years ago by persons unknown ( I can't remember a name.) to the best of my knowledge he was located somewhere in England and as best as I can remember the title of the theory was THE GOYA THEORY. The rudiments of which were that a natural balance exists in nature to wit: that every element attempts to attain a state of equilibrium so that for example hot and cold will gravitate toward a medium temperature.That is simplistic I realize but the best example I could come up with on short notice.
- Any and all help appreciated. Yours truly,Jim Pfaff
- See Gaia hypothesis. Frencheigh 15:41, 4 April 2006 (UTC)
Thanks much,Jim
Dogs Watching Television[edit]
My grandmother used to have a West Highland White Terrier that was able to notice other animals on the television and respond with growls and barks etc. But my attempts to get our Alsation-Collie cross to notice other animals on the television have all failed. He just wont look at the TV. Why wont my dog watch the tele? --Username132 (talk) 15:20, 4 April 2006 (UTC)
- Dogs mental capacity differs from animal to animal. Same with other animals. I used to have a dog that did not like the mirror I had leaned up against the wall. She would go clear up over the bed to avoid walking next to it. When I blocked the bed route, she went past it at a trot with her head down and would not look at it.
- Right, one of the dogs is smarter than the other one. You work out which is which. --Trovatore 21:35, 4 April 2006 (UTC)
The family used to have a cat that would look behind the mirrror to try to find that other cat that looked like her. We used to have a creepy cat statue with eyes that seemed to follow you, the housecat would raise his hackles the first time he saw that. When you say the dog won't look at the TV, do you mean the dog actively avoids the set, or just shows no interest? I suggest running a tape of a barkign dog on TV, and see what that does.
- I can think of two reasons why a dog wouldn't see a TV image as the real thing. One is that they may have a different persistence of vision than humans. That is, they may see the TV image as a series of still images, not as motion, as we do. The other is that dogs rely less on vision, and more on other senses, particularly smell, than humans. So, if the TV doesn't smell like another animal, they don't believe it. With humans "seeing is believing", whereas, with dogs, "smelling is believing". StuRat 02:22, 5 April 2006 (UTC)
- I've grown up around dogs my entire life, and I've always been curious at their reaction to the television. Eventually I learned that animals simply have a greatly different perception of the world from humans. We, for example, live in a world of symbols: we have an intuitive understanding of symbolic representation, such as letters for words and words for actions and things. This symbolic understanding extends to 2-dimensional pictures as 3-dimensional objects: while we take for granted that the illuminated flashing box is showing us representations of real objects (like animals), it is unlikely that most dogs can make that logical leap (they don't drool at dog food commercials, for example). It is far more likely that the terrier was responing to the sounds of the televised animals, and did what dogs do: he barked back. – ClockworkSoul 03:34, 5 April 2006 (UTC)
- You could perform a small test on your dog. Find a copy of the album Animals by Pink Floyd. There's a track on it called, simply, "Dogs". In the background it has sound effects of dogs barking. Over the years I've had a few dogs and some of them reacted to the barking while the rest don't acknowledge it at all. This would at least give you an idea as to whether it's the television he's avoiding or if he's just not interested. Dismas|(talk) 10:01, 5 April 2006 (UTC)
- Here's the status in our household: Stella, a big fluff of a chow/German shepherd/Aussie mix, watches stuff on TV. The most fun was a few years ago when a squirrel ran out onto the field at Pac Bell Park during a Giants game; Stella started acting exactly as she does when she spots a squirrel in the great outdoors. However, she utterly ignored it when we replayed it for her on the Tivo. Mr. Slick, a great big lummox of a boycat who has always lived indoors, is fascinated especially when we put on videos of birds and critters, and even when the TV is off or on the news, will sometimes go and try to find the birdies behind the TV. (We had to stop leaving the bird video on because we worried he would scratch through the screen of the LCD TV.) The other two cats, however, spent a fair amount of their youth as outside cats, and are not in the least bit impressed or even interested in the TV birds. So, yeah, it varies dramatically from critter to critter. --jpgordon∇∆∇∆ 15:29, 5 April 2006 (UTC)
Also, it depends where they grew up. For instance, I had a stray(cat) who would always run from the t.v. Then, her kittens which we had from birth are fine with it.
Pyrite and Sulphur[edit]
Please can you tell me what type of rock (sedimentary rock, igneous rock or metamorphic rock) sulphur and pyrite are? Computerjoe's talk 19:20, 4 April 2006 (UTC)
- Actually, they are both considered minerals, not rocks. --Ed (Edgar181) 19:28, 4 April 2006 (UTC)
- Very well, but would it still be possible to class them under one of the above three? Computerjoe's talk 20:18, 4 April 2006 (UTC)
- I don't think so, because rocks and minerals really have distinct definitions. Minerals have a homogeneous composition and rocks are defined as aggregates of different minerals. A mineral might be a component of different rocks that fall into each of the three classes. --Ed (Edgar181) 20:40, 4 April 2006 (UTC)
- Thank you Ed! :D Computerjoe's talk 21:16, 4 April 2006 (UTC)
- I don't think so, because rocks and minerals really have distinct definitions. Minerals have a homogeneous composition and rocks are defined as aggregates of different minerals. A mineral might be a component of different rocks that fall into each of the three classes. --Ed (Edgar181) 20:40, 4 April 2006 (UTC)
- Very well, but would it still be possible to class them under one of the above three? Computerjoe's talk 20:18, 4 April 2006 (UTC)
weather[edit]
what device points the way the wind is blowing —The preceding unsigned comment was added by 24.182.128.238 (talk • contribs) 19:43, 4 April 2006 (UTC)
- Perhaps a weather vane? Isopropyl 19:48, 4 April 2006 (UTC)
You don't need a weatherman for this. Just keep a clean nose, be careful of the plainclothes. --Trovatore 19:49, 4 April 2006 (UTC)
- Any way the wind blows, doesn't really matter to me. Isopropyl 23:24, 4 April 2006 (UTC)
Politicians use straw polls to determine which way the wind is blowing, which is occasionally affected when one of the gasbags releases more hot air than usual. StuRat 08:56, 5 April 2006 (UTC)
What's the deal with Firefox freezing on Macs?[edit]
In my office I use a mac, and I find that Firefox often freezes if it encounters something it doesn't know how to handle. For instance, if I were to try to go to text twist, a page with, I think, shockwave, Firefox will freeze completely and I have to force-quit out of it. This happens on maybe 20% of pages with odd media formats within them, so at least once a day. I'm using a brand new iMac with a pentium chip, barely used, and the latest version of Firefox. The exact same thing happens on the two-year-old iMac in the office, so I don't think it's just my computer.
When Firefox in Windows reaches something it can't handle, it tells me so or just refuses to load that page. Here on the Mac I have to force-quit, losing all my tabs and anything I've been working on in Firefox. How can I get it to be a bit more graceful in dealing with unexpected formats? — Asbestos | Talk (RFC) 20:03, 4 April 2006 (UTC)
- Actually, I suffer quite a bit with Windows. If you get a chance, use talkback. This enables developers to search BugZilla much quicker. Also, you could search BugZilla. Computerjoe's talk 20:17, 4 April 2006 (UTC)
- Are you using the latest versions of OS X and of Firefox? It is rock solid on my machine. Why are you using Firefox? Is there some specific function you need, or might Camano or Safari meet your needs? For great justice. 23:52, 5 April 2006 (UTC)
- Ahh, but it's Java, not Shockwave, which is probably the reason why you're freezing. In general, the Java VM likes to freeze things. Although, last time I used Mac OS X, browsing with Safari (I think a month or two ago), and used Java, it was fine. Maybe check your Firefox plugins and check that Java is going OK? -- Daverocks (talk) 10:25, 6 April 2006 (UTC)
Recharging Alkaline Batteries[edit]
Are alkaline batteries rechargeable? This website claims that they are. I remember seeing expensive batteries chargers a few years ago that wre supposed to be able to recharge ordinary batteries. --Username132 (talk) 20:04, 4 April 2006 (UTC)
- The website and experiments seem pretty well thought out. I don't see any reason to doubt him. Obviously the manufacturers would warn you against this, both to cover themselves against you over-charging and getting acid on yourself, and because they'd be losing out on the sale of a battery. — Asbestos | Talk (RFC) 20:22, 4 April 2006 (UTC)
- I've got an alkaline recharger. It's great when it works, but when it doesn't, the batteries leak inside the charger or, worse, in use. The problem is the utter randomness of the failure mode. Sometimes a battery lasts for dozens of charges, but the next battery of the same type goes splat on the first charge. It's not too bad for powering cheap devices like torches/flashlights, if you don't mind having to clean them out occasionally. I've also tried "rechargeable" alkalines. They barf less frequently, but the highly caustic stuff that comes out burns your fingers, and the slight extra reliability is not worth the risk and the higher cost. Now I use NiMHs in any device that costs more than the batteries, and they never fail. --Heron 20:28, 4 April 2006 (UTC)
Make database backup using phpMyAdmin[edit]
How do I make a database backup using phpMyAdmin for a mySQL database. I need something I can save on to a CD. Thanks Gerard Foley 21:32, 4 April 2006 (UTC).
- The phpMyAdmin documentation (either distributed or online) is probably a better reference, but you're basically looking to export the relevant database(s) to files (compression is unlikely to matter if you're saving them to CD), making sure to export both structure and data. There may also be some form of "export all" function, but if so, I am not aware of it. — Lomn Talk 22:23, 4 April 2006 (UTC)
- I found an export button, but I can't find where the file gets saved. Gerard Foley 23:27, 4 April 2006 (UTC)
- Try this. --Fastfission 01:09, 5 April 2006 (UTC)
Besides brainstorming processes and other business practices, what's this American Inventor judge's claim to fame? The average American house is supposed to have 18 of his inventions. Yet does this guy publish a list anywhere?
These are the only two relevant links I can find...
http://www.fansofrealitytv.com/forums/showthread.php?s=82a4ef00100cece6411be2fed9b8d48a&t=52829&page=2 http://www.eurekaranch.com/experience/
He's cited in two patent applications, through a "Personal Engineering" article, though not involved with them.[7] He's invented Eveready Lead Free Battery and Crystal Pepsi, but there's no other web references to him, it seems.
So... is he for real? -- Zanimum 21:41, 4 April 2006 (UTC)
- A bit more information "Doug Hall began his inventing career at age 12, inventing and selling a line of magic and juggling kits. After earning a chemical engineering degree from the University of Maine, he joined Procter & Gamble, where he rose to the rank of Master Marketing Inventor -- inventing and shipping a record nine innovations in 12 months."[8]. That's about all the information you're going to get because:
- "3. What are the 18 products or services in the average American home?
- Sorry we can’t tell you. Our contracts with clients require us to remain silent even after the products are introduced.
- The Eureka! Ranch business model is based on “contract inventing.” We are an invention think tank. We are paid a fee for our inventing as opposed to a royalty. We do our work under a “work for hire” agreement. This means that we are not named on patents and have no claim of ownership.
- The basis for the claim of 18 products and services. In truth we have invented or helped reinvent many more than 18 – 18 is the average in each home. And that’s less than half of our work as the majority of our invention consulting work is for industrial and international companies.
- The claim of 18 is based on a nationally representative survey conducted in 1996. The true number today is much higher. When you realize that we work for such clients as Procter & Gamble, Johnson & Johnson, Frito-Lay, Pepsi, American Express, Bank of America, Kraft, Ford, etc., it’s easy to understand how the numbers get so large – so quickly.
- Invention Licensing: In addition, I’ve personally negotiated licensing agreements on behalf of clients with the National Football League, Major League Baseball, Parker Brothers, Henson Associates, Garfield & Friends and Random House." [9]. --Fuhghettaboutit 22:37, 4 April 2006 (UTC)
- Thanks, I missed that FAQ. So there's no way to find out what he created. That really sucks. -- Zanimum 12:51, 10 April 2006 (UTC)
April 5[edit]
Canon Pixma MP780 troubleshooting.[edit]
I have a Canon Pixma MP780. It is an all-in-one printer (copies, scans, faxes, does photos, and prints). It is powered via a USB cable. Out of the blue, it has ceased to power on. The black (3e I believe) cartridge is full, but the cyan, magenta, yellow cartridges, and the other black cartridge is empty, although I don't think that has anything to do with the situation... What could be the cause? What can I do to solve this problem? Javguerre 02:10, 5 April 2006 (UTC)
- Its likely not your ink, I don't want to sound like I'm stating the obvious but check your power cables. If you have a multimeter try checking to see if voltage comes out of the power supply. Let me know if those help, I can look up some more things to try if necessary :) -- Tawker 07:34, 5 April 2006 (UTC)
Try a different USB cable, and try it in a different USB port, or, if possible, on a different computer to elimate failure of your cable or computers usb ports. For great justice. 23:57, 5 April 2006 (UTC)
Dogs[edit]
Why do dogs run? Thanks. The pizzaman 19:03, 5 April 2006 (UTC)
- To get to the other side. --Optichan 20:54, 5 April 2006 (UTC)
- Because they can. User:Zoe|(talk) 23:47, 5 April 2006 (UTC)
- Because they are predators. Before they became lapdogs, they hunted in packs, and had to chase down prey in order to eat. Also, they had to run away from predators. It makes some sense that they are driven to run, even though they may not 'need' to anymore, it's such a part of their biology. For great justice. 23:49, 5 April 2006 (UTC)
- No such thing as a dumb question? This is asking like why dogs can walk. --
 Mac Davis] ⌇☢ ญƛ. 07:16, 6 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 07:16, 6 April 2006 (UTC)
I smell a homework question, and I'll be darned if I'm going to devalue the education of a pre-pre-school student who thinks he can just hop on wiki refdesk for the answers to all his problems!!! --Jmeden2000 15:11, 12 April 2006 (UTC)
Multithreading in Fedora Core 4[edit]
I've successfully installed the second processor in my Proliant 3000, but things don't seem to be any faster than usual. Is there something I need to install or trigger to activate multithreading in Fedora Core 4? If a program wasn't designed for multithreading, can an OS split its requests up across two threads for faster processing? Is there a more suitable distribution to take advantage of the two processors (FC4 takes nearly 10 minutes to load). --Username132 (talk) 03:26, 5 April 2006 (UTC)
- Is this an existing install of Fedora or a new one? -- Tawker 07:32, 5 April 2006 (UTC)
- To take advantage of multiple processors, you need an SMP kernel. I believe that this is included in a Fedora install if the installer detects multiple processors; otherwise you need to install it yourself - Fedora RPMs definitely exist, and shouldn't be too hard to find (it's called kernel-smp or linux-kernel-smp or something like that). Once you've installed the RPM, you need to restart your computer.
- As for the advantages, the only performance increase with most applications will be due to one processor handling the OS overhead while your application gets the other all to itself. Some programs (mostly scientific simulation software) can be recompiled to take advantage of double processors, but most desktop software can't. 61.48.162.166 11:26, 5 April 2006 (UTC)
- When you say "things don't seem to be any faster" can you be specific about what you are measuring? It will be rare (though not unknown) for any *single* program to give greater throughput on multiple processors. Multiple processors benefit multiple programs. If a program wasn't designed for multi-threading, there is no magic available to make it multi-thread. Notinasnaid 11:37, 5 April 2006 (UTC)
- What I meant was that it still takes about 10 minutes to load the operating system. --Username132 (talk) 19:03, 5 April 2006 (UTC)
- Most Unix systems I've seen first run in the Kernel, single threaded. They spend a lot of time checking devices, which is not going to be related to the number or speed of CPUs. Then they start all the daemon processes. There is potential here to use dual CPUs, but the systems I have seen do not; they start them one after another, often with series of built in delays. So boot time isn't very much dependent on the kind of computer. What will improve it is removing unnecessary startup. A bold move would be to identify startup scripts which are independent of each other, and write a framework to multiprocess them. Maybe some Linux distributions have already done this. Mac OS X has done this. Notinasnaid 20:05, 5 April 2006 (UTC)
Basically, it will be noticeable if you run more programs at the same time or, reconfigure some of your software. Unless you get like an quad-core apple, and actually run alot of stuff at once, they are useless IMO, nice bragging rights though!
Nitroglycerin[edit]
I will take this time to ask a foolish question, could Nitroglycerin be used in propelling a craft pecifically a spaceship?
- Not a spaceship: the chemical formula for nitroglycerin is C3H5(ONO2)3. When exploded, it combines with oxygen to form the stable carbon dioxide, water and nitrogen molecules. The vaccuum of space, having very little oxygen with which to react, can't support the reaction. Even in within the atmosphere, however, propulsion with something as terribly unstable as nitroglycerin is a very bad idea: pure nitroglycerin is so reactive that it has been known to explode after receiving even the tiniest of jolts, or even by changing temperature by as little as 1 degree too quickly. Nasty stuff. – ClockworkSoul 03:49, 5 April 2006 (UTC)
- To explode nitroglycerin does not requires oxygen (or anything else). Pure nitroglycerin is too unstable to be used as rocket fuel. -Yyy 06:12, 5 April 2006 (UTC)
- I can't see any reason to think it would be a particularly good fuel even if stability weren't a problem. High explosives don't have particularly high energy density; they just release it very very fast. --Trovatore 06:25, 5 April 2006 (UTC)
- Just for the record, fuel+oxygen reactions are used in rockets, commonly in fact. See liquid oxygen. --Bth 12:44, 5 April 2006 (UTC)
- That's true, but rockets bring the oxygen with them. – ClockworkSoul 13:56, 5 April 2006 (UTC)
- Solid rocket fuel contains all the necessary oxygen within itself and does not rely on additional atmospheric oxygen. It is a relatively simple recipe. You may want to check out some of the amateur rocket sites if you are curious about the properties, stability, ranging, and targetting of such devices. Ande B 22:23, 5 April 2006 (UTC)
- Unless you're using a Hybrid rocket. Solid fuel+gaseous/liquid oxidizer. Nitroglycerin is a liquid anyway. Night Gyr 17:39, 7 April 2006 (UTC)
- Hybrid, now that's cool! I always found it ironic (as have many others) that nitroglycerine, combined with a few binders to make a nice little pill, could keep the heart from exploding. Ande B 01:53, 8 April 2006 (UTC)
- Unless you're using a Hybrid rocket. Solid fuel+gaseous/liquid oxidizer. Nitroglycerin is a liquid anyway. Night Gyr 17:39, 7 April 2006 (UTC)
- Solid rocket fuel contains all the necessary oxygen within itself and does not rely on additional atmospheric oxygen. It is a relatively simple recipe. You may want to check out some of the amateur rocket sites if you are curious about the properties, stability, ranging, and targetting of such devices. Ande B 22:23, 5 April 2006 (UTC)
- That's true, but rockets bring the oxygen with them. – ClockworkSoul 13:56, 5 April 2006 (UTC)
- Nitro would be unsuitable as a rocket fuel mainly due to its instability. The instant you lit off the engine, the shock would set off a sympathetic detonation in the rest of the supply. Also, explosions are less efficient than a steady burn due to the danger of damaging the ship and corresponding need for stronger (i.e. heavier) engines and nozzles. Night Gyr 17:39, 7 April 2006 (UTC)
How is modulation diffrent from multiplexing?[edit]
this is regarding networks communication,i am always getting convuse between these words,can u plz make it clear, it would be so great and thankfulll to u .thanks a lot --- viv
- If I'm reading correctly the main difference is modulation uses one channel whereas multiplexing uses multiple channels.
In telecommunications, multiplexing (also MUXing) is the combination of two or more higher level channels into a single lower level channel such that a reverse process, known as inverse multiplexing, demultiplexing, or demuxing, can extract the original channels. The individual channels are identifiable by a predetermined coding scheme.
vs
Modulation is the process of varying a carrier signal in order to use that signal to convey information. The three key parameters of a sinusoid are its amplitude, its phase and its frequency, all of which can be modified in accordance with an information signal to obtain the modulated signal. There are several reasons to modulate a signal before transmission in a medium. These include the ability of different users sharing a medium (multiple access), and making the signal properties physically compatible with the propagation medium. A device that performs modulation is known as a modulator and a device that performs the inverse operation of demodulation is known as a demodulator. A device that can do both operations is a modem (a contraction of the two terms).
Cheers -- Tawker 07:31, 5 April 2006 (UTC)
Thin[edit]
If you thickened something with starch, is there anything easily available to thin it a bit? 57.66.51.165 10:18, 5 April 2006 (UTC)
According to some places, that makes it thicker owing to the grains swelling. Plus, I kind of need it concentrated. 57.66.51.165 16:23, 5 April 2006 (UTC)
- It's possible that if the mixture is so thick that the starch is not fully disolved, that might be true, but then you would have a really thick paste, almost a solid. Water is the way to go I'm afraid. For great justice. 23:47, 5 April 2006 (UTC)
Small switching PSUs 400 VAC -> 24 VDC[edit]
Hi, I'm trying to find someone selling small switching PSUs (for mounting in an enclosure). Wanted voltages: 400 VAC->24 VDC. I haven't found anything with a lower power rating than approximately 100 W, and most of them are quite bulky (the smallest side can't be bigger than 65 mm or they won't fit). There are loads of alternatives for 230 VDC voltage though. Does anyone know of a suitable inexpensive alternative, available in northern Europe/Scandinavia? 62.119.184.141 12:20, 5 April 2006 (UTC)
- If I were trying to get hold of a not-so-common component like that, I would try RS[10] or Farnell[11] (both links are to their lists of national sites) both of whom generally have a good range. Or if you have a regular supplier who doesn't list what you're looking for, have you tried asking them if they can order something for you specially? -- AJR | Talk 19:16, 5 April 2006 (UTC)
- I had already tried Farnell, didn't find any. I tried RSComponent as well - my search for "PSU 400VAC 24VDC" didn't get any answers (except partial matches - there are lot of 24VDC PSUs). And a custom-made solution isn't really in the question I think - the number of units needed is pretty small. 62.119.184.141 09:51, 7 April 2006 (UTC)
dear sir/madam, I would like to know, what makes the best lubricant to smear on my penis. i have tried sunflower oil, but my girlfriend says that it burns too much. This may sound juvenile, but I really have a problem. Thank you very much for your time,
--86.16.167.4 15:09, 5 April 2006 (UTC)
- There are many products specifically designed for that. You might want to look at personal lubricant. --Ed (Edgar181) 15:14, 5 April 2006 (UTC)
- Assuming you are a young couple, my first impression is you are not taking your time with her enough. If you have plenty of foreplay you should not need a lubricant, as her sexual arousal will be enough to provide that.
- You may be right but many young women actually need the assistance of a lubricant and should not feel that they are somehow "inadequate" because of that. There are plenty of good water based or silicon based alternatives to oil so that the couple can experiment with a number of them to get the right one for their life styles. Ande B 22:32, 5 April 2006 (UTC)
- Right. Maybe he's talking about anal sex?... —Keenan Pepper 17:25, 5 April 2006 (UTC)
Cockpit Automation Philosophy[edit]
Sir or Madam, I am currently writing a graduate-level paper on the differences between the cockpit automation philosophies used by Boeing and Airbus and am attempting to locate greater factual and/or 'learned opinion', vice the readily-available lay-opinion comparing the two. Any assistance would be greatly appriciated; thank you in advance.
- All I can offer is one of those readily available lay-opinions: There is concern that a high level of automation will lead the pilot and copilot to pay less attention, thus causing more accidents. However, this is really just a specific example of one of the problems inherent in human interaction with fault tolerant design (where the "faults" are caused by inattentive humans). StuRat 17:43, 5 April 2006 (UTC)
- You're looking in the wrong place. You need to spend some time with tools like Google Scholar and Web of Knowledge to see what's in the academic journals. A search for "Boeing cockpit automation" in those places turns up dozens of relevant papers. --Robert Merkel 05:17, 6 April 2006 (UTC)
GRR... My upload bandwidth sucks...[edit]
I've noticed that anything that a ISP would sell you has a terrible up-down ratio (excluding SDSL, but that tends to have lower downspeeds). Where do the ISPs get all their bandwidth?--Frenchman113 19:19, 5 April 2006 (UTC)
- Er, this doesn't answer your question, but to my understanding the sum of up + down bandwidth is limited by the quality (i.e. price) of your and their DSL/cable equipment. Since most people are doing things like web browsing and email that don't upload much, the ISPs emphasize download speeds at the expense of the up. For completeness, the answer to what you actually asked is that ISPs rent bandwidth from bigger providers upstream of them (like UUNET) just like you rent it from them. Eventually you reach one of the backbone providers like Level 3 who own the biggest pipes and effectively "make" the bandwidth. --Tardis 20:37, 5 April 2006 (UTC)
- Right. But say one was feelthy rich and could lay one's own data link between continents. What would it take to become a 'Backbone' provider? Is the difference determined by who sells bandwidth to who? 08:31, 6 April 2006 (UTC)
- As to why upstream is slower: I like the picture in ADSL. That's just how frequency allocation has been made. To get a symmetric line: call your local telco providers, they are likely able to lease you a line. That is what most companies do when they want a high volume web site etc. Expect to pay 10x-100x what you pay for ADSL per month (more for really fat pipes). To lay your own lines: consult local legislation; you may need to form a company that is a certified telco provider. Sure that is possible if you have the cash; most telcos are private companies.
- For most home users the ADSL or cable modem the local telcos offer is pretty much the best price/speed point you can get. If there was a magical way to offer faster service some company would have already done that and wiped the competition off the Earth. Market economy in action... Weregerbil 11:06, 6 April 2006 (UTC)
From AIDS back to HIV[edit]
I was wondering if the drugs we have today for HIV patients are so good that they have the ability to make the HIV virus undetectable in the human body, they why can they not bring back a patients with AIDS back into HIV+ status?
- HIV+ is a different mode of operation of the virus -- a dormant phase with no symptoms whatsoever. I imagine that "undetectable" HIV is simply small numbers of viral particles, which can easily grow back to large numbers if the treatment is removed or becomes ineffective. In other words, HIV+ is a different kind of infection, whereas these drugs only change the amount of it (and not to 0). But I am not a pathologist at all... try reading the pharmacological information associated with the drugs? The FDA might have some relevant information. --Tardis 20:46, 5 April 2006 (UTC)
O4[edit]
Have molecules composed of four oxygen atoms been produced? What's up with them?
- In theory, one could have
O-O | | O-O
and fulfill the octet rule requirements. Cyclobutane has this structure (with hydrogens), for instance. But it strains this type of chemical bond to adopt a 90° angle, so it would almost certainly be unstable and fall apart into two normal O2 molecules. --Tardis 21:09, 5 April 2006 (UTC)
- Tetraoxygen does indeed exist at very high pressures. —Keenan Pepper 21:12, 5 April 2006 (UTC)
- There's N4 as well. I'm not sure anyone actually knows if O4 has a cyclic structure though. --BluePlatypus 23:31, 5 April 2006 (UTC)
- My proposition about O4 : since we know that O 2 is paramagnetic, before people can make the high magnetic field, it is anti-magnetic,why don't you try to make the experiment as follows:
- Use two tanks which can sustain a high pressure, a tank contain O2, another is empty, and maybe waccuum Join two tanks by one tube + one siphon + one tube ; ( siphon has the diameter bigger than the tubes); in middle of the siphon,place one thick steel frame which can sustain a high pressure, drill many pores( its diameter is super small as possible) on the steel frame , around the siphon, apply the highest magnetic field with about 60MOe, and then press the O2 in first tank thru the siphon, I think that you can get O4 in the second tank,this is my own opinion, I don't have any equipment for this experiment. If you have the condition to make it, please let me know the result. Or if you have the stuck, I have the time to suppose again . --User:Ngocthuan 06 23:08 , 6 April 2006 (UTC)
Galileo[edit]
My question is easy, but i just can't find answer :( What is the well-known phrase, associated with Gelileo Galilei? "Yet it spins", "Still it rotates" or how? Just i'm not native english speaker, and never interested of it in english. Thank you. ellol 21:48, 5 April 2006 (UTC)
- It's not mentioned explicitly in his article but it is linked in the 'see also' section. He was, apparently, forced to recant his heliocentrism, but muttered E pur si muove! - "And yet it moves" - under his breath. --Hughcharlesparker 22:08, 5 April 2006 (UTC)
- Sorry. Right. Thank you! ellol 03:24, 6 April 2006 (UTC)
- Yes, but this is most likely apocrophal. I mean, he might have said it under his breath (we really don't know), but he was effectively shut up about it. For great justice. 23:42, 5 April 2006 (UTC)
isotopes[edit]
the time it takes for one half of the amount of an isotope to decay to nonradioactive form is it's__________?
- Half-life. --Someones life 23:02, 5 April 2006 (UTC)
- Half-life = how much of my time I spend reading obvious homework questions. StuRat 08:23, 8 April 2006 (UTC)
Constitution font[edit]
On Mac OS X, is there a free font that evokes the kind of script used on old copies of the US constitution? It doesn't need to be exact, or historically accurate, but just evoke that kind of 'feel'. Thanks! For great justice. 23:36, 5 April 2006 (UTC)
- Zapfino is an incredibly flowing cursive font. It should be suitible for what you are doing. --Serie 00:06, 6 April 2006 (UTC)
Wooaaahh! That IS cursive! That's definately the puppy! Thanks! For great justice. 00:07, 6 April 2006 (UTC)
April 6[edit]
Adhesive bonding[edit]
I've asked a few questions on Talk:Adhesive without many satisfactory answers. I would like to know, for example, if I glue two pieces of polished steel with cyanoacrylate glue, how much of the bonding is chemical and how much is mechanical. I am inclined to think that it is largely a chemical bond, but if so, what is the bond involved? —Ben FrantzDale 02:59, 6 April 2006 (UTC)
- I don't think you could give an exact number for that. I'd think cyanoacrylates would mainly be considered to bond mechanically, polymer glues in general work by filling out the surface area while a liquid and then reacting to form a solid polymer. Chemical compounds in general don't bind very well to metal since the metallic bond is a bit of a different animal from most. It can still bind by chelating to the metal atoms on the surface, probably through either the cyanate or carboxyl groups. However, those bonds would be weaker than in the cases where an adhesive can form hydrogen bonds with the surface. --BluePlatypus 05:13, 6 April 2006 (UTC)
Photographic Memory[edit]
Although the eidetic memory page is certainly informative, I'm not quite clear on what answer is arrived at. Is there, or is there not, such a thing as photographic memory as it is commonly portrayed? By that I mean innate, photographic (actually a snapshot, rather than based around important features like 'red shirt' and 'a bit to the left'), essentially effortless, and durable. If not, why is there so much popular certainty that such people exist? Black Carrot 03:28, 6 April 2006 (UTC)
- This is from my own incredibly shaky memory, so may not be totally accurate, but from what I recall people actuallt do take in most of the items in a scene, but the linkage between short-term and long-term memory is better in some people than others; also some people are better able to retrieve items from their long term memory. It's not that some people have photographic memory where others don't; it's more that some people are better able ot store and access whatever memories they have received. Because some people can perform very well in such memory tasks, it is popularly assumed that some "super-special" memory is present in some individuals, shich is why the idea of photographic memory is so prevalent. Anyone able to confirm or deny...? My specialy was perception, not cognition :) Grutness...wha? 07:58, 6 April 2006 (UTC)
- On the off chance it jogs a memory, the only article I can remember ever reading that mentioned photographic memory existing claimed that, when people with such memory (which they apparently could get hold of enough of to do this test) forgot a picture, which did happen over time, it 'shattered' or 'fragmented' or some such rather than becoming vaguer and less detailed. Anyone recognize this? Black Carrot 04:27, 9 April 2006 (UTC)
The period validity of the patent[edit]
The period validity of the patent of the fuel saver by magnet treatment is invalid.Is’nt it ? Wiki? Ngocthuan 06 11:08, 6 April 2006
U.S. Patent 3,830,621 - Process and Apparatus for Effecting Efficient Combustion. U.S. Patent 4,188,296 - Fuel Combustion and Magnetizing Apparatus used therefor. U.S. Patent 4,461,262 - Fuel Treating Device. U.S. Patent 4,572,145 - Magnetic Fuel Line Device. U.S. Patent 5,124,045 - Permanent Magnetic Power Cell System for Treating Fuel Lines for More Efficient Combustion and Less Pollution. U.S. Patent 5,331,807 - Air Fuel Magnetizer. U.S. Patent 5,664,546 - Fuel Saving Device. U.S. Patent 5,671,719 - Fuel Activation Apparatus using Magnetic Body. U.S. Patent 5,829,420 - Electromagnetic Device for the Magnetic Treatment of Fuel.
I don't know the dates of these. For great justice. 04:27, 6 April 2006 (UTC) The Inset Fuel Stabilizer [12] appears not to be patented. For great justice. 04:29, 6 April 2006 (UTC)
It seems that we don't have articles on fuel saving gadgets, magnets round the fuel line or in the air flow (Ecoflow, FuelMAX, FuelSaverPro, etc), catalysts in the fuel line or tank (Broquet, Fitch Fuel Catalyst, Prozone, Fuelstar, etc), platinum-based combustion enhancers (PVI, Gasaver, Ctech3000, etc), ignition enhancers (Fuel Saving & Power Push, Fireball Ignition, etc, air bleed into the inlet manifold (Ecotek, Khaos, Powerjet USA, etc), turbulence increasers (Ecotek, Tornado Fuel Saver, Powerjet USA, SpiralMax, etc), devices to "atomise the fuel better" (Ecotek, Tornado, SpiralMax, Vaporate, etc), oil additives (Slick 50, Duralube, etc), fuel additives to enhance combustion (Acetone, PowerPill, BioPerformance, etc), engine "cleaning" products (10k Boost, Powerboost, etc), electrical modifications (grounding straps, voltage stabilisers, etc) or hydrogen generators. Shame. For great justice. 04:35, 6 April 2006 (UTC)
- In a slightly less oblique way, For Great Justice is suggesting that patent or no patent, the evidence that any of these devices actually work is extremely thin to nonexistent, particularly the ones related to magnets. See snake oil. --Robert Merkel 04:57, 6 April 2006 (UTC)
- Absolutely - although actually, I think an article on these devices wouldn't be a bad idea - explaining the science of why they can't work would be a worthwhile excercise. What would the name of the article be though? Is there a generic word for these? For great justice. 05:06, 6 April 2006 (UTC)
- Aside from "junk", I don't think so. To be fair, injector cleaners don't quite belong here, in certain specific circumstances they can actually be useful. Subaru actually recommends the periodic use of one for my Subaru Impreza WRX.
- But as to the broader point, how about fuel economy accessories, or something like it? A survey of the devices and their purported mechanisms of action would be quite useful. --Robert Merkel 05:11, 6 April 2006 (UTC)
- Fair point on injector cleaners. I still think there's some milage in the phenomenon of snake oil as applied to fuel economy magnets - perhaps only as a section of snake oil though. These aren't really just accessories though - the point is that they are completely spurious... For great justice. 05:18, 6 April 2006 (UTC)
- But as to the broader point, how about fuel economy accessories, or something like it? A survey of the devices and their purported mechanisms of action would be quite useful. --Robert Merkel 05:11, 6 April 2006 (UTC)
- I have been found out the magnet treatment around the fuel pipe from many years ago, but I think that it is the minimium magnetization, because it is very easy for locating the magnet lying perpendicular to another and first and foremost all of the magnets are perpendicularly to the fuel pipe, this thing is the same meaning that they cause the magnetic line perpendicular to the fuel pipe.
The magnetic Flux = B * S * cos (B,n) Here , cos (B, n) =0 ( n : the direction perpendicular to S; i.e: normal line of Surface S) For the maximum magnetization , cos (B,n) must be equal to 1
I.e.: the magnetic lines was caused by these magnets which are paralell to the fuel pipe.
For this raison, we can reduce the volume of the magnets, and follow to their weight shall be reduced noticeable . Another raison, many peoples don't like to use word wrap is because the peoples that don't use it like to force a horizontal scroll bar into your web browser. even the powers of wikipedia and firefox combines cannot wrap the text. Ngocthuan 06 16:25, 6 April 2006 (Vietnam)
Atmospheric pressure (can someone source this for me?)[edit]
An anon added the following to a (featured) page I watch: "Where an atmosphere is less than 0.006 Earth atmospheres water cannot exist in liquid form as the required atmospheric pressure, 4.56 mmHg (608 Pa), does not occur." I didn't see this specific point on the atmospheric pressure page and I don't know an mmHg from a bowling ball. Hoping someone could point to a source. Marskell 08:26, 6 April 2006 (UTC)
- It's the pressure at the Triple point of water. --BluePlatypus 08:33, 6 April 2006 (UTC)
red mercuy[edit]
Dear Sir is red mecury rs 99.999 can be used in medisence or not
- I believe the idea is, that if Red mercury existed (which it doesn't), it could be used in an atomic bomb. --BluePlatypus 08:35, 6 April 2006 (UTC)
- I think you are referring to mercurochrome which can be used as a topical antiseptic. - Cybergoth 03:21, 7 April 2006 (UTC)
Ewww, what the hell popped out of that earwig?[edit]
Hoping someone knowledgeable in earwig entomology can answer this. I just squished an earwig on the wall with a tissue. While pressing down on it (I had to squish it fast but it took a bit of finger-strength to get it to actually squish) there was a sound not unlike a clicking sound.
I take the tissue off the wall and there's two things -- the earwig's corpse, dead but intact. And then there was something else, it was a dark color and with a shape that an eggplant might be if you were to bend it in the middle 90 degrees. I'm guessing it was either something fecal or was it perhaps an egg it was carrying? Perhaps some inside body part of the earwig? Either way it convinced me that killing an earwig is an ickier experience than merely being in the presence of an earwig, and next time I'll capture one in a jar and let it loose in my neighbor's yard instead.
Just what in the world popped out of that earwig when I squished it? It wasn't even messy, just a solid object with no real "splatter" to it. --209.77.244.12 09:39, 6 April 2006 (UTC)
- I heard that earwigs have two penises in case one breaks off during sex. So maybe what you saw was its spare penis. Ah, our article on earwig has that fact as well. You might find some more stuff in that article that might help you. -- Daverocks (talk) 10:21, 6 April 2006 (UTC)
- The earwig article doesn't actually have a whole lot on earwig anatomy. Shame. For great justice. 18:00, 6 April 2006 (UTC)
- Also. WTF happened? Compact Flash cards are now the new standard for size comparison? Come on! Only on Slashdot, surely - what happened to a ruler, or something?! For great justice. 18:03, 6 April 2006 (UTC)
- The earwig article doesn't actually have a whole lot on earwig anatomy. Shame. For great justice. 18:00, 6 April 2006 (UTC)
Nuclear engineering[edit]
How is it possible to get highly enriched uranium from a centrifuge?Wont that Uranium possess critical mass and undergo fisson?What initiates criticality accidents?
- The uranium first goes through some processing which converts it to uranium hexafluoride. This is a gas, and its density is rather low compared to solid uranium, so criticality isn't so much of an issue here. --HappyCamper 11:16, 6 April 2006 (UTC)
- Have a look at Zippe-type centrifuge. The details of the various designs remain a closely-guarded secret. --Robert Merkel 23:17, 6 April 2006 (UTC)
El Nino and La Nina[edit]
Hello, I am writing an article for my high school newspaper about El Nino and La Nina because I read somewhere that we are just leaving a La Nina year and entering an El Nino year. Is this true, because the Wikipedia article doesn't say that. Thanks for your help!! Zach 10:42, 6 April 2006 (UTC)
- The Australian Bureau of Meteorology's climate page has extensive information about the current state of ENSO. --Robert Merkel 23:20, 6 April 2006 (UTC)
Mermaid[edit]
Hi,
Does mermaid exist?--Aju 11:07, 6 April 2006 (UTC)
Nope. How old are you? Loomis51 21:52, 6 April 2006 (UTC)
- Irrelevant. Misinformation can strike at any age, though generally we learn to guard against it better over time. Tzarius 09:07, 7 April 2006 (UTC)
To a very lonely sailor, a manatee can look like a mermaid. StuRat 08:14, 8 April 2006 (UTC)
Silicon Wafers[edit]
I've got a question about the surface of a silicon wafer. Do you get an silicon dioxide layer on the surface? If so, what kind of thickness might you expect? If not, what does the surface of a silicon wafer (outside of the cleanroom environment) look like? 12.106.14.201 14:36, 6 April 2006 (UTC)
- It looks pretty much like a (somewhat dark) mirror. Imagine this sliced up. You won't get an oxide layer at room temperature, you have to grow them at high temperature to the desired thickness. Current research involves MOSFET gate oxides with thickness < 10 nm. - mako 02:14, 7 April 2006 (UTC)
APOLOGY[edit]
Hello! It’s me Nita .I am going to write an assignment about Feasibility report.I am totally ignorant of this term. I had already sent a question to u n u people replied it too. I confessed that I had committed some mistakes that’s why I am sending you an informal apology .I beg your pardon sir,hope you will ignore my mistake. I admit it that my English is not proper but you people will have to accept the fact that on the face of this earth a lot of nations with different languages are residing and none can snub a person on the plea that he is speaking or writing wrong English .I know four languages .ENGLISH, URDU, HINDI, FRENCH and PERSIAN .I guess its enough for a girl who is just 17 .my purpose of sending u this mail is just to give u this explanation that ; I am sorry for using wrong grammar ,I am sorry for not saying PLEASE .now may you tell me what is feasibility report .I am not asking you to make my whole assignment I am just asking for a little assistance so that I may be able to fulfill my task properly —The preceding unsigned comment was added by 203.99.61.48 (talk • contribs) 14:37, 6 April 2006 (UTC) Thank you.
- When touring a strange land, it is best to have a guide, or to learn the local customs. That way, people won't be rude to you. Here, we have the equivalent of a guide at the beginning of this document, and it is best to read it. --Zeizmic 14:47, 6 April 2006 (UTC)
- A feasibility report describes whether something can be done or not, how easy or hard it will be to do, etc. Search for the term on google and you will find hundreds of examples of them. Chapuisat 14:52, 6 April 2006 (UTC)
- No, I don't think that's true. "Feasible" is different from "possible". For something to be feasible, it has to be something that can be reasonably accomplished within practical constraints, particularly economic ones; it's not enough for it to be possible in theory. That said, there is a minority usage which uses "feasible" synonymously with "possible" (makes my skin crawl, personally; it's as bad as "refute" meaning "deny"). But that usage is unlikely to be the one intended in something called a "feasibility report". --Trovatore 23:04, 6 April 2006 (UTC)
- Not sure why you think you're disagreeing with me. That's exactly what I was saying it was. "whether something can be done or not, how easy to hard it will be to do". So a report might come back and say "it can't be done" or it might say "It can be done but it will take 3 years and $400,000 and require extensive paperwork", or maybe "It will cost $0.75 and a trip to the hardware store". Anyways, the best way for the original poster to understand would be to google "feasibility report" and read through some examples. Chapuisat 13:33, 7 April 2006 (UTC)
- Huh? Saying it will take 3 years and $400,000 is information about how hard it is to do, not about whether it can be done or not. As far as I can see you're contradicting yourself in the space of two sentences. --Trovatore 19:59, 8 April 2006 (UTC)
- Not sure why you think you're disagreeing with me. That's exactly what I was saying it was. "whether something can be done or not, how easy to hard it will be to do". So a report might come back and say "it can't be done" or it might say "It can be done but it will take 3 years and $400,000 and require extensive paperwork", or maybe "It will cost $0.75 and a trip to the hardware store". Anyways, the best way for the original poster to understand would be to google "feasibility report" and read through some examples. Chapuisat 13:33, 7 April 2006 (UTC)
- Whoops. My eye seems to have interpolated a "not" that wasn't there. I read it as "whether something can be done or not, not how easy or hard it will be to do. --Trovatore 20:22, 8 April 2006 (UTC)
In the case of a feasibility report on an investment, an important indicator of how good the investment would be is the return on investment. This can either be given as a percentage or number of years. For example, if it costs a million dollars to build a bagel factory, and it can make 200,000 dollars in profit a year, that's a 20% ROI or 5 years to pay off the original investment. That would be considered to be a good investment. StuRat 08:09, 8 April 2006 (UTC)
A more general way to evaluate feasibility would be a cost-benefit analysis. The opportunity cost should also be considered. StuRat 22:41, 11 April 2006 (UTC)
- Hi Nita, the link to Feasibility report, which was a redirect to Feasibility study has just been fixed. Probably you should ask your teacher what is expected of you though, it's not clear what you want to know. You say "I know four languages .ENGLISH, URDU, HINDI, FRENCH and PERSIAN." That's very impressive. Do you count Urdu and Hindi as one language because they are so close? GangofOne 23:01, 11 April 2006 (UTC)
Software to record data through the sound card[edit]
Does anyone know any piece of software to record everything that sounds through the speakers? Something like "redirecting" the data to a file. For MS Windows and GNU/Linux also, if possible. Thanks. --GTubio 14:58, 6 April 2006 (UTC)
- I know there's something for the Mac, and I'm sure there is for the PC or Linux, but don't know what it is - would Audacity meet your needs? For great justice. 17:24, 6 April 2006 (UTC)
- On windows, double click on the speaker icon, go to Options -> Properties, select the option "Recording" and on that list, enable all checkboxes that has something to do with "out" ("Stereo Out", "Mix Out", "Stereo Mix" or something like that). Click ok. The slide bars will change for recording volumes, and below each one there's a checkbox. Mark one of the outs and just hit record with any program, one of them will work. ☢ Ҡi∊ff⌇↯ 18:45, 6 April 2006 (UTC)
Thank you very much to all. --GTubio 08:12, 11 April 2006 (UTC)
Interconnection[edit]
If you connect a simple device designed for 240 V/ 50 Hz (like a British hairdryer) with an adapter to 110 V / 60 Hz (like America), will it work? Will the higher frequency damage it? 57.66.51.165 15:24, 6 April 2006 (UTC)
- No, it wont work....for a long time atleast.... The fuse will probably burn up..if it has a fuse... otherwise the whole device would blow up... :-D ..... Jayant,17 Years, India • contribs 16:05, 6 April 2006 (UTC)
- Well, you can get pretty cheap transformers that will convert the voltage. I have one, but frankly, if you only want a hairdrier, it would be cheaper to buy a small US hairdrier. For great justice. 17:21, 6 April 2006 (UTC)
- It won't work, but putting a 240V device in a 120V (the actual US voltage standard) does not, if I recall correctly, cause spectacular failure. The device just doesn't get enough voltage to do anything meaningful. Now, overvolting a 120V device in a 240V outlet... that will cause sparks to fly. As for frequency, damage is unlikely in either direction. — Lomn Talk 17:23, 6 April 2006 (UTC)
- If you plug a 60Hz device into a 50Hz socket, if it's got a transformer or coil somewhere in the circuit (most devices except incandescent light bulbs do), the lower frequency can cause the coil to saturate and become effectively a short circuit, quite possibly leading to a fire. --Serie 22:08, 6 April 2006 (UTC)
- Having moved around a lot between both sides of the Atlantic, I can confirm most of the above (haven't played much with frequency-sensitive stuff), but wanted to warn about transformers: firstly, they only convert voltage, not frequency, so a 120 -> 240 transformer used in the US will produce 240V 60Hz (not 240V 50Hz, like in Europe) electricity - be warned for frequency-sensitive appliances, they might well go wonky (basically, anything complex). Also, transformers have a maximum wattage rating - a small one might be 50W or 100W, check the label. While this is fine for most small appliances, be warned of anything that produces lots of heat (toaster, hairdryer...) - these use massive amounts of power (a medium-sized hairdryer is often 1000W, check the label), and these will blow your transformer in very short order. I agree with For Great Justice, get yourself one of those 'travel' hairdryers (often sold at airports) which have a voltage selector switch for 120 / 240. — QuantumEleven | (talk) 12:14, 7 April 2006 (UTC)
Orbital feasibility of Counter-Earth[edit]
According to the article the orbit of a Counter-Earth (a planet always on the other side of the sun from us) is just feasible. So would somebody please point out what is wrong with this analysis:
- Consider the earth at the point where the line joining it to the sun forms an exact right angle with the major axis of its orbit. Counter-earth must be at the opposite side of the sun, at the point where it's line to the sun also forms a right ngle with the elliptical axis.
- Now let us advance the earth until it is in the same position on the other side of the sun - i.e. the other point where it's line to the sun forms a right angle with the elliptic axis. In order for counter-earth to still be hidden it must now occupy the position that earth previously occupied. BUT this cannot be the case, because by Kepler's second law the areas swept by the arcs must be identical, and this is obviously not the case (one of the planets must be going round the long side of the ellipse).
- So is counter-earth orbitally feasible or not? DJ Clayworth 20:30, 6 April 2006 (UTC)
- Well, the counter-earth theory doesn't (or shouldn't) say that counter-earth is always 180° offset from Earth, but rather that it is so nearly so that it is permanently occluded by the sun. There's allowance for wiggle room in the orbit proportional to the apparent size of the sun. However, since counter-earth is effectively debunked by observations of other planets, I'm not going to bother to work out the math on this one. — Lomn Talk 21:04, 6 April 2006 (UTC)
- This was my first thought when seeing the problem above, however, as Melchoir pointed out, there is no requirement that Earth2TM needs to orbit on the same elipse. If the ellipse were rotated 180° for the other planet, then the instataneous velocities of the two would always be opposite and a line could be drawn connecting the planets and sun at each point in the orbit. So Kepler's Laws don't seem to prevent such a situation, but it's really a 3 body problem. The Lagrangian point article mentions L3 as a possible place for Counter-Earth, but doesn't say whether or not the system would be stable with two approximately equal masses. Maybe the maths reference desk would be able to help out? EricR 22:50, 6 April 2006 (UTC)
I think if the Hand of God were to place 2 perfect earth copies around a single sun, then it might be stable for a few million years. But instabilities, such as solar flares and giant meteors, would soon knock them out of perfect alignment. The formation of a Solar System is horribly unstable, and single planets sweep out their full orbital band. --Zeizmic 21:06, 6 April 2006 (UTC)
- Meteors schmeteors. The counter-earth would have to have a moon exactly the same size as ours, and at exactly the same distance from its primary, and at exactly the same point on its orbit as ours at all times, created at exactly the same moment - otherwise the two orbis would get out of synch very quickly. And given that the moon was most likely caused by a chance collision of a proto-planetary body with the earth, the chances of that happening are probably about as good as going down to the beach and finding a grain of sand with your name and address carved on it. Grutness...wha? 00:59, 7 April 2006 (UTC)
- And, as a final nail in the coffin, we have several satellites in solar orbit (mostly used for observing the sun), these would have spotted the Earth2TM already if it was there. — QuantumEleven | (talk) 12:07, 7 April 2006 (UTC)
- Ah, yes. It had not occurred to be that there was a different ellipse that might satisfy the conditions. And no, my question was not intended to imply I thought counter-earth might exist. Thanks for your help. DJ Clayworth 15:42, 7 April 2006 (UTC)
Lead and its history[edit]
I am doing a research a report about lead and am having trouble finding out about leads history. ANY information would be helpful.
- If you typed "lead" into the search box on the left of this page, you would have found our article on lead. --Robert Merkel 23:24, 6 April 2006 (UTC)
question on animal species[edit]
Out of all the animal species in the world, what animals are most abundant? The percentage?--24.147.235.177 21:57, 6 April 2006 (UTC)
- You mean the most individual animals or the greatest total mass? —Keenan Pepper 00:19, 7 April 2006 (UTC)
- It would be something small like ants or bacteria (though I can't remember if bacteria are part of the animal kingdom). - Mgm|(talk) 10:33, 7 April 2006 (UTC)
- if you don't count bacteria as animals, nematode worms are a good candidate Malcolm Farmer 09:39, 11 April 2006 (UTC)
Journal Article[edit]
Where can I find the article: " On a supposed proof of a theorem in wave motion" which was written by G.J.Stoney, and published in the Philosophical Magazine in 1897 Vol.5 No.43? I am not sure if I will be able to find the place where your response will be posted, and I don't care if the whole world knows my e-mail address, I get lots of junk mail anyway. So if it's possible, send any information you find,to me at newage@uniserve.com Thank-you Thos
- Unfortunately, I can't find an online copy. (JSTOR doesn't have it.) Your only recourse might be to find a substantial university with an old collection. It's actually quite remarkable how many universities have these incredibly interesting historical journals in the open stacks. (Incidentally, be very gentle with the old documents if you do find the paper. Stuff from the turn of the last century may or may not be in good shape, depending on the paper and binding. You might want to ask for assistance from the library staff before you try to photocopy old bound journals—making copies can be very hard on their spines.) TenOfAllTrades(talk) 00:06, 7 April 2006 (UTC)
- Yeah, can't find it either. Its too old. --
 Mac Davis] ⌇☢ ญƛ. 01:34, 7 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 01:34, 7 April 2006 (UTC)
- I presume this is a US journal? If so, a copyright library might be your call of last resort. For great justice. 02:01, 7 April 2006 (UTC)
- If the homepage of the journal (here) doesn't have it (which it doesn't), then it's most likely not scanned or available anywhere at all online. Most journals don't have online archives that go back farther than a decade or two. Anyway, what you need to do is check with your local research/university libraries to see if they have it (their catalogs are usually online). If not, you can usually have a photocopy of the article sent to you from a library that does, for a small fee (say $5 or so). --BluePlatypus 04:50, 7 April 2006 (UTC)
- I can probably get you a copy of the article early next week, as my university seems to have it available pretty easily for scanning. Let me see what I can do. Also, the title of the magazine from that era was, to be specific, The London, Edinburgh and Dublin philosophical magazine and journal of science. What a mouthful. --Fastfission 12:13, 8 April 2006 (UTC)
Downloading the Yahoo! Toolbar[edit]
When I try to download the Yahoo! toolbar, I am led to the MSN Search engine site without my permission, and, of course, no download takes place. This happens no matter how many times I try again.
Here is the sequence of links
- http://toolbar.yahoo.com/config/slv4_page?.p=featureantispy&.cpdl=net06
- http://toolbar.yahoo.com/config/slv4_done?.act=3&.dflt=1&.intl=us&.region=us&.partner=none&.guest=&.cpdl=net06&.mf1=as&.xpsp2=1&.data=
- http://search.msn.com/results.aspx?q=us
Also, under the subject category of Science on the Wikipedia Reference Desk, the word computing looks like a hyperlink while "medicine" and the others don't. When I click on it, I go through the following sequence of links.
- Many other links on Wikipedia take me to global find in search of something. I have a Windows XP Media Center Edition. I have scanned my computer with the latest version of Norton Anti-virus and found nothing. I have also updated my Windows operating system. I was going to download the Yahoo! toolbar to use its anti-spyware software, but I can't do it.
Can someone help me? When previewing this page, even words that I haven't created links for on the edit page have links after I click "Show preview."Patchouli 22:07, 6 April 2006 (UTC)
- ok it looks like you probably have some sort of spyware? first off, i wouldnt bother with the yahoo toolbar, its not going to help anything. go into start->control panel->add/remove programs. look for anything that might be spyware and get rid of it (get rid of viewpoint media player- your spyware is a bit more malicious than that though, so its gotta be some other stuff). download lavasoft adware, install it, get the latest patch inside the program, and run it, delete what it finds. now go download mozilla firefox for your browser... alot of the spyware that targets internet explorer doesnt affect firefox. if you are still having a lot of spyware problems after these 3 steps, there is a simple solution to it all. backup your desktop, my documents, and any other personal data you have, and reinstall windows. modesty 02:50, 7 April 2006 (UTC)
- I have Ad-aware and have run and deleted everything twice today. Do you think uninstalling Internet Explorer and reinstalling it will help? I can't find IE in the Add/Remove program list.Patchouli 03:10, 7 April 2006 (UTC)
- I obtained a resolution after calling 1-800-HP-INVENT. Here is what I did.
- I opened Internet Explorer.
- I clicked on the Tools menu.
- I chose Internet Options.
- I clicked on the Advanced tab.
- I removed the check mark next to “Enable third-party browser extensions (requires restart)" under the Browsing section.
- Then I clicked OK, closed the browser, disconnected, and restarted my computer.
- I obtained a resolution after calling 1-800-HP-INVENT. Here is what I did.
This was the panacea to all my browsing and file-downloading issues. Patchouli 05:03, 7 April 2006 (UTC)
- It sounds as if this turned off some co-operative adware. Nevertheless, the idea of some unsolicited program in my computer fills me with dread. When I had a hint of this the other day, I immediately disconnected the machine from the internet, formatted the hard disk, reinstalled the system and applications, and then restored my personal data (not programs) from last night's backup. I would recommend you do the same. If you aren't willing to, at least never enter any financial data or important password on this machine, ever again. Notinasnaid 12:16, 7 April 2006 (UTC)
- i know how you feel but remember if you browse in fear you are letting the terrorists win!!! modesty 16:23, 7 April 2006 (UTC)
It's 8 times easier to catch something with IE than Firefox. The troubles with my kids went down to nothing when I converted everybody. Apparently, even 'good' sites are being hijacked and filled with exploits. --Zeizmic 15:52, 7 April 2006 (UTC)
- The Mozilla browser works well only for some time. If you want to keep it permanently, I believe you have to pay $30 for it.
- This is simply not true. Both the Mozilla suite and Mozilla Firefox are free --LarryMac 20:40, 10 April 2006 (UTC)
Dear Gentlepeople:
I herewith assert that visiting every site hosted by blogger.com results in an adware and malfuntions on your computers.
Sincerely,
Patchouli 17:55, 9 April 2006 (UTC)
- I sincerely doubt you could visit "every site" hosted by blogger.com; that would take quite a long time. Perhaps you mean visiting "any" site, although that would be wrong. My crap little blog is on blogger and I have never been had any adware trouble. --LarryMac 20:40, 10 April 2006 (UTC)
You are correct, I meant "any site." The asseveration arose due to the fact that after I e-mailed Matt Dattilo with my allegation, I received an answer saying,"This is blatantly false. My site is hosted by blogger.com; thus, if it installs spyware, then every site hosted by blogger does."Patchouli 23:09, 10 April 2006 (UTC)
Webmail[edit]
How long has G-mail been an experiment? How long does it take? My university give me this ridiculous 2 Mb inbox limit and delete things without notice if you go over (I've checked, and I can't see how I could possibly over the limit). I'm fed up of it, and since Opera wont let me download IMAP mail (what in the world could make this more difficult than downloading POP email which it allows), I need to find an alternative. Can anyone recommend a service that isn't going to try to charge me to download email or whatever? Another thing that sickens me, is when they say they've "unfortunately" (yeah, right; they "unfortunately" want to extort money out of me) had to reserve the ability to download email or use email clients to paying customers only. Email services should be free, like the sun, the air or the daffodils planted by the council.
Since Google insist on increasing their pledge to users to ludicrously high capacities when they could be opening up 250 Mb accounts to everyone, I pledge to never use their stupid email service as long as I live (or until I forget). --Username132 (talk) 23:50, 6 April 2006 (UTC)
- Apart from the privacy issues, Gmail sounds like it could fit your needs, check out Yahoo as well though. BTW, someone pays for those daffodils, most likely through taxes, and someone pays for free email, somehow, sometimes by showing you ads, sometimes by using the info in your emails to better show you ads. You might want to think seriously about what the cost of your 'free' email is. For great justice. 00:17, 7 April 2006 (UTC)
- Mozilla Thunderbird (website) can download IMAP mail, if that helps. It's completely free, too, and has a ton of other great features. -- Daverocks (talk) 07:27, 7 April 2006 (UTC)
- Note that Gmail are playing a bit of a statistical shell game with their claimed capacity. AIUI, they're working on the principle that the vast majority of people aren't going to use anywhere near as much as they've got available, so they can say that the maximum you can have is very high. Couple this with the fact that they are buying more and more disks all the time and you have the basis of the claim. If everybody suddenly "filled" their Gmail mailbox the servers would collapse in a gibbering heap. (Or, more likely, the allowed capacity would drop very quickly until you'd suddenly met it.) --Bth 11:26, 7 April 2006 (UTC)
- If you want to keep your school's "free" email (how much of your tuition money is going towards those 2Mbs?), then I'd go with Daverock's solution and get Thunderbird. A mail client will allow you to use your own hard drive as your email's space, and so you could certainly fit 250 MBs or whatever you need. I'm not sure I understand your anger at Gmail, though. It is indeed open to everybody, they just liked the viral marketing strategy they went with. If you'd like an account, just email me or check out any of the Gmail spoolers that are now chock-full of invitations. But like FGJ pointed out, there's no such thing as a free lunch. — Asbestos | Talk (RFC) 13:52, 7 April 2006 (UTC)
Relative Velocity[edit]
Given that a person standing still on earth would nonetheless be moving at several thousand kph due to the earth's rotation, and given that this velocity would only be exponentionally increased by the Earth's revolution around the sun, and given the massive speed at which our solar system revolves around the centre of the milky way, and finally, given the milky way's incomprehensible speed in which it drifts away from the centre of the universe, is it possible to estimate the relative speed at which we're actually moving? If so what is it, and how close is it indeed to the speed of light? Loomis51 23:55, 6 April 2006 (UTC)
- There is no "center of the universe," although one can sensibly establish a "universal" reference frame based on the frame in which the surface of last scattering of the cosmic microwave background is stationary. It turns out we're moving at about 0.2% of the speed of light, or about 600 kilometers per second [13], relative to that. -- SCZenz 00:01, 7 April 2006 (UTC)
- Wow, interesting. I was about to say the question made no sense but your answer's better. Isn't the surface of last scattering expanding, though? So it's not really stationary in any reference frame, just expanding equally in all directions. —Keenan Pepper 00:07, 7 April 2006 (UTC)
- The "surface of last scattering" I believe means the surface at which the CMB last scattered (on average) at the time when it scattered. Thus it's a specifically-defined surface in both space and time. Yes, if you trace the CMB into the present day, obviously it's being spread out in all directions, so you can think of the speed I gave as (in rough terms) our speed measured relative to the average "rest frame" of the CMB near the earth (where the expansion of the universe has little effect). All this was determined based on the measured dipole of the CMB, as is indicated in the reference I added above. -- SCZenz 00:10, 7 April 2006 (UTC)
- I also read here that the major source of this movement may be the Local Group of galaxies orbiting the Virgo cluster of galaxies. -- SCZenz 00:13, 7 April 2006 (UTC)
- Relative velocity is the velocity of the body, this body interacts to another nearby it , as the earth gravity attracts a man, a man drives round the lake by car, the route of the lake is a curve line.
- But all of the surveys of motion always observe based on a nearest object , as a man and earth, and the interaction still exist (i.e.: the gravity extraction). Relative velocity can use to compare something which estimates motionless, when you use the term of relative velocity in this case, it is exactly . But if you can win the gravity extraction, it means you leave the earth into univerve, the relative velocity is not the notion which is applied for discussion. Here, the power is enough to win the earth gravity, it already issues the initial velocity , and from here, when you go slowly, it means your initial velocity is reduced some value which is you must register for your itinerary(or you have to use the formula : v(t)= a v(t)+ v ini ) …
- Ngocthuan 06 08:59 2006-04-07 (UTC)
- I'm not sure I follow your argument; can you clarify? -- SCZenz 20:25, 7 April 2006 (UTC)
- If you are an observer standing motionlessly at a corner of the route, you can see a car going with the speed about 50 km/h. It means you and the observed object which are the same condition:- on the earth and very near together.On the earth, it means you rotate around an earth axis (an axis is thru the north & south pole), although you still stand motionlessly , because the earth turn around itself, and the earth goes progessively in the ellipse orbit around the sun, you also have such progessive motion. And a car is same, it rotates around an earth axis.... and goes with the speed 50 km/h.
- I'm not sure I follow your argument; can you clarify? -- SCZenz 20:25, 7 April 2006 (UTC)
But when you observe a car, you have to get the origin for the observation, a car run with 50km/h towards only you (still stand motionlessly at this corner), it is the relative velocity.Ngocthuan 06 2006-04-9 T 11:37 UTC
April 7[edit]
Brake Fluid and Clorox[edit]
Hi. i was reading an email when it mentioned something about if you mixed Brake Fluid and Clorox it would make smoke.i was wondering if it would make smoke.if not, is there any other household stuff that when mixed will make smoke?(i like pyrotechnic stuff..)
Thanks, --Shannon 00:46, 8 April 2006 (UTC)
- I'm not sure about the brake fluid and bleach, but a Google search will yield some results, such as this. In redox reactions like this, there is an oxidizer and a fuel source to be oxidized. There are too many of these chemicals to list them all, but in the example I gave you above, potassium nitrate is the oxidizer and sugar is the fuel.--Chris 01:12, 7 April 2006 (UTC)
Thanks.do u know where exactly i can get potassium nitrate from?
- There is no way to get it easily from household items. It must be purchased specifically. I recommend ScienceLab.com. Please be sure to review the MSDS for potassium nitrate before deciding to work with KNO3. I don't suggest that go ahead with this "experiment" if you do not plan to follow proper safety precautions and have some sense of responsibility. Please follow all directions exactly as they are stated and use your good judgement.--Chris 03:28, 7 April 2006 (UTC)
You might also find it in the local hardware store as 'Stump Remover', where you are supposed to soak the stump in it, let it dry, and then light it. Never worked for me. --Zeizmic 11:56, 7 April 2006 (UTC)
- I thought stump remover is straightup black powder, which does have KNO3 in it, plus charcoal and sulfur. --Chris 22:41, 7 April 2006 (UTC)
Hmmm. i will try to find the chemicals and all safety precautions will be observed. Thanks for helpin me find the answer to it =)
--Shannon 00:45, 8 April 2006 (UTC)
Is sleep deprivation good for anything?[edit]
I'm often very tired, and don't have time to get an adequate amount of rest. I'm wondering, is there anything good about sleep deprivation? Flea110 04:22, 7 April 2006 (UTC)
- Well, according to sleep deprivation, it seems that in amongst all the bad things it can do, low sleep levels might help fight depression... temporarily. Seems like the depression usually comes right back after a normal amount of sleep, though. -- Filliam H Muffman 05:10, 7 April 2006 (UTC)
- After 36 hours or so you start hallucinating. GangofOne 07:24, 7 April 2006 (UTC)
- As this PubMed article suggests, sleep deprivation increases serotonergic activity in the brain, which temporarily relieves psychological disorders such as depression. As a person with social anxiety disorder (which is also linked to low serotonin levels), I can confirm that this is true from experience. You should note, however, that you will only get a noticable effect if you have a chemical imbalance in the first place. Oh, and of course eye candy hallucinations are also encountered with sleep deprivation, although I wouldn't describe them as pleasant. --Aramգուտանգ 08:30, 7 April 2006 (UTC)
- I agree entirely with aram's comments, and for exactly the same reason. I would add, though, that some artists have used the hallucinations associated with sleep deprivation to good purpose - most famously Salvador Dalí. Grutness...wha? 10:37, 7 April 2006 (UTC)
- I doubt that. I've been up for 36-48 hours straight quite a few times, and never ever had a hallucination. You can get flashes in front of your eyes, but I wouldn't call that a "hallucination". A migraine can give you that too. --BluePlatypus 15:15, 7 April 2006 (UTC)
- Someone here's an outlier then. I've definitely had hallucinations from that sort of length of sleep deprivation (though mine were auditory -- water in pipes seeming like voices, that sort of thing). --Bth 16:02, 7 April 2006 (UTC)
- i heard radiohead frontman thom yorke would use sleep derivation sometimes when writing lyrics modesty 16:20, 7 April 2006 (UTC)
Sleep deprivation helps me get work done, as I get too tired to be anxious, and my anxiety contributes significantly to procrastination. moink 21:26, 7 April 2006 (UTC)
Space-Time Curvature under General Relativity[edit]

This is a question about how non-scientific explanations of the Theory of General Relativity are supposed to explain gravitation. Layman explanations of space-time curvature usually have a diagram (apparently always the same one) which shows a two-dimensional surface streched (downwards) into a third dimension at the location of a body having mass. The story goes that a second body going by with no acceleration will follow a curved path because space-time is curved by the first body.
What I don't understand is why this would cause one body to curve towards another rather than away. When I try to figure out what the curved path will actually be (from the diagram), it looks like the it should curve away. Apparently, it is implied that the second body would fall into the 'depression' in the original two-dimensional surface because of.... what? the influence of gravity? It's apparently using gravity to explain gravitation. How is this explanation supposed to work? The Wikipedia article hints it's not a simplistic as the picture, but doesn't seem to go beyond that.
- If someone is able to post a complete and thorough reply to this question, please forward it to the Nobel Prize committee. Seriously, though, the curved sheet model just takes advantage of the fact that objects rolling around on a big sheet in a 1 G field happen to move in a way similar to what general relativity predicts for objects moving past planets and the like. It's meant to show you how things move, not why things move. To really understand why things move, you will have to understand nasty things like metric tensors and the Einstein field equations. -- Filliam H Muffman 07:24, 7 April 2006 (UTC)
- Well.. I don't quite see why you'd think it would curve away. If you have a downward "dimple" in a tablecloth or similar and roll a ball towards it, moving in a straight line, it will go around the rim in a curved path once it hits the dimple and continue away in a straight line in a different direction, having been deflected somewhat inwards. That analogy is the point of the picture. If the dimple was raised instead of lowered, then it would be deflected in the opposite, outwards, direction. But gravity doesn't act in that direction, which is why you've got a lowered dimple and not a raised one in the picture. --BluePlatypus 07:54, 7 April 2006 (UTC)
- The diagram also gives a nice visual analogy for the inverse square law, since the gradiant of the curve is much steeper near the object than far away. But like the others have said, this is a way of visualizing how the objects would move so, not why. — Asbestos | Talk (RFC) 13:36, 7 April 2006 (UTC)
- In other words, it's just a cool picture? It's describing the exactly the same behaviour as Newtonian gravitation. Peter Grey
- Pretty much. It's also meant to give an idea that general relativity deals in spacetime curvature, which, among other things, predicts that light is also affected by gravitation. That makes gravitational lensing possible. Newtonian gravity claimed that light doesn't bend because it's massless. -- Filliam H Muffman 03:03, 10 April 2006 (UTC)
- One of the byproducts of Special Relativity has been the famous equation, E= mc², that first stated the fact of mass and energy being only two sides of the same coin, instead of what previous physicists have conceived. While Newton had previously thought light to be massless and thus insupsceptible to gravitation, nonetheless certain details within the mathematical framework he had produced for gravity as a force did predict a certain amount of bending occuring when light approaches gravitational bodies (a third less than what Einstein later proposed), which, although he knew was true, was never able to explain away with his theory. The new definition in modern physics, now, for the word massless, also meant something different. It refers only to those particles with no rest mass, but not necessarily no remaining kinetic energy whatsoever. Due to Einstein's equation, as previously indicated, you may say that light does have mass if you wish to, since its energy could be easily converted to mass via the constant of c². However, the energy of the photon is constantly changing during its flight, so the records for mass you will be able to obtain will never be invariant, thus referred to as the relativistic masss. In modern physics, however, it is no longer considered appropriate to define the particle on terms of its relativistic mass for obvious reasons, and thus it suffices to say that light is massless in the fact that it has no rest mass. Nonetheless, as long as the photon still possessed the kinetic energy/relativistic mass necessary for its existence, then it shall be supsceptible to curvatures in space- time like other particles with rest mass. Luthinya 10:21, 10 April 2006 (UTC)
- Erm, where have you got that "third less" from? The Newtonian prediction for the deflection of light around a point mass is half the value given by GR. --Bth 10:34, 10 April 2006 (UTC)
Sorry, got my figures wrong. Thanks for correction. Luthinya 10:45, 10 April 2006 (UTC)
- This kind of picture is indeed confusing, but there is another reason for it that hasn't been addressed fully above. The key is the mechanism by which "curved spacetime" affects the motion of objects (particles, satellites, light, whatever). General relativity says that an object moving only under the influence of gravity follows a geodesic in spacetime, which is the analogue of a straight line in Euclidean geometry. Since the spacetime itself is curved, these "straight lines" have properties we do not expect, but they are, in a precise sense, the "least curved" lines you can have within curved spacetime.
- Roughly speaking (see below), one of the important properties they share with Euclidean straight lines is that they are the shortest distance between two points. This means you can picture a geodesic in the following way. Take a curved surface, such as the dimpled fabric surrounding the ball in the relativity diagram asked about above, and fix two points, one to be the "pitcher" and one to be the "catcher." We then want to draw the geodesic on the curved surface connecting the two points, representing the path of an object from the pitcher to the catcher. We do this by using a rubber band, stretched taut: this automatically follows the shortest path between the points.
- Now suppose we look at the result as viewed from above. If there were no curvature, the rubber band would be a straight line between the two points. Since the ball is there, dimpling the surface downward, the rubber band will not take a path that appears straight as viewed from above, since that path will be rather long due to the dimpling. Instead, the rubber band will curve around the ball slightly, to avoid the trip into the depths of the dimple. When seen from above, it appears that the ball is affecting the path of the object with some "force", when in fact the object is trying to follow the best approximation to a "straight line" that it can in the circumstances.
- All that is rather hard to say without additional pictures, hope it comes across. Anyway, very little of that is usually included with the usual picture, which is why it is easy to come to very inaccurate conclusions about what the picture is trying to say. If you want a better version of all this, look at Taylor and Wheeler, Spacetime Physics.
- Note for experts: in spacetime, with its Lorentz metric, geodesics are actually local maximizers of proper time; but part of the point of the kind of picture we're discussing is to give a Riemannian picture. --Spireguy 20:12, 11 April 2006 (UTC)
- Still, for most layemen beginners, it does offer a much more approcheable beginning for the subject, even if much of the important details have been left out. As far as the geodesic problem is concerned, since we as humans possess four dimensions (including Time), and yet space- time may curve in a way that is impossible for us to conceive or imagine- only "talk about", it immediately shows that space- time curves into at least one more dimension than that to which we are accustomed of in our daily life, i.e. the 4D bodies. And just as the 2D figures upon cardboard cannot stand up vertically upon it, since they have no motion or conception of 'depth', we as 4D people cannot cross the barriers of dimension and traverse into the 5th or other higher dimension freely, as we now have the ability to in our 4D world. We therefore have to kind of traverse with the curvatures of the surface of higher- dimensional space- time, which is to us expressed in a 4D fashion like differently laid card boards are to the 2D beings. Thus, for us, the easiest way between two places in space- time may not necessarily be a straight line, but more usually a curved 4D geodesic adapted from the curvatures of space- time from gravity. Luthinya 20:34, 11 April 2006 (UTC)
- That's what I'm getting at. Isn't a path curving away from the point mass shorter? What we would need to visualize would be a contraction of space, not a stretching, right? Peter Grey 04:25, 12 April 2006 (UTC)
- Bear in mind that we're measuring "shortest" in the coordinate system defined by the grid drawn on the sheet. The deformations of the sheet deform the coordinate system itself -- that's the whole point of the analogy. What looks longer to us from outside the sheet is shorter, when measured in a system where the side of a "square" on the sheet is a constant however stretched it looks to us. --Bth 11:12, 12 April 2006 (UTC)
- The shortest path "in the coordinate system" is the straight line. Or is something still missing in the story? Peter Grey 16:13, 12 April 2006 (UTC)
- Um, no. In order to walk the "straight line" in which you are depicting, we must abandon the 4th dimensioned (including Time) curvature of the "surface" of the grid (space- time), and seek to traverse into extra dimensions in order to ignore the curved influences of gravitational objects. However as 4D beings this is pratically impossible for us, so instead of this the shortest route for us will have to be the the curved surface of the grid, which at least is 4D and possible to traverse. Therefore, instead of what Newton has previously proposed, the shortest route between two things is not a 'straight line' in the usual sense of the word, but a geodesic varying according to the shape of surrounding space-time influenced by gravitational fields. At least a longer way is shorter than the impossible, so to speak. The thing is that on the diagram you cannot see the grid curving into extra dimensions the way it should, which is what makes the analogy slightly hard to come to mind. Remember the analogy is only a start to understanding; try eventually to draw away from the picture and just let the ideas flow accross your head- aided by some mathematics, you'll find this much easier.
PS May I add that my above language is extremely inaccurate in depiction, especially without the mathematics to compensate for it. Hope it comes through anyway. Luthinya 18:17, 12 April 2006 (UTC)
- The point I'm trying to underline is that, unless gravity is assumed (as part of the illustration of gravity), then there is no 'up' or 'down', and the diagram should demonstrate the same behaviour whether the deformation is 'down' or 'up'. Peter Grey
- I'm afraid I cannot quite catch your point. What exactly do you mean by down or up? The deformation is curved into extra dimensions for which we may only talk of, yet never be able to obtain direct conceptions of for which we will understand properly since, as I said before, we cannot cross the barriers of dimensions. One should also be careful between the shortest path and the easiest path. Traditionally, we tend to think that the shortest path between two things, usually a "straight line", is also the easiest path to traverse between the two objects in question. However, because of this simultaneous involvement in meaning our brain has got accustomed to thinking that the shortest journey must necessarily be the easiest one to achieve, taking seemingly the smallest period of Time (if you can put it like that). Unfortunately, this stops being true as soon as we step out of the world of the Greek Geometres- the flat world of 2Ds where the curvatures made we can entirely ignore, since we are one dimension (in Space) higher. Already in the 3D world we are encountering trouble. When a tourguide shows you the fastest way to cross a mountain, he shows you the curved path just by the foot of the mountain and fitted to its topological curvatures. However, who would not agree that the shortest way is actually to tunnel through the mountain centre itself, and come upon the other side? True, this may be the shortest way, but it is by no means the easiest. Far easier it is to adapt to the geodesic of the landscape than to delve in it.
Space- time itself possesses more dimensions than our race, so delving becomes completely impossible. All we can do now is follow, once again, the geodesics of the landscape, and trust them to be our easiest way. (or trust maths- whichever)There will be no talk of picking or choosing either- all objects follow intuitively the easiest way to traverse between two points. When there is a stone that curves a rubber sheet downwards the ant naturally walk as close to the stone as possible in getting its way round to the other side, not even waste time climbing or delving into the stone. The same it is for us- we naturally follow the curvatures already made in the fabric of space- time. When a gigantic gravitational body curves space-time into extra dimensions unreacheable, the easiest way to cross to the other side is to the follow the geodesics and walk as close to the body as possible, giving the effect of being "gravitated towards it", rather than away from it where the journey will naturally be harder. Luthinya 09:55, 14 April 2006 (UTC)
TO CLEAR UP THIS DISCUSSION, WHICH SEEMED TO HAVE CREATED A GOOD DEAL OF GENERAL INTEREST, I SHALL REMOVE THIS QUERY FURTHER NOW TO THE BOTTOM OF THIS LIST, AT APRIL 14TH. THANK YOU FOR YOUR UNDERSTANDING. Luthinya 09:58, 14 April 2006 (UTC)
Sports Science[edit]
How can the diet of a body builder be compared to the diet of a jockey?
- In many ways. You could compare the number of calories, or the proportion of carbohydrates, or even the sheer weight of the two diets. Grutness...wha? 10:35, 7 April 2006 (UTC)
It's probably a polite fiction that people can significantly bulk up without an Anabolic steroid. Just compare the body builders of yesteryear with the Rambo's of today. --Zeizmic 14:52, 7 April 2006 (UTC)
- It's definitely possible. Depends what your goal for bulk is, but with enough protein and exercise, one can add major muscle mass without resorting to drugs. You may not end up looking like the hulk, but rambo is in reach, given the right genes. Night Gyr 17:35, 7 April 2006 (UTC)
Time dilation[edit]
What is the easiest way to understand length contraction and time dilation?
- Here on Wikipedia, a good starting point might be our Introduction to Special Relativity page, which takes a fairly unusual but (IMO) particularly clear geometrical approach. For more details, try our articles on length contraction and time dilation. Elsewhere online, sites like this and this take the more common playing-ping-pong-on-a-train approach. Do come back here if you have anything you want cleared up. --Bth 11:20, 7 April 2006 (UTC)
Create a super computer by many used computers[edit]
I hear that we can create a super computer by many used computer, it means that If I have about 10 pcs of Pentium III Pc, I can create one super computer. The Operating System now is open source.User: Ngocthuan 06 2006-04-7 19:52 UTC
- If you had looked at the supercomputer article, you could have probably seen Beowulf (computing), which is the standard model for making a supercomputer cluster. The links and external links in that article will probably tell you most of what you're looking for. — Asbestos | Talk (RFC) 13:19, 7 April 2006 (UTC)
Logical way to finish this Sudoku?[edit]
I got annoyed with the Sudoku today because it took me more than 2-3 stops on the T to solve, so now I've decided that it was a bad puzzle (obviously). I solved the puzzle by supposing one box was one number, working through it all and seeing that it didn't lead to any contradictions. My question is: Was there a logical way to finish this sudoku, without starting with "Suppose this box is a ...", and then showing presence or absence of a contradiction? I realize that that is an ok way of finishing a tough sudoku, but I far prefer it when they can be solved more elegantly:
7 2| 6 | 98 5 |928| 71 98 |74 | 2 ----------- 6 8|29 | 17 273|156|849 19 | 87|2 6 ----------- 3 |872|964 427|639|185 869| 1 |723
Thanks! — Asbestos | Talk (RFC) 13:16, 7 April 2006 (UTC)
- Probably not, the puzzle looks like it's been designed with the "pick one of two choices and see if it leads to a contradiction" method in mind. Parts of it can be solved independently, though: for eample, one of the two possible choices for filling the central square leads to a fairly obvious contradiction in the central columns. —Ilmari Karonen (talk) 17:12, 7 April 2006 (UTC)
- I'd agree, I can't see a way to do it that doesn't rely on guesswork^W proof by contradiction. As such, it doesn't count as a well-formed sudoku to my way of thinking. --Bth 17:18, 7 April 2006 (UTC)
- All solutions depend on proving-by-contradiction. Some of the problems just have more obvious contradictions, making it easier to find the correct one. It's an NP-complete problem, so one solution isn't really much more elgant than the other since they all more or less imply testing all the possibilities. So the perceived elegance is more about whether you can solve it within your mental 'search depth', or whether you have to resort to writing the numbers down. --BluePlatypus 18:23, 7 April 2006 (UTC)
- I see what you mean, but solving using the various other "standard" techniques (listed at length in our sudoku article) doesn't feel like guesswork/proof-by-contradiction in the way that having to employ the "what-if" method does. I've never seen a sudoku before that had to be solved by what-if. (OTOH, I've often solved them by that and then used my knowledge of the solution to see what I'd missed in my application of the other techniques.) --Bth 18:54, 7 April 2006 (UTC)
- Well, yes and no. Personally I feel that a sudoku should be solvable by deduction in that you should logically be able to deduce simply by examining the puzzle what particular number will go into a space. If you are left with a situation where the only way of finding out is to plug in numbers and see whether the correct solution can be reached you've gone beyond deductive reasoning and into inductive reasoning - a different matter entirely, my dear Watson. Grutness...wha? 03:41, 8 April 2006 (UTC)
- I see what you mean, but solving using the various other "standard" techniques (listed at length in our sudoku article) doesn't feel like guesswork/proof-by-contradiction in the way that having to employ the "what-if" method does. I've never seen a sudoku before that had to be solved by what-if. (OTOH, I've often solved them by that and then used my knowledge of the solution to see what I'd missed in my application of the other techniques.) --Bth 18:54, 7 April 2006 (UTC)
- Cheating, and using http://sudoku.sourceforge.net/, and adding in all of your available numbers. There is no solution. Sorry! Kilo-Lima|(talk) 16:54, 10 April 2006 (UTC)
Desktop & Laptop screen placement[edit]
1)In a desktop, the monitor is more or less perpendicular to the desk, and we view that monitor with 3 feet distance. If we use a laptop or a tablet pc whose screen is placed at 45 degrees to the desk/lap, should the same distance of 3 feet be maintained? Or simply, if viewing angle changes, should there be a difference in viewing distance?
2)What is the reccommended viewing distance and reccommended angle for placing laptop screens?
When we place the screen on the laps in a slate tablet PC, the viewing distance is 1 to 1.5 feet. Does that say that viewing distance is related to angle of viewing?
- Laptops and tablets are not particularly ergonomic. Get an external keyboard and jack your laptop up on some phone directories when you are using it as a desktop replacement. For great justice. 18:38, 7 April 2006 (UTC)
- You can get stands that hold the laptop screen vertically at eye level (the ones we use do so by having a 30° tray to put the laptop base-part on, with the hinge furthest away from you) and plug in an external keyboard and mouse. Ojw 20:36, 8 April 2006 (UTC)
- Your screen is probably not at a 45 degree angle; many laptops are barely even capable of bending that far. Night Gyr 06:29, 9 April 2006 (UTC)
Can Envelopes Be Composted?[edit]
Paper can be recycled or composted but envelopes cannot be recycled due to the glue. Can envelopes (without plastic windows) be composted? --Username132 (talk) 15:16, 7 April 2006 (UTC)
- Theoretically, most things can be, but consult your local recycling company for their facilities / policies on this. For great justice. 18:36, 7 April 2006 (UTC)
- I'm sorry - you said composted, not recycled! They will certainly break down if you put them in a composter - the question is, not knowing exactly what chemicals are in it, you might not want to use the compost on vegetables etc you want to eat - otherwise, go for it. For great justice. 22:24, 7 April 2006 (UTC)
- The recommended constituents of compost are: 1) vegetable waste (and some miscellaneous kitchen waste like tea bags, eggshells, etc), 2) lawn trimmings or leaves, and 3) shredded newspaper, straw, or wood chips. Bleached paper (normal office paper, including envelopes), cardboard, other paper, cooked food, animal-based waste (except eggshells), fats, etc while capable of decomposition, are NOT recommended for composting.
- In the case of office paper (as per your question), it is both the chemicals in the paper and the density of the paper. 'Perfect' compost is alternating layers of dense vegetable matter (kitchen scraps), dry vegetable matter (lawn trimmings or leaves), and cellulose (straw, wood chips or SHREDDED newspaper), not necessarily in that order but definitely including all three. The combination of the three types of elements is ideal for the cultivation of all the different types of organisms that turn waste into soil. If you don't follow the 'formula', your compost will still decompose, but it will be slower, smellier, and grosser.
- Newspaper is included for two reasons; one is that it aerates the pile, and it is actually nutritious to worms. White paper is like refined sugar, it has no nutrient value, so worms wouldn't eat it. It's also dense and soaked with chemicals, which would inhibit the movement of the worms and maybe poison them. IF the paper decomposed, it would be a slow process facilitated by bacteria which would be acting on the organic drippings from other parts of the compost that would 'soak in' to the paper. It's waaaaay better to leave it out, most compost experts say leave it out. Recycle your office paper through your city's recycling programme, or send it to the dump to follow its own decompositional path, away from your compost pile.--Anchoress 00:55, 11 April 2006 (UTC)
- Thank you - compost without the envelopes it is! :) --Username132 (talk) 04:06, 11 April 2006 (UTC)
- No problem. I also forgot to mention that - as far as animal waste is concerned - manure is fine, but not dog, cat or human feces. Also some documentation says cardboard is OK, I think the main thing is unbleached, unpressed paper, and ideally it should be shredded. In my jurisdiction they say only newspaper, no other kinds of paper.--Anchoress 04:34, 11 April 2006 (UTC)
Dr. Keith Black neurosurgeon[edit]
Why is there no information on Kieth Black M.D. on wikipedia ?
- Because no one has added anything about him yet. I didn't know who you were talking about so I looked him up. He doesn't seem terribly notable to me, but if you feel like adding information about him, go ahead. Be Bold. Chapuisat 16:55, 7 April 2006 (UTC)
- He seems sufficiently notable to me to warrant an article (and to displace the drag-racing Keith Black to a disambig link), so I've created a stub for him based on a quick Google search; I'd strongly encourage the questioner to add some info if they have any. More generally, to expand on Chapuisat's point, Wikipedia has gaps and omissions because it's entirely the result of volunteer contributions and constitutes a permanent work in progress. --Bth 17:00, 7 April 2006 (UTC)
- If one added every living and dead physician, lawyer, and dentist, then we would have maybe over 10,000,000 articles on these folks alone. Unless that person made a breakthrough and accomplished something meaningful, I don't think such articles would be needed.Patchouli 23:54, 7 April 2006 (UTC)
Linux[edit]
From a Linux tutorial; "Most modern Linux distributions encourage a practice in which each user has a specific directory for the programs he/she personally uses. This directory is called bin and is a subdirectory of your home directory."
Would it not be inefficient for many users to have different copies of the same program? --Username132 (talk) 16:42, 7 April 2006 (UTC)
- Well, if it's a big multi-user system, each user probably has a disk quota that they can use to store whatever they want. If the programs they want to run aren't available in /usr/bin or /usr/local/bin or whatever, they can put them in /home/whoever/bin. —Keenan Pepper 17:07, 7 April 2006 (UTC)
- And the particular situation of everyone having a local copy of SuperWhizzyUtilityX shouldn't arise if the sysadmin's on the ball. They should be putting anything that several users want into /usr/bin and such places. (Incidentally, if you want to see where the shell searches for executables when interpreting command lines, type "echo $PATH" to see the (colon-separated) list stored in the PATH environment variable.) --Bth 17:15, 7 April 2006 (UTC)
- It may be a tad inefficient, but it's a lot better than sharing the same installation - what if someone deletes the program? Bth's point is also a good one - commonly used applications get installed into a shared directory they can access but not modify.
Gold in sea water[edit]
I have read somewhere that there is approximately 9 tonnes of gold per km cubed in sea water. --
-Is this true for all seas (apart from where there is large amounts of fresh meltwater)?
-Is the same true for fresh water and what is the amounts?
-Is the gold not worth anything (like industrial diamonds)?
-Is there an efficient/cost effective way of extracting this gold, taking into account; positioning (what sea/ocean), labour, building/machienry etc.
-If you discover anything "good" please don't make it "exceptionately clear" to anyone else and put it on the website. I cant force you to but pretty please do.
Im slightly mad but if it wil work i will be slightly rich....Yipee!!!
I think im ment to do this: --William Dady 16:58, 7 April 2006 (UTC)
- The presence of trace amounts of gold and other precious metals in seawater is not a secret, and it is not economically feasible to extract it because the concentration is so low. 9 tonnes may seem like a lot, but a cubic kilometer is a ridiculous amount of water. See [14] and Fritz Haber, a brilliant chemist who tried and failed. —Keenan Pepper 17:12, 7 April 2006 (UTC)
- To put it into perspective, a cubic km of water weighs about a trillion (1,000,000,000,000) kg, or a billion metric tonnes. So you got a concentration in the 9 parts per billion (mass) (by your numbers).
- Actually, according to this article [[15]] ,the highest concentration found was 0,05 ppb and average was 0,013 ppb. To put it into a more "real-world" size perspectve: One olympic pool of "good" sea water (3 million liters) should give you 150 milligrams of gold, worth US$ 3. --Rodrigo Novaes 15:42, 14 April 2006 (UTC)
- Thanks for your query. We figured it out but sorry we're not sharing. alteripse 14:59, 8 April 2006 (UTC)
- Finally, there are two metals that might one day be economically viable to extract from seawater - uranium and vanadium. See this abstract. Arthur C. Clarke wrote a short story about the idea decades ago. --Robert Merkel 08:12, 9 April 2006 (UTC)
Centrifugal Force[edit]
If a space station were to have a ring with people inside and it was spinning fast enough then it should create artificial gravity. So my question is if a person inside this ring where to jum up would he or she be pulled down to the spot where the jumped up from? Patrick Kreidt
- Essentially, yes. You should draw a force diagram of the situation you are talking about. I think you are interested in whether the ring would 'spin' under the person while they were in the air? Sketching out a diagram will show that there are no forces that will do this in the example you mention. For great justice. 18:34, 7 April 2006 (UTC)
- I don't think so. Maybe I have not thought this completely out, but here is how I see it. At the time a person jumps 'up' (and 'up' is defined as perpendicular to the surface) a person will be moving forward at a certain speed. Ignoring air friction the person will continue to move forward at the same speed the floor is moveing forward. Since the floor moves in a circle and the person does not, for the person to come down in the same place would require the floor to travel a longer distance in the same time the person requires to land. From this I conclude the person will land in a spot a little ahead of the take-off point.
- Why doesn't the person move in a circle? As the ring spins, it emparts momentum to the person at an angle tengental to circle, that means they spin, with the ring, and are pushed away from the center of rotation. If the person jumps towards the center of the ring, they already have momentum emparted by the ring that will carry them 'forward' at the same rate that the ring is spinning. For great justice. 20:49, 7 April 2006 (UTC)
OK - so take a look at the diagradm - the black arrow shows the force vector of the spinning ring. The red arrow the direction of spin, and the green arrow the arc of the jumping man. Because he has the same forward motion as the ring, the arc he describes, even if he jumps 'up', will put him back where he began. For great justice. 21:06, 7 April 2006 (UTC)
- That's roughly true, but remember that the ring moves in a circle, whereas the person's initial velocity (aside from the "jumping speed") is tangential to the circle at the time of the jump. I think you land in roughly the same place, for small jumps, but getting an exact answer requires actually calculating it out. -- SCZenz 21:21, 7 April 2006 (UTC)
- Er, I don't think it quite works like that. You have to remember that there's no force 'down' (outward) on the jumping man, just whatever residual momentum he carried when he left the ring.
- Picture the following situation. The ring sits in the plane of this webpage, like this: → O
- The ring rotates counterclockwise. The top of the ring is moving left (←) and the bottom is moving right (→).
- Our hypothetical spaceman is standing on the inner surface of the rotating ring. Assume that we conduct our experiment just as he reaches the bottom of the circle. He, and the ring, are both going to be moving to the right (→) with equal speed.
- The spaceman jumps. He retains the original horizontal component of his speed → and adds a modest upward component (↑), assuming he pushes off normal to the ring surface. The path he follows will then be straight along the vector sum of those two components, taking him diagonally up and to the right on our diagram until he smacks into the wall again (er, lands).
- So, what happens? Let's follow the spot of ring from which our spaceman started. Its horizontal velocity will be
- vhoriz(t) = vmax·cos(ω·t)
- where positive velocity is to the right. In other words, its velocity is at a maximum at the bottom of the circle, and decreases as the centrifuge turns.
- Our spaceman, on the other hand, retains all of his initial horizontal velocity, so he lands ahead of his starting point. TenOfAllTrades(talk) 21:21, 7 April 2006 (UTC)
- Hmmm...looking at that explanation, I think I can cut it down a bit by looking at the problem in a slightly different way.
- The launch point on the ring travels at constant speed along a curved path.
- The astronaut travels at a constant speed greater than the speed of the ring surface (remember the vector addition of his jump velocity to the ring's velocity) along a straight path.
- The two paths intersect at some point after the jump.
- The astronaut gets there first — he followed a straight path at higher speed. Therefore, the astronaut will land at a point on the ring ahead of his departure point. TenOfAllTrades(talk) 21:26, 7 April 2006 (UTC)
- So, my diagram is wrong, because the spaceman describes a straight line, not a parabola? For great justice. 21:30, 7 April 2006 (UTC)
- Huh. Thanks, that'll teach people to trust anything that they read on the internet! So, why does this not work on earth, which looks like the opposite case? For great justice. 21:27, 7 April 2006 (UTC)
- I think it does work on earth, but the difference is so small it can be ignored. Keep in mind, on Earth you will only be jumping maybe 3 feet, on a ball with a 4000 mile radius. I will try to explain, though. Remember that circumferance increases in direct proportion to the radius. This means the arc of a circle with 4000mi radius will be slighty shorter than the same number of degrees of arc of a circle with 4000mi+3feet radius. This means your jumper will have to move a longer distance in the same time the earth does to traverse the same arc.
- How about this version? For earth, actually I think the difference is that you do describe an arc, not a line, because gravity is continually acting on you - no? For great justice. 21:34, 7 April 2006 (UTC)
- I think it does work on earth, but the difference is so small it can be ignored. Keep in mind, on Earth you will only be jumping maybe 3 feet, on a ball with a 4000 mile radius. I will try to explain, though. Remember that circumferance increases in direct proportion to the radius. This means the arc of a circle with 4000mi radius will be slighty shorter than the same number of degrees of arc of a circle with 4000mi+3feet radius. This means your jumper will have to move a longer distance in the same time the earth does to traverse the same arc.
- The Earth case is quite a bit more complicated due to gravity, yes. Where you land (ahead or behind your starting point) depends on the size of the centrifuge and speed of rotation (note that it has to be fast enough that you don't fall off when you go over the top....) as well as the impulse you give yourself during your jump. In the special case where you jump while the centrifuge is at the bottom center (as described in my first though experiment above), you'll land ahead of your jumping off point—it comes down to the person maintaining a constant horizontal velocity while the jumping off point is losing the horizontal component of its velocity. I'm too tired to work through the consequences for anywhere else on the centrifuge right now. TenOfAllTrades(talk) 03:41, 8 April 2006 (UTC)
- The easiest way to figure out the direction of these effects, and their approximate magnitude, is to pretend the Earth (or other spinning body) is actually at rest, but apply the Coriolis force. --Trovatore 16:40, 8 April 2006 (UTC)
- 1) There is no such thing as actually at rest.
- 2) The very fact that you may apply the Coriolis force to a body already implies that it's spinning.
- The field of 'artifical gravitation' generated by the spinning ring must be understood as a fairly universal field of 'gravitation', at least certainly one in which the Equivalence Principle may apply, in making this question at all possible. Yet when the astronaut jumps vertically from the ring's frame of reference, the artifical gravitational field acting upon him draws slightly weaker effects due to the longer distance, but the field itself still remains fairly universal for our purposes. However, when the astronaut is jumping up, though he shall still ultimately be drawn to the ring, during his time of suspension he is no longer travelling in the direction of its spin or with its velocity. As we have settled that the field shall be considered universal for our purposes, then it follows that he shall not be drawn to the particular place from which he jumped more than any other. Thus, following the above discussion, it appears plausible that the astronaut shall land in a slightly different place from where he jumped, the exact position determined by other factors and certainly noticeable if the ring was spinning fast enough- and he had not jumped too far away!
- Galileo himself actually suffered the same kind of inaccuracy due to the spinning of the Earth from his famous experiment on the Tower of Pisa, however in that case the indescrepancy was too small to mar the ultimate judgment of the experiment. Luthinya 10:38, 9 April 2006 (UTC)
Chemical structure formula in Word?[edit]
Does anyone know of any (free) application able to draw chemical structure formulas and save them into the WMF or EMF format, so that they can be inserted in for instance Microsoft Word? --Andreas Rejbrand 18:39, 7 April 2006 (UTC)
- You might try ChemSketch.[16] There's a free version, but I'm not sure what file formats it can save in. ChemDraw can save in those file formats, but there aren't any free versions, as far as I know. --Ed (Edgar181) 18:47, 7 April 2006 (UTC)
- Thanks; I'll have a look at it. --Andreas Rejbrand 18:49, 7 April 2006 (UTC)
- Now I've tested it, and it really looks great. Thank you for informing me, Edgar181. --Andreas Rejbrand 19:12, 7 April 2006 (UTC)
Total human decomposition in Canada[edit]
A tall order, I know, but I was wondering how long it would take a human body to totally decompose in a four-season (with a "real" winter, which is to say at least one or two months below zero degrees Celsius) environment.
I've read up on decomposition and eco-cemeteries (the latter being the reason I'm interested in the question), but neither go into enough detail to really tell me how long, pillar to post, it takes before a body is entirely gone. Skeleton included.
I'm aware that there are factors like ground moisture, limestone, etc. involved, but a ballpark-by-decade would be great. 10 years? 20? 60? Thanks! --MattShepherd 20:13, 7 April 2006 (UTC)
I'm no expert but i know the location where you bury the body really matters, they've found human skeletons and even bodies still fully formed (in admitedly extreme conditions) that are hundreds of thousand of years old.
- The tricky part is the skeleton, which generally requires salt-water or something rather caustic to break it down. The rest is broken down fairly easily, unless there is some extreme condition in which microbes can not exist. StuRat 07:57, 8 April 2006 (UTC)
- Skeletons can remain for ages in good conditions. Your best bet would be to contact the Body Farm in Tenessee, but you'd better explain why you want to know to them... - Mgm|(talk) 19:40, 8 April 2006 (UTC)
Even cremation does not destroy the skeleton completely. See the article for details. --Rodrigo Novaes 13:19, 14 April 2006 (UTC)
what if..[edit]
if every single human being on the face of the earth decided to jump 1 foot to the right, at the exact same time would it be enough force in one direction to shift the earths orbit? tilt? tidal forces? register on a ricter scale?21:13, 7 April 2006 (UTC)
- The Straight Dope answers the question for the specific case of China - seems like not much would happen. [17]. Answerbag has another demonstration of why this is bogus [18]. For great justice. 21:17, 7 April 2006 (UTC)
- nah thers no way, but i mean if you do think about it, every particle in your body is exerting gravity on everything... all the time modesty 03:58, 8 April 2006 (UTC)
- I think this is an example of the falacy of equivalence of large numbers. The earth is really big, and the number of people on it times their weight is really big. Therefore they are equivalent, and one will automatically effect the other. For great justice. 22:19, 8 April 2006 (UTC)
- nah thers no way, but i mean if you do think about it, every particle in your body is exerting gravity on everything... all the time modesty 03:58, 8 April 2006 (UTC)
Why does DNA only replicate in the 5' - 3' direction and not vice versa?[edit]
I know this is kind of a homework question and you guys don't like that which is fair enough, but i'm revising for my degree and really have no idea why. I am generally quite interested anyway. Cheers, Mark west 80.42.104.21 22:23, 7 April 2006 (UTC)
- I believe it is because the enzyme that catalyses the the replication process can only grip on the "5" end of a strain. Since it can't start at the "3" end, 3-5 replication does not happen. Note that this is from my sketchy memory of biochemistry 5 years ago. SanderJK 23:27, 7 April 2006 (UTC)
- WRT homework questions, there's no problem with asking specific factual questions (like yours seems to be, though it's an area I know nothing about). What we object to is when people post an essay topic, or a physics homework problem, or the like, and expect us to do the task for them in its entirety. --Robert Merkel 00:28, 8 April 2006 (UTC)
The reason that synthesis can only occur in the 5'-3' direction is based on the reason these names exist in the first place. If you are an applicant for a degree in molecular biology or something similar, then you know that these names, 5' and 3', refer to the carbon positions in the deoxyribose backbone. Both the 3' and 5' carbons have hydroxyl (alcohol) groups attached, however, wheras the 3' carbon is part of the ring, the 5' carbon is sticking out (read: much more reactive). Therefore, when DNA is split into monomers, deoxyribose molecules with purine or pyrmidine residues, the 5' carbon loses a bond, whereas the 3' carbon is not directly affected (instead the oxygen in the hydroxl loses a bond). Now consider this, since the residue separating the 5' end of one monomer and the 3' end of the next is a phosphate, the electron in the bond between a phosphate (which is completely resonance stabilized) and the sugar monomer is much more likely to go to the phosphate. Thus the positive charge can stick on the 3' oxygen (unlikely) or the 5' carbon (very likely). So wiht all this information, we can explode a diagram of DNA: you have a sugar monomer with a + charge at the exposed 5' carbon and a stable 3' carbon with full bonds and a stable hydroxl; and a phosphate with a - charge. Thus the most likely eventuality is a deoxyribose monomer with a phosphate attached at the 5' carbon. Since the active sight on the polymerase protein is reactive to the hydroxl side, it sits on the 3' end of the DNA chain and waits fot another monomer to come by, then attaches it via the floater's 5' end. Look at this attachment form far a way, and the chain appears to be growing from the 5' to the 3' direction.Tuckerekcut 16:23, 9 April 2006 (UTC)
Awesome answer, that helped a lot, thanks Tuckerekcut.
What is the alloy grade of cast steel which used for cylinder head?[edit]
In low speed diesel engine, the cylinder head is Manufactured from cast steel. Please, I need to know the alloy grade of cast steel and the folloing properties: 1- denisty 2- specific heat 3- thermal conductivity
thank you
- That's a toughie. You might have to ask a manufacturer of low-speed diesels like MTU to find out - or get a hold of a sample and take it off to the metallurgy lab. If you're interested because you want to set up in competion to them, the latter might be your only option. --Robert Merkel 00:35, 8 April 2006 (UTC)
April 8[edit]
Electrical Storms[edit]
Why do electrical storms only seem to occur in a rainstorm, but never in a snowstorm? Loomis51 00:19, 8 April 2006 (UTC)
It's possible to see lightning in a snowstorm. I saw it several times in New Hampshire. Brian G. Crawford 01:10, 8 April 2006 (UTC)
- See the environmental lapse rate page. A thunderstorm derives some of its energy from air rising in an unstable atmosphere, and moisture condensing as it cools. Warmer air on the bottom can hold more moisture and is more unstable than cooler air. EricR 01:55, 8 April 2006 (UTC)
Lightning in a snowstorm is referred to as thundersnow, and we had it here this winter. Night Gyr 03:27, 8 April 2006 (UTC)
It depends a lot on other factors, probably - such as the location of the storm, wind currents, etc etc etc. Here in southern New Zealand, for instance, thunderstorms are almost always accompanied by hail rather than rain. And yes, lightning quite often strikes over the sea (where I live I've got a great view over the Pacific), with no obviously spectacular results. Grutness...wha? 04:37, 8 April 2006 (UTC)
Also, does lightning strike in the sea, and if so does anything special happen compared to striking land? --Username132 (talk) 01:28, 8 April 2006 (UTC)
- Yes, lightning does strike at sea, and many boats are damaged in this way ([[19]]).
At land, unless the lightning strikes at you or very close, you're in no trouble. In the sea, because of salt, electricity is conducted (see Electrical conduction). You will get a severe shock from a relatively distant ray if you are taking a bath or even standing at a wet part of the beach.
Figures are that 70-90% of people will survive a lightning, but even a 10% chance of death doesn't look very appealing to me. --Rodrigo Novaes 13:38, 14 April 2006 (UTC)
Oxidizing Heavy Metals Salts[edit]
I would like to know in details about the oxidizing heavy-metal salts as this relates to corrosion of copper and copper alloys. Copper alloys resist many saline solutions, alkaline solutions, and organic chemicals. However, copper is susceptible to more rapid attack in oxidizing acids, oxidizing heavy-metal salts, sulfur, ammonia (NH3), and some sulfur and NH3 compounds.
We have a corrosion problem in one of our gas engines of Caterpillar and the service engineer identified the occurance of oxidizing heavy-metal salts on a engine part made of copper alloy.
I would appreciate your helping me out of this situation by providing details obout oxidizing heavy-metal salts.
Thanks & Regards,
Ahmed Mohiuddin Caltex Oil (Pakistan) Limited A chevron Company
- The obvious answer is to replace the copper with something less reactive, like gold or platinum. Another option would be to supply a "sacrificial rod", say made out of aluminum or magnesium, which would react with the heavy metal salts in place of the copper. The rod would need to be replaced as it corrodes, of course. One option might be to place the rod in the oil reservoir. Hopefully, the oil will carry the salts to the rod before they can attack the copper. The rod could be attached to the oil cap, but must be long enough to extend down into the oil in all conditions. Water heaters often use a sacrificial rod. StuRat 07:28, 8 April 2006 (UTC)
- You may be looking for the article on sacrificial anode. TenOfAllTrades(talk) 14:28, 8 April 2006 (UTC)
Adobe Photoshop Album Starter Edition 3.0[edit]
okay....im really starting to get mad at my Adobe Photoshop Album Starter Edition 3.0 because every time i go to a website any pictures that are on the site are automatically saved to it. Does anyone know how to stop it from doing this?I dont even have to look at the picture specifically...it just automatically saves it....really annoying.
Thanks for any help,
Shannon 03:15, 8 April 2006 (UTC)
This is NOT a science question. Ohanian 04:46, 8 April 2006 (UTC)
- No, but it's a computer question. I don't know the software, but look for a preferences tab on programs icon bar, failing that, what do you use it for? Why not uninstal it and use a free image editor like The Gimp? For great justice. 06:49, 8 April 2006 (UTC)
- Ohanian, please note this text from the main ref desk page, referring to the Science desk: "To ask questions about science, medicine, computing, and technology" --LarryMac 20:17, 10 April 2006 (UTC)
okay ill do that thanks.
Shannon 01:14, 9 April 2006 (UTC)
What is hafnium's cosmic history?[edit]
What is hafnium's cosmic history? I've looked everywhere I think I could find that information, but I can't seem to get any information that I need. Could you help me find the information I need, please?
- What do you mean by "cosmic history"? The only thing I can think of is how it was originally formed, which it shares in common with all the heavier elements, so I don't understand why hafnium has been singled out. You may want to look at our article on nucleosynthesis for more on that. --Bth 07:12, 8 April 2006 (UTC)
Ear ringing[edit]
My ears ring when I have earplugs in, I read in the wikipedia article that theres no cure for it, but I was wondering if this was normal, and if there was anything I could do to make them stop ringing, thanks.
Flents
- I have used Flents foam ear plugs which I have learned reduce ringing more than any other type of earplug. However, after wearing earplugs for over six hours every day for some time, I noticed that the ringing sound stayed even after I took the earplugs out of my ears; this is tinnitus. I my case, it ceased after I stopped putting earplugs in my ears for a month. Try to use the earplugs only if it is necessary.Patchouli 09:44, 8 April 2006 (UTC)
The ringing you are experiencing is indeed called [tinnitus], but it is not caused by the earplugs themselves. Most likely your tinnitus is constant throughout the day, however you dont notice it because of the ubiquitous background noise of daily life. Even in the dead of night, the hum of environmental control systems and various electronics in your environment distracts your attention from the ringing sound. However, when you put in earplugs, these sounds are muffled, and the ringing sound, which has been present as a tiny, unwavering signal from your ears to your brain, becomes relatively large compared to the background noise, and you notice it. Unfortunately, tinnitus tends to get worse as we age, mostly from incremental damage to our sensitive ears. Ringing due to acute damage may fade away after a few hours or days, but the underlying chronic tinnitus described here tends to be permanent. Tuckerekcut 15:54, 9 April 2006 (UTC)
Not enough sleep...[edit]
having "black lower eyelids" (i don't know its real name) has always been frustrating to many people. i always wonder why they appear when we don't get enough sleep, and perhaps there might be some other reasons?... i would be more than grateful if you could just stop by and answer this question for me. Thx! --219.77.165.58 11:18, 8 April 2006 (UTC)
- The skin around the eye (especially directly under it) is extremely thin and has a lot of blood vessels in it. While you are awake, your eyes stay open most of the time and you get a buildup of gunk (wax, dust, salt, etc...) in your eye. Tears help keep it clean, but they work best at night with your eyes closed and with plenty of REM. That buildup does two things - pushes blood vessels closer to the surface of the skin and blocks flow, making the vessels expand slightly. The more visible vessels are what you are seeing when you see shadows (and puffiness) under you eyes. --Kainaw (talk) 16:49, 8 April 2006 (UTC)
genius v.s. lunatics[edit]
many great mathematitians and well known scientist have the tendency of being nuts, but why is that? Thx :)
- A simple answer to your question is that people who are capable of making brilliant new developments (in any field whether it's math, science, philosophy, etc.) tend to be people that think differently than others, that look at problems in a new way, or don't simply accept the traditional point of view. Society tends to think of people with that kind of outlook as nuts (or at least "eccentric" in polite company). --Ed (Edgar181) 11:42, 8 April 2006 (UTC)
- I'm also fairly sure that the genius/lunatic thing is incorrect. In my recollection, there is no correlation between true mental illness and intelligence, but the cases in which they do correlate are generally so interesting and noteworthy that we end up letting them dominate our perception of it. --Fastfission 12:07, 8 April 2006 (UTC)
- I'd agree with both statements, and would like to add the fact that from my runins with various scientists i would say that many fields tend to attract certain kind of people, and that the studying of some fields can really change your view of the world. Combined with a certain social ineptness that does seem more common among researchers then in most circles, and the relatively often portrail of autistic savant in popular media, and the fact that abnormal people (including scientists) will get more media attention in general, it is easy to understand how such a picture of scientists would become widespread. Most of all, they are just people, perhaps with a little workaholic nature engrained. SanderJK 13:00, 8 April 2006 (UTC)
My cynical hemisphere will bet it's 99% a matter of noticing. Ordinary person has mental illness-- no news. "Brilliant" mathematician (is there any other kind?) or scientist has mental illness, and everybody can feel reassured that they are better off and savor the irony of the smarter guy's misfortune. Eccentricity and poor social skills are usually distinguishable from major mental illness. (what is the dsm-iv for "nuts," anyway?) alteripse 14:56, 8 April 2006 (UTC)
- In the case of a "genius", poor social skills and a constant preoccupation are what others call "mental illness". As an example, Einstein has terrible people skills. After his wife died, he became a shut-in. When in public, he either did his obligatory presentation or stayed away from the crowd, preoccupied with other things. Over and over, the genius form of antisocial behaviour has been explained as a disdain for the stupidity of humans in general. Einstein's quote, if I remember correctly, is that "Only two things are infinite, space and human stupidity. I'm not sure about the former." As for the preoccupation, they are working on problems that they find much more interesting than what to have for dinner or which politician do we want to raise our taxes next year. All in all, I see it as an adult trying to fit in with a class of preschool children. The children aren't really stupid, they are normal. The adult isn't mentally ill either. They are just focused on different things. --Kainaw (talk) 16:44, 8 April 2006 (UTC)
- OTOH, a large part of Einsteins 'genius' status is the fact that he fit so well into the stereotype. I'd say he was a large part of defining it. By comparison, Bertrand Russell for instance, shared a similarily negative view of humanity but was quite social. Given the political activism of both, it's quite obvious that they weren't actually disinterested in humanity itself. All in all, I think the 'insane genius' myth says more about people in general than about the geniuses. First, it displays our need for 'heroes', placing some people on pedestals way above everyone else, even though they're actually just at one end of a continuum. (Einstein was a genius, but was Niels Bohr? Feynman? Gell-Mann? Weinberg? Aage Bohr? Any Nobel-prize winner? Etc) Second, it illustrates a human tendency for 'justice'. People who are very smart must somehow 'pay' for that by having diminished ability in other areas. So geniuses are anti-social. Athletes are stupid. Etc. Sure, there are lots of 'geniuses' who were single-minded and anti-social, but there are those people on any job that doesn't require those skills. And as Alteripse said, there's a big difference between eccentric behaviour and real mental illness. Another factor might be cranks - people might reason along the lines of a crackpot simply being a genius who's wrong or misunderstood. (that's certainly how they see themselves!) A lot of them do seem to have some form of personality disorder, and they also seem to have a rather homogenous set of personality traits. (dogmatism, delusions of grandeur) But those personality traits aren't the ones that make a good scientist in the real-world. --BluePlatypus 20:40, 8 April 2006 (UTC)
- As a friend of mine likes to say: "The line between genius and insanity is very thin. In Mexico, we call it the Rio Grande." Grutness...wha? 01:25, 9 April 2006 (UTC)
- Someone asked me once whether I suffered from being crazy. I replied that I don't suffer, I love every minute of it. Of course, that was a quote from someone so I can't say I actually made it up, but it demonstrates a distinct lack of feeling by most people towards those who are different. I would never think I suffer from being like I am (ie. a crazy scientist :-) ), but others would instantly think that because they could never like my situation that I mustn't like it myself. Ansell 01:25, 9 April 2006 (UTC)
- I think your comment demonstrates a certain lack of feeling towards those who have a genuine mental disorder. Many of them do suffer, profoundly. --BluePlatypus 03:50, 9 April 2006 (UTC)
- Boy, I just don't know where to start with this one. What exactly do you mean by "lunacy" or madness, anyway? I think we have to be careful to distinguish between mere eccentricity and clinical psychopathology. Just because a highly intelligent, academically oriented person has no social skills and little sense of fashion does not mean they are "nuts," although the tabloids would have us believe that was the case. And not all types of madness have been deemed favorable to achievment. The "mad genius" stereotype has been around for a long time, especailly as applied to artists. The art majors on college campuses know that they can get away with all sorts of odd behavior and have it explained away as a manifestation of "artistic genius." Similarly, slovenly, asocial maths geniuses are often excused for their faux pas because such things are somehow a mark of their superiority in the intellectual heirarchy. People like Kay Redfield Jamison have written books about the correlation between bi-polar disorder and certain types of "creative genius." And the media has been playing the "Asperger / Engineer" connection for about 10 years now. Correlation is not cause, however, and it may be that the reason some of these famous "mad" geniuses have been so productive is that, in addition to having psychiatric disorders, they were highly intelligent and highly motivated. This may have enabled them to not simply "work around" their problems but to incorporate some of their supposed problems into a successful combination of skills. For example, many math and music prodigies experience synaesthesia, which gives them a unique perspective and provides an alternate path to understanding their fields. Manic episodes allow some people to work compulsively for days on end until a particular problem or project reaches a satisfactory conclusion. I seem to recall some evidence that in the bi-polar brain, some of the excess neurochemicals that build up in one portion of the brain "spill over" into adjacent areas associated with creative and other activities. And depressives have been found to have an enhance ability to recognize the true consequences of personal and political actions. I'd better stop now. This topic seems to be making me feel a bit crazy. Ande B 09:02, 9 April 2006 (UTC)
The whole problem may be solved on a point of perception. Unless you believe in absolute normality, there really is no single frame of reference from which one may judge sanity. From the scientist's point of view, it may the casual person who is mad. This lunatic behaviour was only caught on the exaggeration of the media and the apparent inpenetrability of many famous scientists/mathematicians. In many cases, misunderstanding and misinformation has always tend to crowd scientists with an air of mystery which others, in failing to understand, classifies as 'eccentricity'. The problem is that scientists are not always interested in making the general public understand themselves, and thus solve the mystery.
Then one must stress that the scientist does not necessarily have a mental illness such as schizophrenia, but his novel ways of perceiving the world, and often autistic moods, sometimes deceives the passer- by into thinking that this is so. In many cases their ways of percieving the world may even be stereotypical enough to be one step from the ideas of a lunatic, save that it is also usually supported by reasoning. The truth is scientists are not always willing to conform themselves in order to undeceive others, and our society does not always enjoy the company of those that do not follow its rules. Luthinya 10:06, 9 April 2006 (UTC)
This discussion is too lengthy to be worth reading. The title thereof implies that every Nobel Prize winner, Fields Medalist, Wolf Prize inner, Shaw Prize winner, Abel Prize winners, etc. + inventor, computer designer, corporate leaders or anyone who didn't watch soap operas, sitcoms, party, and goof off is moron.Patchouli 02:03, 10 April 2006 (UTC)
Unfortunately, this is pretty much how most of our society tends to view especially technological geniuses. Don't get me wrong- I'm often avoided as a nerd for reading maths/physics books all the way through lunchtimes in my high school. Luthinya 10:29, 10 April 2006 (UTC)
dynamic programming[edit]
how to implement dynamic programming using 'c' language —Preceding unsigned comment added by 59.92.34.98 (talk • contribs)
- Our article on dynamic programming is quite general; the ideas should be readily applicable to C. --Bth 14:27, 8 April 2006 (UTC)
C# Adding Machine[edit]
Please can someone give me the code required to make an adding machine in Microsoft Visual Studio C# 2005 express edition? Computerjoe's talk 12:53, 8 April 2006 (UTC)
- sounds like a homework question. have you tried asking your classmates? Night Gyr 19:18, 8 April 2006 (UTC)
- No, it's not a homework question. I'm a good faith editor, and I know this isn't the place to get h/w done. I'm learning C# by myself, and have made an adding machine before; but forgot the code. Computerjoe's talk 21:06, 8 April 2006 (UTC)
- wouldn't it just be a matter of creating code for each button, so pushing a key puts a number-symbol onto the end of a string, then when an operation key is pushed the string is converted to a number and the specified operation is performed? it doesn't seem to hard to write if you know how to create button controls. I know java and C/C++, not C#, though, so microsoft may be pulling something different. Night Gyr 05:56, 9 April 2006 (UTC)
- Yep. I need to know the code to transform e.g. TextBox1 and TextBox2 into a string. This has float.Parse in I think. Computerjoe's talk 09:02, 9 April 2006 (UTC)
- Er, turning the contents of a text box into a string? Should already be a string if it's in a text box. If you're really emulating a calculator (with graphical buttons to provide digits, particularly), you shouldn't be doing any string-parsing anyway. (Just handle the input yourself, forming or in response to a digit .) Then format floating-point numbers for output only, retaining the number for further computation. In other words, you want to do as few data-conversions as possible. (You also want to be using double precision, probably.) --Tardis 14:58, 10 April 2006 (UTC)
- Yep. I need to know the code to transform e.g. TextBox1 and TextBox2 into a string. This has float.Parse in I think. Computerjoe's talk 09:02, 9 April 2006 (UTC)
Poincare's radiation paradoxes[edit]
Hey folks! I was just reading Olivier Darrigol's paper from the journal Isis on the Einstein-Poincare priority dispute, and I came across the phrase "radiation paradoxes" as an item of importance. What are these radiation paradoxes? I'm a layman, so I was hoping someone could explain it in ordinary english. Thanks in advance! 65.95.139.89 18:49, 8 April 2006 (UTC)
- The very short version is that if you try to work out the equations of electromagnetism -- particularly for the propagation of electromagnetic radiation (ie light) -- for different observers travelling at different speeds, but use Galilean relativity (that is, the intuitive idea that the speed of an object moving at according to one observer is according to another observer in whose reference frame the first observer is moving at ) you will get different answers for different observers -- hence "radiation paradoxes". Postulating an invariant speed of light fixes this, but forces you to use a more complicated equation for relative speeds (albeit one that is very close to the Galilean one at low speeds). --Bth 19:26, 8 April 2006 (UTC)
Diseases[edit]
How many diseases affect the man most and the woman most? —Preceding unsigned comment added by 70.53.95.183 (talk • contribs)
- Red/Green Colorblindness is overwhelmingly male, since it is caused by a recessive gene on the X chromosome, of which women have 2 but man have 1 (They have XY instead of XX). 21:14, 8 April 2006 (UTC)
- I'm not sure how to answer this question. Men, for example, are incapable of contracting cervical cancer. And with regards to "how many", you can't really say "six" as an answer. Could you rephrase? Isopropyl
- Isopropyl is right; for more examples, see also Sex and illness. Melchoir 22:40, 8 April 2006 (UTC)
Thousands of diseases affect people of different sexes differently statistically for thousands of known and unknown reasons. In other words, it may be more unusual for a disease to have exactly the same sex distribution of the population than to have at least a small sexual association. alteripse 02:07, 10 April 2006 (UTC)
Data compression?[edit]
I was wondering, are there any lossy text compression algorithms? he he... it would be a bit like censorship if you think about it.--Frenchman113 21:12, 8 April 2006 (UTC)
- Pitman shorthand is another one. Four candles, anyone? --Heron 21:31, 8 April 2006 (UTC)
- A lossy text compression system wouldn't be very much use, but if you wanted to play, you could run text through an mp3 or jpg compression system, and see what came out - the results would probably show you why there really isn't one in the sense you mean it. For great justice. 22:04, 8 April 2006 (UTC)
- Pitman shorthand is another one. Four candles, anyone? --Heron 21:31, 8 April 2006 (UTC)
- you could use abbrev. to repr. words. They lose some data (i.e. gain ambiguity) but as long as context makes up for it, you can save space at min. qual. loss--same prin. as other lossy algos. Night Gyr 06:01, 9 April 2006 (UTC)
- If you have Linux, rm * gives 100% compression. -- Filliam H Muffman 03:55, 11 April 2006 (UTC)
- Another linux option... cat file | tr -d ' ' whichwilleffectivelycutoutallthespaces.thiswillgiveyoumaybea10%sizereduction,butyoucouldprobablystillmakeoutthetextright?
--Jmeden2000 15:17, 12 April 2006 (UTC)
Another question[edit]
Hate to be a nuisance, but how can I force WMP to save video that's being streamed from the web? I'm totally missing something here...--Frenchman113 22:31, 8 April 2006 (UTC)
- Try asfrecorder, http://sourceforge.net/projects/asfrecorder/ 202.58.62.4 00:27, 9 April 2006 (UTC)
Projectile, Missile, and Rocket[edit]
What is the difference among these?Patchouli 23:15, 8 April 2006 (UTC)
- A projectile is any object launched into the air by any given power source (includes asteroids, arrows, cannoballs, meteors, etc). A missile is a projectile launched by a human (in the modern sense, usually an explosive-bearing rocket). A rocket is a device powered by Newton's third law of motion (exhaust=action, rocket movement=reaction). Hope that helps.--Frenchman113 00:02, 9 April 2006 (UTC)
- In a general sense, a missile is a general term encompassing all human-launched projectiles, including stones flung from slings and shells from battleship cannons, but in a modern military context, it's the subset of rockets that have their own guidance systems, i.e. guided missiles. Night Gyr 06:04, 9 April 2006 (UTC)
Thank you.Patchouli 10:58, 9 April 2006 (UTC)
- Many people disambiguate rocket and, say, jet engines, by the idea that rockets carry their own fuel and reaction mass. -Fangz
April 9[edit]
latex in xfig diagrams[edit]
I'm trying to make a diagram in xfig which includes some LaTeX formatted text. I'm following the instructions here.
Here's what I do:
- draw my diagram
- make a text box with text "$\int f(x)\, dx$"
- set the "special" flag to "special" of the text box
- choose export, then select "combined PDF/Latex both parts", then export, resulting in two files intbox_t and intbox
- change the filename of intbox to intbox.pdf; my system won't work without the extension
- change the line "\includegraphics{intbox}%" to "\includegraphics{intbox.pdf}%", so account for the change in filename above
- in my tex source file, I include the header "\usepackage{graphicx}"
- I input the file with "\input intbox3.pdftex_t"
- then I tell latex to do its work. It seems to find the file and import the pdf, but then it barfs with:
loading : Context Support Macros / PDF (2004.03.26)
) (./intbox3.pdftex_t <intbox3.pdf, id=1, 258.9675pt x 177.66376pt>
<use intbox3.pdf>
! Undefined control sequence.
\color ...vevmode \csname fi\endcsname }\@ldc@l@r
l.14 }}}}
?
I'm using an Mac OS X system, using TeXshop frontend to pdflatex.
I'm a hair away from giving up. This is for a wikipedia article, so if you can straighten this out for me, you're helping grow an article. -lethe talk + 00:23, 9 April 2006 (UTC)
- Are you using \input{intbox3.pdftex_t}? If you want to email me the sources I could have a look. I use TeTeX under Linux but it doesn't seem to be an operating system problem. Ansell 00:54, 9 April 2006 (UTC)
Another solution is to export the xfig doc in "LaTeX picture + epic macros" (or "eepic macros" if you need them). Then you have to \usepackage{epic} (or eepic), but everything is in LaTeX format from the start. So for the text you just put placeholders in the xfig doc, and then go into the exported source and hack the text in by hand. The biggest downside is that if you need eepic, it won't work with PDFLaTeX and you'll have to do the two-step process to get a pdf file, which may not have hyperlinks. --Trovatore 18:47, 9 April 2006 (UTC)
LASIK and losing vision[edit]
I overheard someone saying that you lose part of your vision or something like that when you have LASIK surgery, is this true?
- Our article on LASIK might be helpful to you, in particular the Complications section. -- Daverocks (talk) 02:53, 9 April 2006 (UTC)
BMW Bluetooth[edit]
I have a BMW with bluetooth, and I had the code but I lost it. BMW says they dont have it and want $120 to retreive it from my car, is there any other way to get it? I used to use a motorola v600 and the code is in the phone, I just cant retreive it (I dont know how), how can I get the code?
- Fortune cookie say: He who can afford BMW, can afford to get it fixed. --Zeizmic 15:19, 9 April 2006 (UTC)
- Heh. But anyway, if you can get the code without paying, then so can anybody else. OMG CAR HAX!!!!1 Tzarius 09:40, 10 April 2006 (UTC)
- I'm just waiting for the "Wikipedia - where car theives go for advice" story to crop up somewhere now. — ceejayoz talk 17:49, 13 April 2006 (UTC)
- Try taking the phone to the dealer and ask him to retrieve the code from it...it'll probably be a lot cheaper and faster.. :) Jayant,17 Years, India • contribs 18:10, 13 April 2006 (UTC)
Cloning[edit]
Is it possible at this time to produce a clone of someone?
- I think so, yes. According to the article on human cloning, ACT was the first to sucessfully clone a human embryo. There are many claims of success beyond the embryo stage, but none of them have been verified. --Bowlhover 04:54, 9 April 2006 (UTC)
- No, not quite, but we are getting close. Part of the problem lies in defining "clone" in a consistent way, and the media has failed to do this. But I assume you mean "Is it possible to make a human being who would have the exact DNA as another." In a somewhat trivial sense, the answer to that is yes. We have long had the ability to perform artificial insemination in a culture dish. And one of the most frequent reasons to do this is to avoid conceiving children with an inheritable disease. By removing a single cell from the early zygote, and allowing that cell to grow for a while before (destructively) analyzing its DNA, we have created a clone of the original zygote. Many such clones can be made from these early embryonic cells and each of them has the potential of growing into a healthy human who would have DNA identical to the other humans who were derived from that same original zygote. But making a human clone from an adult does not yet seem possible with current techniques and understanding. Just convincing the early cell to make the initial divisions has been problematic. Developmental biology is not a simple field. In fact, it's in its infancy. Until we understand the numerous developmental hurdles and chemical cascades that determine human development, we wont be able to undertake this task without high risk. Once we do reach that stage of technological capability, the entire process will likely seem so incredibly obvious we'll be baffled at why it took us so long. Ande B 09:18, 9 April 2006 (UTC)
- Yes, but I have a feeling that it's going to take us a very long time to reach that stage, certainly much longer than needed. The world's most advanced nation, the U.S., has prohibited federal funding for human cloning research. Some U.S. states have even banned all forms of human cloning. Religion got into the way of scientific research, just as it did in the past. --Bowlhover 17:35, 9 April 2006 (UTC)
- The world's most advanced nation, the U.S.
- (much coughing and spluttering and muttering about damn Yankees) ;) — QuantumEleven | (talk) 09:31, 10 April 2006 (UTC)
- Yes, but I have a feeling that it's going to take us a very long time to reach that stage, certainly much longer than needed. The world's most advanced nation, the U.S., has prohibited federal funding for human cloning research. Some U.S. states have even banned all forms of human cloning. Religion got into the way of scientific research, just as it did in the past. --Bowlhover 17:35, 9 April 2006 (UTC)
- No, not quite, but we are getting close. Part of the problem lies in defining "clone" in a consistent way, and the media has failed to do this. But I assume you mean "Is it possible to make a human being who would have the exact DNA as another." In a somewhat trivial sense, the answer to that is yes. We have long had the ability to perform artificial insemination in a culture dish. And one of the most frequent reasons to do this is to avoid conceiving children with an inheritable disease. By removing a single cell from the early zygote, and allowing that cell to grow for a while before (destructively) analyzing its DNA, we have created a clone of the original zygote. Many such clones can be made from these early embryonic cells and each of them has the potential of growing into a healthy human who would have DNA identical to the other humans who were derived from that same original zygote. But making a human clone from an adult does not yet seem possible with current techniques and understanding. Just convincing the early cell to make the initial divisions has been problematic. Developmental biology is not a simple field. In fact, it's in its infancy. Until we understand the numerous developmental hurdles and chemical cascades that determine human development, we wont be able to undertake this task without high risk. Once we do reach that stage of technological capability, the entire process will likely seem so incredibly obvious we'll be baffled at why it took us so long. Ande B 09:18, 9 April 2006 (UTC)
How accurate are the Heavens Above predictions?[edit]
In terms of time, how accurate are the Iridium flare predictions? I know my latitude, longitude, and elevation to within 30 m, so the errors in my position shouldn't affect the results too much.
I'm curious about this because I plan on photographing tomorrow night's magnitude -2 flare, using a 15-second exposure time. I'm going to use an accurate clock to tell me when to press the shutter button--there needs to be 7.5 seconds of exposure before maximum brightness, and 7.5 seconds after. --Bowlhover 04:42, 9 April 2006 (UTC)
- I successfully photographed the iridium flare whose maximum brightness was at 21:17:08 (according to the Heavens Above prediction). Here is the 4.1-megabyte photograph--the flare is the streak of light in Cepheus, and to the left of the photo is Cassiopeia. I pressed the shutter button at 21:17:00, so the shutter opened at 21:17:02 due to the 2-second self-timer. It closed at 21:17:17. As you can see from the photograph, the flare was already very near its maximum brightness when the shutter opened. So the prediction was off by about 5 seconds. --Bowlhover 02:22, 10 April 2006 (UTC)
- Nice work! Ande B 21:14, 11 April 2006 (UTC)
Tinted CD-Rs[edit]
I saw and purchased some tinted CD-Rs yesterday in Taipei. The writing surface of these discs is in bright orange or bright green (they are colored like highlighter marker pens) and they cost NTD6 each (less than US$0.20 cheap). Do they employ newer dyes? Or are they just ordinary CD-Rs with tinted polycarbonate plastics? -- Toytoy 07:15, 9 April 2006 (UTC)
- I actually just saw one of those the other day for the first time. As you guessed, it's just tinted polycarbonate. Chapuisat 15:08, 11 April 2006 (UTC)
Open University Recommendation[edit]
Can anyone help in recommending a free Open University that allows under- 18 pupils to enjoy the studies of Mathematical Modelling and Theoretical Physics? I shall be deeply grateful for any suggestions. Luthinya 10:10, 9 April 2006 (UTC)
- If you want an Open University, you're probably in the UK, but MIT has a lovely site over at OpenCourseWare, where lecture notes and readings and other materials are posted. Isopropyl 15:38, 9 April 2006 (UTC)
Angular Momentum[edit]
In a book I have read recently concerning the spin of subatomic particles, I have heard Max Born say that though particles do not actually have spin in the usualy sense of the word, yet they still behave as if they have angular momentum. I am afraid I have not been able to decipher this remark, and shall be grateful if anyone may help in understanding it. Luthinya 10:15, 9 April 2006 (UTC)
Subatomic particles do have angular momentum. They can rotate about a linear axis in three dimensions, with such rotation obeying conservation of momentum, affecting collisions (although not always the same as for molecules and solids; baryons and fermions are very different in this respect because of their different properties) and wobbling with gyroscopic effects including when rotation interacts with field effects such as electromagnitism. Particles also have quantum spin which is a quantum number which is completely disjoint from angular momentum. "Spin" was named after experiments with polarization suggested that the quantum number was similar to angular momentum. As a quantum number, spin has conservation laws based strictly on small or simple fractional multiples of integers. Note that the angular momentum of a low-mass electron is unlikely to ever have much of an affect on its behavior when compared to electrostatic forces. For nuclons, though, angualr momentum can be very significant. See, for example, Cold fusion#Current understanding of nuclear processes. --James S. 11:30, 9 April 2006 (UTC)This is a bad explanation for several reasons. Please see Spin (physics), Spin quantum number, and Angular momentum. --James S. 13:15, 9 April 2006 (UTC)
- Ok, well.. There are a lot of levels of explaination that could be given here. The simplest, most common one is to describe spin as 'intrinsic angular momentum'. That is, an amount of angular momentum that is built-in to the particle, so to speak. So spin does work 'as if they have angular momentum', but it's a different property. (they can, however, interact. (Spin-orbit coupling) --BluePlatypus 01:49, 10 April 2006 (UTC)
Sheep[edit]
Can a human physically make a sheep pregnant by sexual intercourse?
- No, in general, different species can not interbreed. (But he can try.) --James S. 11:17, 9 April 2006 (UTC)
- But, but I can make it happen. It's actually doable.
- Give me some sexually matured male sheep and female sheep. I can physically force the female ones to pregnant. In fact, they may just go pregnant with or without my efforts. -- Toytoy 14:30, 9 April 2006 (UTC)
See Hybrid and Horizontal gene transfer and HeLa (a single cell species created by humans from a human) for interesting examples of what is possible, and Category:Mythological hybrids for what people used to think was possible. WAS 4.250 14:44, 9 April 2006 (UTC)
- Chimera (genetics) is also an interesting read. Isopropyl 15:36, 9 April 2006 (UTC)
- Do I detect a sense of urgency in this question? I hope not. Phileas 06:19, 10 April 2006 (UTC)
astronomy - colour correction[edit]
In colour images taken of the night sky, do astronomers ever attempt to correct the colours to allow for redshift? Obviously this would be tedious, difficult and probably impossible where the redshift of objects is not known. However for something as large as the Orion Nebula, is this ever done? --Paul venter 14:34, 9 April 2006 (UTC)
- The Orion Nebula is in our own galaxy, and therefore can be expected to have a negligible redshift. In general, quite a bit of processing is done on most astronomical images, and correcting for redshift would be relatively easy. See this link for information about how the Hubble Space Telescope does it. In any case, computing an object's redshift is relatively easy; if you have a spectrograph, you're most of the way there. -- Filliam H Muffman 03:14, 10 April 2006 (UTC)
- You don't even need (expensive and time-consuming) spectroscopy; if you have good photometry at multiple wavelengths you can get a reasonable estimate from photometric redshifts. (We should have an article on them; maybe I'll add it to my to do list.) --Bth 07:17, 10 April 2006 (UTC)
Can we defeat radiocarbon dating?[edit]
Can we defeat radiocarbon dating? I think it is very possible.
- Build a totally enclosed greenhouse similar to the Biosphere 2 (with positive pressure).
- Buy lots of coal.
- Grow Cyperus papyrus in the greenhouse using hydroponics equipments.
- Burn the coal to provide 12C-rich CO2.
- If you need other organic fertilizers, grow alfalfa at first and use it as the fertilizer.
- Make your papyrus with your 14C-poor Cyperus papyrus.
- Let an expert create the forgery.
- Hire an antique dealer to inform scholars.
- Let them see the v1.0 fake which may not be good enough.
- Let the antique dealer to ruin the deal.
- Let the forged item sit in a bank safe for years.
- Take your time to create and age your v2.0 fake to the desired status of corruption.
- Let the scholars buy it.
I think you can always find cheap and aboundant materials that are not polluted by post-WW II radioactive fallout (e.g. antartic ice to provide water). Scientists can only conduct destructive tests on unwritten parts of the speciment. I guess you don't even need to make 14C-poor ink. I think it is possible to make something to defeat 12C and most other scientific dating techniques. You can mass-produce multiple copies of the forged document (each created and aged a little differently) and test them with all the tools available. The copy that can fool all tests will be released to the scholars. Maybe you can breed your own "ancient" Cyperus using DNA fragments extracted from real antiques. - Toytoy 16:09, 9 April 2006 (UTC)
- I like it! Seems like it would be possible, but with your method the forgery would be just as old as the coal you bought (by C-14 dating). I'd add another step about regulating the C-14 concentration by adding outside CO2 to get just the right date you want for your papyrus to have died. -Snpoj
- Yes, you're right. I'll build a window. It's easy to regulate the amount of 14C. You can build a window or buy some charcoal. You may also want to filter your coal burner because unfiltered smoke may contain too much sulfur. Anyway, I think it is very possible to cheat scientists. All you need is a great expert of ancient literature. You can open the window and harvest your Cyperus every 12 hours. Sooner or later, you'll get a batch of Cyperus that's dated to the desired time period. These grasses are growing fast! Maybe you can sell the unused but dated portions to other law breakers! :) -- Toytoy 16:37, 9 April 2006 (UTC)
- But why fool scientists now if you can fool them thousands of years in the future? Why rewrite the past when you rewrite the future!? Using a mass spectrometer, isolate C-14, burn it and infuse it into the atmosphere of the dome. Now when the papyrus dies it will have a healthy stock of C-14 with which to pass the time. Eventually, in the year xxxx so much C-14 will have degraded as to appear that our Cyperus died just last year in xxxx-1! Now in the year yyyy documents written by us on the Cyperus back in 2006 will be found. The unsuspecting scientists will believe they must rewrite the history of xxxx with our falsification and we'll be laughing in our forgotten graves! -Snpoj 03:48, 10 April 2006 (UTC)
- I don't think anyone claimed it to be impossible, either. But you leave out the problems with aging. There are no doubt chemical markers (e.g. various decomposition products) which can be used to distingush something which has aged normally and something which has been bleached or similar. Then there are of course all the other usual methods of detecting a forgery. I don't think radiocarbon dating is terribly important for dating documents either - from what I understand from reading bibliophile literature, old paper is in relatively good supply. --BluePlatypus 18:00, 9 April 2006 (UTC)
- Unfortunately the problem you have is that C-14 is created from C-12 by solar raditation.
http://www.ndt-ed.org/EducationResources/CommunityCollege/Radiography/Physics/carbondating.htm Thus the ration of C-14 in your papyrus would still have the correct C-14. Now add to the fact that your Ancient language experts are few and far between. Most of them are scholars, and they all know each other. -Tollwutig 14:56, 10 April 2006 (UTC)
- If you build a greenhouse at near sea level, and you burn fresh coal to produce fresh 14C-poor CO2, solar radiation can do nothing. To create 14C, you need high energy cosmic ray that's only available very very high above.
- You can always obtain genuine unused ancient papyrus or Chinese paper from antique dealers. In fact, many high-end Chinese art counterfeiter have their personal stockpiles of unused paper aged for at least a couple hundred years. However, if you have an endless supply of 14C-poor papyrus, you can mass-manufacture history-making documents and age them by a trial and error approach. If you take time and money to do it, you will have some really great speciments in a few decades that can fool almost everyone on Earth.
- This method is surely difficult. An ordinary counterfeiter will not do it, but a determined government or religious group may have the will, expertise, time and resources to create something to support its own position (we own this land, we are better than you, our god was ...). -- Toytoy 15:41, 10 April 2006 (UTC)
- I have an even better idea, I could just use my time mahcine to go back to 1192, and drop off a reem of printer paper in an easy to find location, then travel back to the future, and pick it up there after it's had a few thousand years to age, then write whatever the heck i want to on it —Preceding unsigned comment added by 64.12.116.74 (talk • contribs)
- With your specifics I take you are discussing the recent Gospel of Judas release by National Geographic? If so you'll have a hard time finding someone who knows Coptic, there are what 5 people who can readily translate it?--Tollwutig 19:50, 10 April 2006 (UTC)
- You're damn right. I am very skeptical to the NG's Gospel of Judas TV show. I think it is difficult to create such a scholar bait but that's not totally impossible. In my opinion, NG failed miserably by creating a two-hour-long show that does not interview reasonable skeptics. I mean they jump to the conclusion too soon or they want the audience to buy the theory that the Passion could be simply wrong.
- Logically, you may also say the Gnostics had build a myth to counter other Christian sects or you may even say both sides lied when they talked about the death of Jesus. NG failed to present a more neutral POV with their dog and pony show. It didn't even say Jesus could turn himself to the Romans rather than asking Judas to betray him. The NG is telling a lousy detective story.
- As to the "only five people on Earth could write ancient Coptics" statement, I really cannot disprove it. However, I am very skeptical. Let's say the text was created by a counterfeiter. If you put it down in ancient Greek, the expert pool will be much more larger and there will be more skeptical eyes to review your text. And by the way, I don't think ancient Coptics is some sort of regulated language. You don't need a license to learn it. You just don't see many college job offers. A determined person previously trained in related languages may learn the language from various sources. Logically, you may also say some Evil Theologist discovered a Medieval Coptic text that tells such a story. He hired an Evil Linguist and an Evil Scientist to rewrite the text to match 3rd century grammer and put it on a piece of 3rd century papyrus to make the text closer to truth. Did I ever mention the Black Helicopter?
- Personally, I have only seen statements such as: GoJ said Judas was not a bad guy. The existence of the GoJ was proven by ... . I did not see them to advertise other previously unknown findings from the GoJ. If you unearth a document like this, I expect to learn some unknown things that are trivial in scope such as "The Apostle Peter had ulcer." or things like this. I don't think all genuine documents shall carry such information, but a forger may invent a story with all known facts and insert just one think to prove his point. After all, I think NG failed to take a more neutral stand in this case. It only took me minutes to devise a way to counter 14C dating. To a determined expert with money to burn, it could be even easier. The NG shall be more careful and skeptical with their discovery. -- Toytoy 00:31, 11 April 2006 (UTC)
- Frankly, you seem to have a little bit of a chip on your shoulder about this, Toytoy. If it makes you feel any better, just because the document is authentic doesn't mean you have to believe what it says. Why not just say "this is what the Cainites believed but they were full of crap", instead of camping out on the grassy knoll? --Trovatore 00:46, 11 April 2006 (UTC)
- Personally, I have only seen statements such as: GoJ said Judas was not a bad guy. The existence of the GoJ was proven by ... . I did not see them to advertise other previously unknown findings from the GoJ. If you unearth a document like this, I expect to learn some unknown things that are trivial in scope such as "The Apostle Peter had ulcer." or things like this. I don't think all genuine documents shall carry such information, but a forger may invent a story with all known facts and insert just one think to prove his point. After all, I think NG failed to take a more neutral stand in this case. It only took me minutes to devise a way to counter 14C dating. To a determined expert with money to burn, it could be even easier. The NG shall be more careful and skeptical with their discovery. -- Toytoy 00:31, 11 April 2006 (UTC)
- I believe some questions can never be fully answered even if we travel back in time with all kinds of evidence collection tricks and tools. If we cannot fully answer today's questions, how can we be so sure about yesterday's unsolved mysteries?
- However, I think some scholars are extremely gullible. I have first hand experience with an easily debunkable pre-Columbus historical discovery. Some scholars, I mean some of them, are just too eager to believe.
- Based on what I have seen, I believe the QC Dept. of the National Geography Society failed to do their job. Instead of showing us non-experts a less loaded version of the story, they made the show as if it was almost the truth. It didn't answer some trivial questions. I think it is natural for some earlier sects to pro Judas and some others to bash Judas. A skeptic may require more solid evidence before jumping on the band wagon. Too willingly to accept can lead to miserable mistakes. Do I have to make up a text that says "All apostles other than Judas betrayed Jesus. They took the money and framed Judas"? If you're willing to believe, someone somewhere may be willing to cook you some tasty truth.
- If the text was genuine, it would be more insightful to study the societal structure of the writers rather than to ship us another version of the truth. I mean the original Gospels were created by the 1st generation followers. By the time the stories travelled to Egypt, it must had been modified a little bit here and there to please local Christians. It's like making locally adapted versions of Hamlet for 17th century Japanese or 18th century Persian viewers. -- Toytoy 07:02, 11 April 2006 (UTC)
- Popular presentations of academic subjects, particularly on TV, tend to be slanted towards providing some sort of "narrative" (often, as in this case, "everything you thought you knew is (probably) wrong", though "person X's wild speculation is vindicated centuries later because it happens to sound a bit like what we now understand" is also popular), and have been for decades now. All that's happened is that you've seen one that touched on a subject that you have some knowledge of, or at least strong feelings about. Almost every show like this makes people who know the field feel the way you do right now. You should probably bear that in mind next time you watch one.
- On the other hand, I'm fairly sure I was aware of these ideas about Judas having been around in some of the now-extinct branches of Christianity well before this flap, so I'm not sure there isn't something in it. --Bth 07:20, 11 April 2006 (UTC)
Inductance[edit]
How to make 1 henery inductance coil?
- Did you check induction coil? I don't know what a Henry or Henery induction coil is. --
 Mac Davis] ⌇☢ ญƛ. 12:46, 10 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 12:46, 10 April 2006 (UTC)
- Here's a site which discusses design and construction of coils. EricR 14:02, 10 April 2006 (UTC)
- Self of Solenoid
L= (m0mr S N*N) / l An above Equation can use to calculate a self of a coil with the defined length and l>> D( D : is a diameter of the coil, S: Surface of coil ; S= (1/4)*4*3.14 D*D) m0= 4*3.14*10-7 ( here mr=1, a core is air ) Supposed: S=4cm2 = 4*10-4 m2 N= number of turn ( round) l= length of coil= 10cm=0.1 m For L =1 H , we must have N= 4461 rounds --User:Ngocthuan 06 18:25, 10 April 2006 (UTC)
April 10[edit]
electrical potential of the sun[edit]
My question is based on the following, which is from "Beyond Velikovsky", by Henry Bauer, page 59. He says that Velikovsky attributed some things to electromagnetic forces between astronomical bodies. In particular, in 1952 astronomer Donald Menzel calculated that the potential of the sun would have to be 10^19 volts to account for some of Velikovsky's claims (also in Worlds in Collision), which Menzel said is impossible. In 1960 physicist V. A. Bailey (unaware of Velikovsky's work and Menzel's calculations) proposed a theory that had (as a consequence) a potential of 10^19 volts for the sun. Bailey found a mathematical error in Menzel's calculations.
I have two questions: (1) what became of Bailey's theory? (2) What is the electrical potential of the sun (if it is known)? Bubba73 (talk), 01:42, 10 April 2006 (UTC)
- Take a look at Electric Universe. ☢ Ҡi∊ff⌇↯ 02:10, 10 April 2006 (UTC)
"[The "electric star" model proposed by Ralph Juergens in 1970s (in Pensee II, IX & X, SIS Review, & Kronos) and revived by Wallace Thornhill in The Electric Universe (1998), part of his "holoscience" project, (in which the Sun is a non-convecting, isothermal ball of plasma powered by infalling galactic electrons and many craters in the Solar System are the result of gigantic electric discharges, etc.) [as deus ex machina] cannot rescue the "polar configuration" from its fatal flaws because the model is a non-starter. It is disproved by practically everything known about the actual behavior of the Sun and heliosphere. This was first explained by this writer in Kronos X:3, 1985, pp. 15-23, and recently in more depth on e-mail list-serves by Robert Grumbine, Karl Hahn, Burch Seymour, Tim Thompson, and Wayne Throop. Thornhill either ignores or dismisses all the negative evidence such as (i) the absence of x-rays in coronal holes (which should be produced by infalling electrons for which no evidence exists beyond the wishful thinking of Thornhill and star-struck acolytes such as Amy & Mel Acheson writing for Thoth and Atlantis Rising, and Don Scott, an electrical engineer, who in parroting Ralph Juergens in Kronos IV:4, 1979, also fails to understand the importance of the Reynolds Number in defining turbulence in photospheric granulation.), (ii) the proof that granulation in the Sun's photosphere is an expression of convection, (iii) the mere existence of the solar wind in which no inflowing electrons have been detected, (iv) the absence of characteristic particles from the nuclear fusion claimed to occur in the photosphere, etc., etc. The model lacks rigorous mathematical support. No one has ever shown that the electric charge required to produce the cited craters, e.g., Aristarchus on the Moon, is feasible, while rigorous mathematical modelling to explain the high temperature in the Sun's corona, a favorite anomaly cited against standard theory, in conventional terms is progressing steadily. The simplistic analogies to plasma and electrical discharge phenomena that are invoked to support the model [as in Talbott & Thornhill's Thunderbolts of the Gods (2002)] cannot nullify the verdict of the overwhelming negative evidence and serve only as an example of invincible ignorance, showing the proponents do not know, for example, the difference between a plasmoid and a pair of opposed lotus blossoms used by the Greeks to represent the thunderbolt held by Zeus. Other examples of so-called electric discharge effects on planets, asteroids, and satellites (such as Europa) can be explained by conventional means without invoking cosmic electricity.]" from AN ANTIDOTE TO VELIKOVSKIAN DELUSIONS WAS 4.250 12:46, 10 April 2006 (UTC)
http://www.asap.unimelb.edu.au/bsparcs/physics/P000031p.htm doesn't seem to identify this theory with BAILEY, Victor Albert although it seems to be a list of all his papers. WAS 4.250 12:46, 10 April 2006 (UTC)
- This page of notes for a Velikovskyite essay references a Nature paper by Bailey from 1960 which isn't on that list: vol 186, p508. Not in any online archives that I can see, annoyingly. But it's probably the paper in question here, if anyone can get to a good library. --Bth 13:16, 10 April 2006 (UTC)
Science. RF Power Measurement.[edit]
Dear Sir/Madam,
I wish to know in detail the method of RF power measurement in different modes such as a. Timeslot Mode b. Continuous Average Mode c. Buffered Continuous Average Mode d. Burst Mode & e. Scope Mode
Kindly help.
Regards Pavan
- Our article on Radio frequency might be of interest to you, as well as this page about measuring RF power. -- Daverocks (talk) 09:02, 11 April 2006 (UTC)
Website Hosting[edit]
Is it possible to host your own website on your own computer? Would this require a fixed IP address? Is it possible to demand a fixed IP address from your ISP? --Username132 (talk) 05:22, 10 April 2006 (UTC)
- To answer my own questions, yes it is possible and a fixed IP is not necessarily necessary. http://www.no-ip.com for example, offers ways around dynamic IPs, although I don't really understand how it works (anyone else?). Some ISPs offer a fixed IP as standard or for an extra fee, or even not at all, but some users report that they have had the same IP for up to two years even with switching equipment on and off multiple times. What is it that dictates when a persons IP is changed?
- Would an admin on this website be able to compare the IPs used to make my first ever edit and this edit I'm typing now, for me? There may be other IPs inbetween due to editting done at university accomodation. Thanks :) --Username132 (talk) 06:31, 10 April 2006 (UTC)
- I've used http://www.dyndns.org which gave me a domain name that I could attach to my (current) IP. I also had a program running on my computer that constantly updated the information about my current IP to dyndns, keeping the domain name attached to my computer. I would assume no-ip works like this, too. My current hardware firewall is also capable of updating its IP to dyndns by itself. –Mysid 06:40, 10 April 2006 (UTC)
- Thanks. I have the option of
- a) hosting through the WinXP machine my mum uses; or
- b) hosting through a Proliant 3000 server with Fedora Core 4 installed which would then have to relay internet access to mum's computer as required.
- Is setting up a web server difficult? I'd like to be able to receive email to my domain name aswell. --Username132 (talk) 07:24, 10 April 2006 (UTC)
- I've used http://www.dyndns.org which gave me a domain name that I could attach to my (current) IP. I also had a program running on my computer that constantly updated the information about my current IP to dyndns, keeping the domain name attached to my computer. I would assume no-ip works like this, too. My current hardware firewall is also capable of updating its IP to dyndns by itself. –Mysid 06:40, 10 April 2006 (UTC)
- Setting up a web server is not trivial - especially as you want to configure it so that it's secure against attacks - but with a bit of knowledge and lots of reading of help documents, it should be doable. Check out Apache HTTP Server, the most popular (and free!) webserver available. If you want to receive e-mail through your domain, you need to also run (at the same time) an e-mail server on your computer.
- A more general note - is there a reason why you want to set up your own server? There are plenty of hosting sites which offer you ample web space, already set up, for a modest monthly fee - most of these have e-mail facilities, too. Obviously, it's up to you, I'm just wondering if you're not going to more hassle than you need to. Also, you need to make sure your server is on 24/7, and that your internet connection can handle the outgoing traffic. For instance, an ADSL connection can only handle very limited outgoing traffic, you can easily saturate your link if you are serving large files or many users. Just something to think about. — QuantumEleven | (talk) 09:28, 10 April 2006 (UTC)
- Admins can't see your IP, only developers and those with checkuser access can. If you want to see what your public IP is, go to DNSstuff.com. -- Finlay McWalter | Talk 14:39, 10 April 2006 (UTC)
- I see. I wanted to know what my IP was a few months ago and I don't think there's anyway to do that except contact a forum where I posted. I'll try somewhere else. --Username132 (talk) 16:42, 10 April 2006 (UTC)
- To see your IP address as the Wikipedia servers see it, click here and wait ten seconds. Note that this logs you out of Wikipedia, so you'll have to log back in afterwards. —Ilmari Karonen (talk) 12:14, 11 April 2006 (UTC)
Some questions about C programming.[edit]
Well here are some of my doubts about some basic concepts of C programming. I have divided them in different queries.
QUERY 1)
i have two programs prg1 and prg2 as follows
//prg1 main() { int i,j,k; }
//prg2 main() { int i; }
First i compile (not run) prg1, then i compile prg2. Now, after the compilation of prg2 is over, what happens to the space which was reserved by variables like j and k? As we have not used memory allocation functions, does that memory get freed on its own?
- If you don't run either of these programs, the memory used by them isn't relevant. The compiler certainly allocated some memory to deal with your source code, and probably had the names "i", "j", and "k" written down somewhere (except not "j" and "k" for prg2, of course!). But once you've compiled them, the compiler has exited and all that memory has been long since released to the operating system. --Tardis 18:30, 10 April 2006 (UTC)
A second question about this same query, in any of the above programs, when i would print the values of any variable i,j or k, a random or garbage value would be printed. But wat is the basis of that random value? I mean some logic would have been built up in the writing of the code for the functions like rand() srand(), etc. So wats the logic there and also here?
- Garbage values and random values are totally different. Random numbers are supposed to be evenly distributed and to have no pattern. Garbage probably will have a pattern, but not a dependable or useful one because it's just what happened to be left over from the last program to use that memory. —Keenan Pepper 16:46, 10 April 2006 (UTC)
- Variable declarations in C literally assign a name to a chunk of memory. That physical memory already existed (as in silicon), and had something stored in it: possibly all 0s, or maybe the hex pattern 0xDEADBEEF, or part of the Wikipedia logo. The point is, you have no control over it, so you shouldn't assume anything about it. --Tardis 18:30, 10 April 2006 (UTC)
QUERY 2)
Now this one is about arrays. consider the following statement,
int a[x]; //x is a valid no., i.e non-negative, etc. etc.
now wat is the limit of "x", wat is the maximum value of "x" which i can use? Or does it not have any limits?
- First, note that in standards-conforming C, x must be a compile-time constant; not even
const int x=10;
will work. But assuming you didn't mean a variable "x" but just some quantity, Keenan's prior comment below is (mostly) right. --Tardis 18:30, 10 April 2006 (UTC)
Secondly, if "x" has a limit, on what does it depend? Does it depend on the type of array, like here its an integer array, so does it depend on the type of the variable defined?
- It depends on how much memory is in the computer you run it on. If it's too big, it will compile, but when you run it the memory allocation will fail and your program will crash. —Keenan Pepper 16:48, 10 April 2006 (UTC)
- It only depends on the type of the array in that 5 doubles may take up more space than 5 shorts. Also, if it's initialized, or if the operating system/executable file format require it, the memory may become part of the executable file, and perhaps may be part of the compiler's memory space. Then it would fail to compile, or it might fail to be written to disk for disk space reasons. Beyond that, it's restricted by the data type of array indices. In Java this is int, but in C it might be size_t instead (check a book). --Tardis 18:30, 10 April 2006 (UTC)
- Explore your system. Make files a10000.c, etc:
- It only depends on the type of the array in that 5 doubles may take up more space than 5 shorts. Also, if it's initialized, or if the operating system/executable file format require it, the memory may become part of the executable file, and perhaps may be part of the compiler's memory space. Then it would fail to compile, or it might fail to be written to disk for disk space reasons. Beyond that, it's restricted by the data type of array indices. In Java this is int, but in C it might be size_t instead (check a book). --Tardis 18:30, 10 April 2006 (UTC)
a10000.c is void main(){int a[10000];printf("10000\n");}
a100000.c void main(){int a[100000];printf("100000\n");}
a1000000.c void main(){int a[1000000];printf("1000000\n");}
a10000000.c void main(){int a[10000000];printf("10000000\n");}
Run. On MY system:
-> ./a10000 10000 -> ./a100000 100000 -> ./a1000000 1000000 -> ./a10000000 Segmentation Fault (core dumped)
On unix, now see the ulimit command. Explore variations on the theme, try long instead of int, etc. Contemplate the implications for portable programming. GangofOne 22:08, 10 April 2006 (UTC)
- main() should return int, not void. —Keenan Pepper 00:03, 11 April 2006 (UTC)
QUERY 3)
This one is about memory addresses and pointers.
Consider following:
int i; printf("%u",&i);
Now, whenever the address of "i" is printed, its always less than 65524 (or some value near that). Why is it so? even if many variables are created, each variable's address value would be less than 65524. Why is it so? Is it because of the reason, that the disk segments are always divided in sizes of 65524 units?
- First, pointers are properly printed with the '%p' conversion specifier. It has nothing to do with disk organization at all; perhaps the best answer is "don't print pointers because they won't mean anything". Pointer values will differ between different systems, will differ because of compiler choice or different compiler options, may differ between different runs of the same program (simultaneously or in sequence), and don't have a well-defined association with any particular point in physical memory (see virtual memory for more on that). As a stab at answering your question, 65524 is very close to , automatic variables are typically located on the call stack, and such stacks often grow downwards in memory; perhaps you have a 64K stack, and your pointer is (for any of a variety of reasons) relative to the bottom of that stack's memory area. --Tardis 18:30, 10 April 2006 (UTC)
- Explore your sytem. while(i<1000000}{printf("%p ",&(i++));) This will generate another question in your mind. Write a program to answer it. This will generate another question... Repeat until (2038 AD). GangofOne 22:08, 10 April 2006 (UTC)
the structure of a dimerized pair of thymine molecules in a chain of DNA[edit]
could you please draw me this structure?
- Can you use Google Image search? --Tardis 18:35, 10 April 2006 (UTC)
car audio - necessity of a capacitor[edit]
i was considering getting a small subwoofer for my girlfriends car to improve the sound quality. we listen to the music loud sometimes, but i mean it doesnt have to be ridiculously loud, just sound a little clearer when the volumes up and hear the bass tones a little better. anyway i talked to my dad about this because his neighbor runs a car audio store, but its a 'pimp my ride' kind of place (hes from romania). my dad said that it will need an amp and a capacitor. i know ill need an amp but do i really need a capacitor for a small subwoofer? i feel like i cant trust his opinion because his last car had a tv that slid out of the dash, this ridiculous sound system (my dad doesnt even listen to music) that soudned terrible when it would be put up loud to impress you, and vertical doors (he now admits the vertical doors were a mistake) anyway im sure ive seen many cars with subwoofers and no capacitors. my question is at want point do you have to install a capacitor, and for a small single amp will i need one? modesty 22:02, 10 April 2006 (UTC)
The purpose of a Cap is, like in any load circuit, to buffer power between a source and a load. A cap allows the voltage drops from the battery or alternator to be filtered out, which is important if you are running a lot of power. If the amp is running only 200-300W RMS (likely for a single sub 10-12") then there is generally enough capacity in the battery and alternator, so a big external cap is superfluous. Once you get past 500 WRMS is when you see issues with voltage drop along the wiring, and overloading of alternators and batteries (to the point of destruction) so extra provisions (a cap is among them, but shouldn't be the first) may be needed. --Jmeden2000 15:36, 11 April 2006 (UTC)
- thank you --modesty 19:11, 11 April 2006 (UTC)
"The Critter"[edit]
I was reading a book about the Vietnam War the other day and a section of the book referred to the portion of the war in Laos. The author of the book referenced a certain animal that one of the other Americans had as a pet. For a long time nobody knew what the animal was called until one of the people stationed there happened to see the animal "Critter" on a Laotian postage stamp with the name "Panis Auritas". I can find no information on this animal.....I did find a picture of the postage stamp but no "Critter". Can anyone help with this information search.--67.98.38.212 22:53, 10 April 2006 (UTC)Benny
so what was on the postage stamp? alteripse 22:56, 10 April 2006 (UTC)
It looks a lot like a Pangolin to me -84.9.46.44 23:14, 10 April 2006 (UTC)
- ...which is genus Manis. I suspect a typo somewhere down the line. There is such a thing as a golden pangolin (though I note it's not listed in the Pangolin article), which might well have something like Manis Auritas as its taxonomic name. Grutness...wha? 02:31, 11 April 2006 (UTC)
April 11[edit]
Electromagnetism and force?[edit]
I have read in some places and seen some examples that electro-magnetism , specifically ionised air and static electric fields can move objects of any kind of matter. and also is the radiation pressure of light [which i know can move matter as have read article in scientific american on it] electromagnetic. How does it work if it does. I think it does as have read an interesting article on it. Am looking into it as a skeptical answer to poltergeist phenomena. this is the interesting article. it is about an electrostatic wall
".7 CASE STUDY - LARGE PLASTIC WEB ELECTROSTATIC PROBLEMS, RESULTS AND CURE, D. Swenson, 3M Company Tremendous static charge generation on a plastic web causes unique physical phenomena and special problems. Solution was simple and cost effective.
David Swenson of 3M Corporation describes an anomaly where workers encountered a strange "invisible wall" in the area under a fast-moving sheet of electrically charged polypropelene film in a factory. This "invisible wall" was strong enough to prevent humans from passing through. A person near this "wall" was unable to turn, and so had to walk backwards to retreat from it.
This occurred in late summer in South Carolina, in extremely high humidity. Polypropelene (PP) film on 50K ft. rolls 20ft wide was being slit and transferred to multiple smaller spools. The film was taken off the main roll at high speed, flowed upwards 20ft to overhead rollers, passed horizontally 20ft and then downwards to the slitting device, where it was spooled onto shorter rolls. The whole operation formed a cubical shaped tent, with two walls and a ceiling approximately 20ft square. The spools ran at 1000ft/min, or about 10MPH. The PP film had been manufactured with dissimilar surface structure on opposing faces. Contact electrification can occur even in similar materials if the surface textures or micro-structures are significantly different. The generation of a large imbalance of electrical surface-charge during unspooling was therefor not unexpected, and is a common problem in this industry. "Static cling" in the megavolt range!
On entering the factory floor and far from the equipment, Mr. Swenson's 200KV/ft handheld electrometer was found to slam to full scale. When he attempted to walk through the corridor formed by the moving film, he was stopped about half way through by an "invisible wall." He could lean all his weight forward but was unable to pass. He observed a fly get pulled into the charged, moving plastic, and speculates that the e-fields might have been strong enough to suck in birds!
The production manager did not believe Mr. Swenson's report of the strange phenomena. When they both returned to the factory floor, they found that the "wall" was no longer there. But the production workers had noticed the effect as occurring early in the morning when humidity was lower, so they agreed to try again another day. The second attempt was successful, and early in the morning the field underneath the "tent" was strong enough to raise even the short, curly hair of the production manager. The "invisible wall" effect had returned. He commented that he "didn't know whether to fix it or sell tickets."
- Bill Beaty
It later claims that this could be ionized air? how would this work? Robin
- The story was made up. --
 Mac Davis] ⌇☢ ญƛ. 01:21, 11 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 01:21, 11 April 2006 (UTC)
- Here's the origianl onlien, it was easy to google. http://www.amasci.com/weird/unusual/e-wall.html which references http://www.esdjournal.com/articles/final/final.htm , with pictures. GangofOne 02:14, 11 April 2006 (UTC)
- FYI amasci.com is the site of User:wjbeaty, mentioned above.
- Here's the origianl onlien, it was easy to google. http://www.amasci.com/weird/unusual/e-wall.html which references http://www.esdjournal.com/articles/final/final.htm , with pictures. GangofOne 02:14, 11 April 2006 (UTC)
Uuuuuum so now we have established that i didnt make the story up and its from a competant source, exactly how would it work? or am i just gona get accused of fraud again instead of getting the answer i asked for? I thought thats what this part of the site was for. Robin
- Actually, you are going to be accused of being unable to properly respond. I moved your comment from the bottom of the page to this section. --Kainaw (talk) 15:38, 11 April 2006 (UTC)
- Those aren't authoriative sources. This Beaty character seems to be a crackpot as well. His page is linked to by crank.net. What else need I say? --BluePlatypus 16:56, 11 April 2006 (UTC)
- That's interesting. I know Bill Beaty's site, and I wonder whether crank.net listed him as an "anticrank" or a "crank". Could you give me the context for the link that you found on crank.net, please? I tried the obvious Google searches like "beaty site:crank.net", but without success. I spend a lot of time battling real cranks on Wikipedia, and IMO Bill isn't one of them. --Heron 19:23, 11 April 2006 (UTC)
The answer Robin is looking for can be found in human psychology, not physics. Humans mispercieve things all the time. Humans misreport things all the time. "How would it work?" It doesn't. It can't. Wouldn't it be cool, useful, and a source of immense profit if it existed? Of course it would. The actual profit here lies in selling books and ads on sensationalistic sites. Always follow the money. WAS 4.250 17:37, 11 April 2006 (UTC)
Thank you for a civil reply. Actually if you study the evidence, and arthur c clarke did in his book "world of mysterious powers" not all poltergeists can be put down to misunderstanding, illusion and fraud. Secondly their seems to be many effects that can do things described in some poltergeist cases. I have been told electromagnetic fields, ultra and infra-sound, ionizing radiation,radiation pressure and static charging can shoot things about. But the complete expulsion of the case i mentioned as fraud without a proper incquiry bugs me. It seems like a genuine case. and remember ball lightning was thought to be non-existant by scientists at first but lo and behold http://physicsweb.org/articles/news/10/2/6 , http://www.newscientist.com/article/dn1720.html in fact one of these thinks that poltergeists and them may be completly related natural phenomena. not paranormal or magical or spiritual, just misunderstood. And the only bill beaty articles i can find on crank .net are critisms of over skeptical science, no listing of his site on either science, paranormal, electromagnetism, or antigravity pages which he should be listed under. Robin 22:45 april 11th
- I toss this out as speculation. Since the fields where so high and the 2nd guy felt crackling over his skin, maybe the effect was physiological. Maybe the fields where so high they were interfering with the action potentials of his nerve-muscle synapses. He said he couldn't turn around , he had to back out. Lost control of some muscle groups. This could be new information for the biophysics journals, if it were followed up with more testing. --GangofOne 23:30, 12 April 2006 (UTC)
Hmmmm i doubt it was something like that as its gave no mention of loss of muscle control, it states he leant his full weight against the invisible barriar, he coudnt do that if he had lost muscle control. Also have done some research and i think that electromagnetic / static electric and static magnetic fields can produce force to move things, maybe this was a large, stationary static magnetic field? poltergeist explanation aside, i would like to know how this works. Robin 16:37 14th april
- Speculating, I see three best possibilities: one with high probability and two with low. As mentioned above, very likely the effect was caused by "electrotetanus" where high-volt pulses of many mA applied to the skin will cause muscles to lock, as mentioned above. Since the original "e-wall" article, a company called "HSV Technologies" developed a similar phenomenon into a beam weapon; essentially a Tazer using UV laser-ionized paths rather than wires. A second possibility is far less likey: that the "e-wall" effect is genuine but was caused by perfectly normal physics, perhaps by small pressure gradients of ion clouds mentioned in the article, or perhaps it's akin to the recently-discovered "hot ice" effect, and is caused by an aerogel of water molecules stabilized by strong e-fields. Very interesting if true! A third possibility: the effect is genuine and is completely outside of current physics. Such events are rare but not unknown, as when Roentgen noticed a glowing chemical on his lab bench, or when Henri Becquerel put a piece of uranium ore against a photographic plate and accidentally founded an entire new field of physics.
- But note well, all of this is pure speculation. There are no "answers" here, only unknowns and probabilities. Comments of skeptics aside, nobody can KNOW that it's mere electro-tetanic effect, any more than we can KNOW it's an earthshaking physics discovery. To banish the unknown, we're required to reproduce the original phenomenon and then verify its causes. (The first thing I asked Dave Swenson was whether he tried throwing an object at the "wall." Nope. Too bad, since the result would have told us a lot.) --Wjbeaty 20:23, 14 April 2006 (UTC)
Hmmm thank you for your input mr Beatey, but as i stated above, the acount talked about leaning his whole weight against it, that could not have been done with just a loss of muscle control, and also if this were true he would not haven been able to walk himself out. So we are stuck with the other two, i am more inclined to beleive the idea that it was normal physics, just in an unusal manifestation. But i have a question which is what kinds of electromagnetic [ or purely electric or purely magnetic] fields can have an effect on objects and people, not a wall effect per se, just moving or knocking about by concussive force etc? Robin 00:27 15th april Robin
Eusing Free Registry Cleaner[edit]
Does anybody have experience of using Eusing Registry Cleaner? My registry must be in need of serious cleaning out but I know that tinkering with one's registry is a dangerous activity, so I'd really want to know I can rely on any software that's going to effect it. Casual reviews much appreciated. --bodnotbod 01:25, 11 April 2006 (UTC)
- If in doubt, always make a full backup first (open regedit, File -> Export, and make sure to select "All" as "Export range"). Then make small, incremental changes, restarting your computer between changes to ensure it still works. I have no familiarity with the program you mention, but check if it has a backup or undo option - most registry editors do. But, in any and all cases, make backups! :) — QuantumEleven | (talk) 06:16, 11 April 2006 (UTC)
- This one looks OK (softpedia give it a clean rating though like Quantum I have no direct experience of it), but you're right to be cautious; the whole malware arms race has led to increasing amounts of malware masquerading as anti-malware tools. --Bth 10:12, 11 April 2006 (UTC)
- The software claims to back up for you (or offer a restore point), but I shall follow your backup idea as best practice, thank you. Anyone with direct use of it? Oooh! ALternatively, can anyone recommend a highly regarded open source app for the same sorts of things? --bodnotbod 13:03, 11 April 2006 (UTC)
- This one looks OK (softpedia give it a clean rating though like Quantum I have no direct experience of it), but you're right to be cautious; the whole malware arms race has led to increasing amounts of malware masquerading as anti-malware tools. --Bth 10:12, 11 April 2006 (UTC)
Constructivist learning theories[edit]
Does anyone know of exmaples of cognitive and social constructivist learning theories? i understand the concepts but need to exmaples to understand them clearly.
- There is a very large selection of pedagogies (approached to teaching) that trace their origin to constructivist learning theories. At the base level an approach that uses hands on, discovery learning, can be considered constructivist in origin (students being the architects of their knowledge). There are many sophisticated examples (try doing a google search for constructivist pedagogy). One example I am familiar with is Covis. -Fermion 02:17, 11 April 2006 (UTC)
Yahoo! and computing services[edit]
The first page of the Yahoo! article states that Yahoo! is a "computing services" company. However, the provided link, computing services, seems to be a redirect to outsourcing. I was hoping to learn from the article what Yahoo! does, since I'm under the impression that they "run a website". I assume that the outsourcing link is inaccurate? -- Creidieki 02:29, 11 April 2006 (UTC)
- The outsourcing link is accurate. Yahoo! hosts and maintains one of the e-commerce web sites for one of the companies that I work for. So we outsource the work to them. They do this for lots of companies. I'm not real familiar with the company and its services but they do perform at least some outsourcing. Here's the address for that service: http://smallbusiness.yahoo.com/merchant/ Dismas|(talk) 04:17, 11 April 2006 (UTC)
- I believe Yahoo! is also an internet service provider and provides it's own search engine. StuRat 22:29, 11 April 2006 (UTC)
Could you contain the current from a lightning strike[edit]
If you ionized the clouds (somehow), would it be possible to store the current from an lightning stike, given the amount of power discharged by them?
- Your terminology "store the current" isn't what I think you mean. I think you mean "store the charge". While you could store some charge from a lightning strike, it would be very little of the overall charge. To date, devices that store charge require time to store up a charge. Lightning happens so quickly that there is no time to store much of the charge. However, it may be possible to steal a little charge from the clouds over time. There is a clear difference in charge between the top of tall buildings and the bottom. It isn't a huge amount, but it may be enough to trickle charge a battery. I've often considered doing an experiment with a lead on top of my offfice (13 floors) and a lead on the ground. The problem is that I don't have easy access to the roof and I'm sure someone would get upset if I hung a cable from the roof to the ground. But, you are free to try it. You may be able to do the same with a kite, using insulated wire for the 'string'. --Kainaw (talk) 13:37, 11 April 2006 (UTC)
- Sorry, I must object. Flying a kite where there is possibility of lightning strike is potentially lethal. We all know Benjamin Franklin did it and became famous for it, but he was lucky. He was smart enough to know to use a silk thread (a nonconductor) for a distance to the cotton thread that went to the kite. The cotton , when wet, was a conductor, was connected to the famous key. He did NOT hold the cotton thread. He held the silk thread that was tied to the cotton thread, according to what I understand. I recommend some library research before messing with lightning. Trying to store the charge of lightening has been tried and done, by Franklin, but it is not practical. Read all about it first. No point in dying redoing 18th century science experiments. GangofOne 23:35, 11 April 2006 (UTC)
Thanks...and no I won't fry myself.
- I did not say "fly a kite in a thunderstorm". I suggested that a kite be used to get an electrical lead off the ground to see if there is enough potential between high altitude and the ground to charge a battery ... when lightning is not present. As for storing the charge, there is much doubt that Franklin performed such an experiment. He flew a kite in the rain (after Thomas d'Alibard did a nearly identical experment with a tall iron rod). Then, there were many failed experiments around the world with lightning rods and jars of various substances. Many house fires followed. --Kainaw (talk) 23:46, 11 April 2006 (UTC)
Linux system keeps setting clock time wrong[edit]
I am running a Fedora Core 3 Linux system with Gnome 2.8. Every time I power up the system, its clock is at the wrong time, it's usually about 40 minutes in the past. I've tried setting the correct time with both date and hwclock but that only helps for the current session, when I reboot the computer the clock is at the wrong time again. Is it some weird service I'm running or is it a bug? I usually have fairly long uptimes, up to over a month, might this have something to do with it? How would I go about diagnosing it? JIP | Talk 06:42, 11 April 2006 (UTC)
- Fedora might be synchronising the clock with an NTP time server every bootup. I haven't used Fedora on a day-to-day basis, but quite a few distros seem to do that. Check the ntpd service and if it's starting on bootup. -- Daverocks (talk) 09:07, 11 April 2006 (UTC)
- I've had (acutally, have) the same problem. It is caused by
hwclock(seeman hwclock). On my previous install I fixed it byrm /etc/adjtime, but I think that removing the--adjustparameters whenhwclockis loaded should fix it as well. —Ruud 11:01, 11 April 2006 (UTC)
Another possibility is that the hardware clock is bad. They don't last forever. StuRat 22:16, 11 April 2006 (UTC)
- But this is a fairly new system. I only bought it fresh from the store, assembled from off-the-shelf parts (new, not used) a year ago. JIP | Talk 15:36, 12 April 2006 (UTC)
- The next step in diagnosis is to note exactly in what way it is bad. Is it always shifted by a fixed amount from the correct time? Is it always a particular date, like January 1970? Is there some other pattern? Notinasnaid 18:38, 12 April 2006 (UTC)
- It is always shifted by a fixed amount of time (approximately 40 minutes in the past). JIP | Talk 13:27, 13 April 2006 (UTC)
- Is ntpd running and if so whats its server list set to? it sounds like you may be syncronising off a bad time server. Plugwash 13:48, 13 April 2006 (UTC)
- ntpd was not running. I activated it and set it to connect to ntp1.kolumbus.fi. This fixed my date and time settings. But as for rebooting, I am still not sure, because I use my company's private DNS server, which is only available through a PPTP connection, which is not started at bootup. So I fear that when I reboot the system, ntpd will not be able to find the NTP server, and will just quit, leaving my system back to the incorrect time. JIP | Talk 19:04, 13 April 2006 (UTC)
- Is ntpd running and if so whats its server list set to? it sounds like you may be syncronising off a bad time server. Plugwash 13:48, 13 April 2006 (UTC)
- It is always shifted by a fixed amount of time (approximately 40 minutes in the past). JIP | Talk 13:27, 13 April 2006 (UTC)
Timing Diagram[edit]
Hello,
I would like to write an article on Digital Electronics Timing Diagrams.
I have found a stub: wiki/Timing_diagram, but this relates to the new UML 2.0 Timing Diagram.
Where should I start my article.
Regards,
Mark
- If there are two clearly different subjects that could fit into a title it can be handled using the diambiguation guidelines. Basically with two articles, you put links up the top of each page, explaining its context, and directing people looking for the other context, to the other page. A likely target for starting your article could be Timing diagram (electronics), as a suggestion. Ansell 09:01, 11 April 2006 (UTC)
UML Aggregation and SQL[edit]
I've been set the task of implementing a database schema given in UML as a MySQL database. In terms of design, what does the composition/aggregation relationship on the UML diagram translate to in terms of the tables required? The specific problem is the classic "library" system - there is a table Book which has the book details, then a relationship to an entity BookCopy, which has only one field listed. Is this extension, or inheritance, or neither? - does the arrow imply a foreign key? There is then another entity with the aggregation symbol - LoanedCopy. Could anyone give any advice on the structure of this, or a pointer to somewhere which has a decent tutorial covering UML -> SQL.
Cheers. QmunkE 12:54, 11 April 2006 (UTC)
- It sounds like there would be a one-to-many relationship from the Book table to the BookCopy table. Perhaps each copy has a unique serial number ? In that case that would be the primary key to the BookCopy table. The ISDN would be the primary key in the Book table and also a foreign key column in the BookCopy table. The LoanedCopy table would be similar to the BookCopy table, but would also have info on who checked out the books, when they checked them out, when they returned them, etc. StuRat 22:09, 11 April 2006 (UTC)
Spacetime[edit]
Could anybody popularly explain the concept of four dimensional spacetime?
- Time is just a dimension - like depth, width, and height. The best explanation I've heard is the "flatworld" example. Get a piece of paper. Put a penny (or something flat) on it. That is your flat man. He cannot see up and over anything because his world is completely flat. Draw a line on the paper. He can't see over it. It is a wall to him. You can make a box with an opening for a door and call it his house. When he is in his house, he thinke he is boxed in on all sides. But, you know different because you see in three dimensions. You know that you can pick him up and put him outside his house. The little flat man's point of view (lack of being able to see up) is similar to our point of view (inability to see forwards and backwards in time). --Kainaw (talk) 16:14, 11 April 2006 (UTC)
- Kainaw's example is greatly explored in the book Flatland by Edwin A. Abbott, a book I'd certainly recommend. EWS23 | (Leave me a message!) 01:10, 12 April 2006 (UTC)
- Or The Planiverse for a more detailed and mind-blowing view of what life would really be like in 2D space. —Keenan Pepper 04:06, 12 April 2006 (UTC)
- The allies of space and time was first perceived by the German mathematician Hermann Minkowski, when he studied his former pupil Einstein's Special Theory of Relativity and realized that their unique connection in behaviour due to the states of motion of the observed objects must be that they are ultimately one thing altogether- space- time, neither one nor the other. This idea was so radical and useful that Einstein incorporated it himself later for inclusion in General Relativity, ten years later. For a better mathematical understanding of the subject, please consult the special introductory page on the Special Relativity page and spacetime. Luthinya 18:32, 12 April 2006 (UTC)
If time is accepted as the fourth dimension, which it apparently is from the above responses, is that the limit of dimensions that "exist" in the universe? Is there speculation on what might be the fifth dimension? Loomis51 23:25, 15 April 2006 (UTC)
- String Theory assumes 11 dimensions and others assume more. Check it out. Luthinya 10:04, 17 April 2006 (UTC)
Size exclusion chromatography - De-salting a protein solution[edit]
I am trying to desalt a protein solution using a column of Sephadex G-25. Is there a rule of thumb for how concentrated this protein solution can be? (I know that if the solution is too concentrated, it causes problems) ike9898 17:08, 11 April 2006 (UTC)
- Salt concentration or protein concentration? --BluePlatypus 18:29, 11 April 2006 (UTC)
- Protein concentration. ike9898 20:51, 11 April 2006 (UTC)
- Well, it all depends on the flow, eluent, the protein itself, etc. But 25 mg/ml seems to be the maximum recommended[20]. --BluePlatypus 21:42, 11 April 2006 (UTC)
If you have a concentrated, salty protein solution the best desalting choice might be dialysis. Simply put the solution inside some moistened dialysis tubing and place the whole thing into a low salt buffer, which you can replenish as necessary. You can leave it overnight or longer. The dialysis membrane traps the protein inside while the tiny salt ions will diffuse through the membrane into the low salt solution by osmosis. This is a routine technique in protein prep.
Another option may be using a centrifugation based device. This allows you to desalt and concentrate the protein in a single step. Refer to the manufacturer for more details (e.g. Centricon).
Size exclusion chromatography is a good technique for purifying one protein from a mixture of proteins and is seperates based on molecular weight. As in all chromatography, the amount you load on this column depends on how big the column is. The bigger the column, the more you can load. Refer to the manufacturer's instructions for your particular brand of packing for the recommended loading amount.
- I'm desalting a protease and I get autolysis if I dialyse. SEC is very quick, and easy to set up in a cold room. But, my protein peak is spreading WAY too much. I thought it might be due to too high a protein conc (increasing viscosity). This technique should be routine, but it is new to me and it's giving me a royal pain! ike9898 17:55, 12 April 2006 (UTC)
LG L1515S monitor[edit]
I use a LG LCD monitor LG L1515S. Is it a low end, low quality monitor? This is the cheapest LCD monitor which I saw and I thought it does not matter to buy the cheapest monitor? Is there any problem buying cheap LCD? whats the problem? Comparing cheapest LCDs and mid/high quality CRTs, which is better?
- LCDs tend to be dim, only give a good quality pic when viewed straight on (not at an angle), and occasionally have a few pixels which are some random color. CRTs don't have these probs, but might be harmful to your eyes, can be blurry, tend to be rather heavy, and may not last as long. A plasma screen display is the best of all, but most expensive, too. You should look at the max resolution (1280x1024, for example), screen size (15 inches, for example), and the refresh rate (60Hz, for example) to evaluate a monitor. Ideally, they should all be as high as possible. Note that the refresh rate often varies with the current resolution setting. A low refresh rate will make the screen appear to flicker, especially when a white screen is displayed. A low resolution will make it difficult to display much on the screen at once and a small screen will make you need to squint to see anything. StuRat 21:50, 11 April 2006 (UTC)
- Refresh rates aren't really relevant to LCD monitors. One thing that does matter with LCDs is response times: the lower, the better. If you want to play videos or games, 8 ms (milliseconds) is great, and anything under 16 ms is good. In this day and age, the benefits of LCDs generally outweigh the benefits of CRTs: in fact, most places barely sell CRT monitors any more. Sum0 15:22, 13 April 2006 (UTC)
- Also with lcds you have to be quite carefull about the resoloution. Higher ≠ better Windows at least (i can't speak for linux or mac) pretty much relies on changing resoloution to change the displayed size of text (there is a font size setting but the user interfaces or lots of app break if you dare change it). Running an LCD at any resolotion other than an exact fraction of its native means a blurry display that gets very hard on the eyes.
- This means several things
- you must be happy with the size of things on your normal desktop at your lcds native resoloution
- if you run games you must be happy with them on your screen, lukilly games tend to use much bigger text than productivity apps so the blurring isn't such an issue but it still helps if you are happy with your games at the native resoloution or a fraction therof (happy with includes both framerate and detail, normally you balance theese by adjusting resolotion but LCDs put you in something of a straightjacket).
- If your vision deteriorates (or if an older relative wants to use your computer) you can't just lower the resoloution to compensate.
- All in all this adds up to a lot of lost flexibility when moving from crt to lcd Plugwash 00:18, 14 April 2006 (UTC)
- This means several things
Call termination charge in USA[edit]
Is there call termination charge in USA? In India, telecom companies from which calls originate pay a call termination charge of Rs.0.30 to the network in which the call terminates. Is there any call termination charge in USA? If yes, what is the call temination rates? How come companies offer unlimited calling to other companies's phones when there is a termination charge which metres by the minute?
- The US telecom industry places all sorts of goofy charges and surcharges on telephone calls. While our article doesn't enumerate many, the FCC has a sample phone bill complete with charges and explanations for a typical US monthly phone bill. As for termination charges specifically, I think that varies by plan. As I recall, such charges may exist but are frequently handled by a long-distance provider who then provides the end-user with a flat per-minute rate. — Lomn Talk 19:46, 11 April 2006 (UTC)
Difference between Google news & topix.net[edit]
What is the difference between topix.net and Google news? Both seem to aggregate news. But topix.net serves ads and no one minds while there has been lawsuits against Google news and it is not able to serve ads. Whats the reason and whats the difference between these two?
- First, money. Google is a cash cow for lawyers to sue. Second, methodology. Google searches anyone they like and they post results of their search. News sites have to partner with Topix (see their 'about us' page for info on the types of partnerships they have). --Kainaw (talk) 23:58, 11 April 2006 (UTC)
A medical query?[edit]
Taking my place at the urinal this afternoon, I was joined by my newest coworker setting up shop at the adjacent recepticle. Somewhat contrary to my own restroom M.O., he asked how I was doing, and out of politeness I returned the question. However, despite the divider between the urinals (which really ought to be law), I was struck by that phenomenon commonly known as "stage fright." That is, even though I had a full bladder on (in) my hands, I was incapable of micturating until immediately after he left. Why/how does this occur?
- The bladder is controlled by the autonomic nervous system. Parasympathetic neurons innervate the wall of the bladder (the detrusor muscle, trigone and sphincter). Parasympathetic stimulation results in contraction of the bladder muscle and relaxation of the urinary sphincter resulting in urination. This is opposed by the sympathetic division of the autonomic nervous system.
- Anxiety or fear, including social anxiety, results in decreased parasympathetic stimulation and increased sympathetic stimulation of the bladder, resulting in relaxation of the bladder proper and tightening of the urinary sphincter, making it more difficult to pee. There's always the stall. - Nunh-huh 18:47, 11 April 2006 (UTC)
I believe this is an evolutionary adaptation. That is, urinating or defecating in the presence of a stranger (or someone you don't fully trust) is a bad idea, as it leaves you vulnerable to attack. Thus, humans (and other animals) tend to delay elimination until they feel safe to do so. I suggest you use a stall, instead. StuRat 21:37, 11 April 2006 (UTC)
Indeed this phenomenon is contrary to the popular train of thought: that fright causes urination. As noted above, in humans, a sympathetic response to a stimulus will basically keep you from urinating, it is when you get to a safe place and the sympathetic stimulus stops that you will urinate (in a situation where your body decides you are safe again, although you still may be "scared") often uncontrollably. This reaction is actually a situation rather specific to humans: in canines, for example, sympathetic stimulation will usually cause urination when the bladder is anything but empty. Tuckerekcut 01:20, 12 April 2006 (UTC)
- I believe in dogs, urinating on themselves is used as a sign of submission. StuRat 03:49, 12 April 2006 (UTC)
- Urination is construed as such by other dogs, and rightly so. if a dog is scared, the sympathetic nervous system is activated, which causes urination. Thus fear causes submission. That this cascade is not under voluntary control is not particularly unusual.Tuckerekcut 03:25, 14 April 2006 (UTC)
There is also another possible explanation for this, which you may find slightly disturbing (but you shouldn't, it's quite natural). I was once at a fully packed pub where there was a lineup for the ladies' room but not the men's (as is so often the case), but this time the girls decided what the hell and started to use the men's room. There was no divider between urinals, and they were the type where the drain is at the bottom, so basically you're urinating against a wall. In any case, being out in the open (literally!) in front of all those female strangers was, to be polite, somewhat titilating. It also had the result of making it impossible to urinate. The medical explanation for this is simple: when aroused, the bladder is completely cut off from the urethra, and its impossible to urinate. (You guys know what I mean!). It's also common for heterosexual men to become involuntarally aroused in certain situations. Bottom line: Even if you're totally straight, the fact that your genitals are hanging out of your pants in the presence of another person (in this case, a male) can lead to difficulty in urinating. Loomis51 23:50, 15 April 2006 (UTC)
benefits of organic foods for immunno-compromised individuals...[edit]
I would like to know what the benefits are, if any, of organic foods are for immuno-compromised individuals, those with HIV/Hep C in particular. Thank you.
---BR.
- I'm not sure that they are. You may want to check out our article on organic food. The crux of your question revolves around how organic foods differ from ordinary foods, which itself is the center of a firestorm of debate. However, the article does mention that organic foods grown in manure actually increase the risk of contamination with E. coli and other bacteria, which is not a benefit at all. Isopropyl 19:29, 11 April 2006 (UTC)
- No body knows. No rigorous research has been done on this. Some doctors believe that there may be benfits, but it's highly controversial. For great justice. 01:23, 13 April 2006 (UTC)
- There was some research in the UK last year sometime which found that there was no scientifically identifiable benefit in earing organic fruit & vegetables. AllanHainey 10:01, 13 April 2006 (UTC)
computer repair?[edit]
what is the best way to fix up an old computer?Cooliabeanias 19:26, 11 April 2006 (UTC)
- Using old computers to run Linux is always a good choice. It runs well even with limited resources, and you can learn to use it on an expendable machine. Isopropyl 19:29, 11 April 2006 (UTC)
- If you are technical enough to figure out what type of power supply and memory you have, you can look into getting an upgraded motherboard and CPU that will use the same power supply and memory. You'll benefit from extra CPU speed with little investment. The common mistake is to buy the motherboard first - then finding out that you need to buy a new power supply and new memory because the old stuff is incompatable. Then, the cost is so much that you could have bought a new computer. --Kainaw (talk) 19:53, 11 April 2006 (UTC)
- Many "old" but workable computers get put out with the trash. It is possible to get quite usable parts from such machines. Power supplies, drives, cases, sound cards. Sometimes the machine works perfectly, just is old. This is a wasted opportunity, when so many people, mostly kids, could learn a lot from having such a machine. Out of date for the lastest stuff perhaps, but plenty good for learning. If you can put together such machines, you can just give them away, if nothing else. GangofOne 23:15, 11 April 2006 (UTC)
- I've tried to give away old "refurbished" computers for email/web browsing. It is difficult. Beggers quickly become choosers. I set up four computers at the homeless shelter with a 5th providing a shared dial-up connection. The problem was that the shelter didn't want to provide a line for dial-up. So, they just became machines for playing solitaire (which was easier to do with the many decks of free cards laying around). Also - on a distantly related topic - I was with a professor doing a talk on Beowulf Clusters. A reporter at the conference asked if these clusters will make use of all the old computers out ther. The professor's response was memorable: "Sure, if you want a huge power hungry heat box with nearly as much processing speed as a standard home PC." --Kainaw (talk) 23:38, 11 April 2006 (UTC)
- There are charities exporting refurbished computers to developing countries (see eg here). But I can't help wondering whether the economics of this really work, given that most new computers are manufactured closer to the people being sent the old ones ... (And the "it reuses them which is better than the environmentally-unfriendly components getting junked" argument has to be balanced against the fuel used, surely.)
- The only really practical use I can think of for old boxes is as middleboxes if you're running a small LAN off a shared broadband connection or something. --Bth 10:29, 13 April 2006 (UTC)
Chemicals[edit]
how do chemicals reacted in diffrent ways when mixed together thanks alot tomas
- They react in a predictable fashion depending upon their chemical properties, hope this helps.
- There are several good articles where you could start looking to find what you need: chemical reaction, list of reactions or the category Category:Chemical reactions. --Ed (Edgar181) 20:30, 11 April 2006 (UTC)
- Well, if there was an easy answer to that question, there wouldn't be much need for chemists, would there? :) --BluePlatypus 21:31, 11 April 2006 (UTC)
Library Classification of books[edit]
I have the responsibility of classifying our monastery library. I have been able to find a conversion of Library of Congress numbers with the Dewey Decimal numbers for an annual fee of over $300. Out of the question for such a small library. Is there a conversion table or some kind of rule of thumb that would help quicken the process? I have not been too successful wiht an internet search.--216.129.236.59 19:44, 11 April 2006 (UTC)
- Is the Poor Clares Monastery in Great Falls? How large a library do you have? Does the existing library have Dewey call numbers, and you want to switch over to Library of Congress? If so, and if the library is not too large, would it be useful to use the LOC website to look up the books and obtain the LOC call number? Some of the external references in the linked articles may help. - Nunh-huh 20:18, 11 April 2006 (UTC) You may also want to consider joining the "Project:Wombat" mailing list, where a lot of librarians hang out, and ask this question there. - Nunh-huh 00:31, 12 April 2006 (UTC)
I have an old book called Dewey Decimal Classification and Relative Index - published by Forest Press Inc, Lake Placid, NY. My copy is dated 1959, so whether it's still being printed or not I have no idea. Unfortunately, given its age, it has no ISBN (ironically it has a LoC Call number - 59-11569). It is designed for libraries and provides a list of all the dewey classifications both alphabetically by subject and numerically by code. Understandably, given its age, it has certainly been considerably revised since then (where would you file books on DVD recording, OCR, or even digital watches?), but if it's still out there it might be exactly what you need. Grutness...wha? 02:36, 12 April 2006 (UTC)uhh. skip that - I misread the question. Grutness...wha? 02:42, 12 April 2006 (UTC)
I am familiar with the Forest Press book. I used it in school eons ago. I don't need a detailed list as we are going with a modified Dewey Decimal classification. And, good heavens, Nunh-huh, why would you come up with Great Falls Montana and specifically a Poor Clare Monastery at that? Isn't that a little obscure and wild? Our books are unclassified at this point. So I am starting from scratch. 12:22, 12 April 2006 (UTC)
- Your IP address (216.129.236.59) is listed as being in Great Falls, MT. --Kainaw (talk) 13:24, 12 April 2006 (UTC)
- I found this site which gives the Dewey classification if you enter the ISBN. If you can get your hands on a bar-code scanner (maybe an old CueCat somewhere?), there seem to be several free utilities around to help in creating a catalog. --LarryMac 15:09, 12 April 2006 (UTC)
- Speaking as a cataloguer for a small-medium library, we use DDC20 at work - it's two decades old, but as long as you're willing to be creative with some sections (we have a heading which technically parses as "21st century history of the Soviet Union"!) that's not much of a problem. Anyway, the point there is that older editions of Dewey work fine, and if you try asking around it's quite possible a larger library (or library school) still has the old cataloguing manuals gathering dust on a shelf somewhere after they went to a more modern version - they're not much use after you've changed, except for training purposes, so asking nicely might well just get them as a donation.
- Of course, real classifying might be more effort than you want... if all you need is a very simple "high-level" classification, OCLC do publish a list of the "thousand sections", the top-level sections of Dewey (PDF), which should give you a baseline to be going on with. For individual books, you could try running them through the Library of Congress online catalogue - they have Dewey numbers listed for most of their stock - but it's probably just as quick to give them a general classification yourself once you have the hang of it. Shimgray | talk | 15:28, 12 April 2006 (UTC)
Thank you, for all your help. With the information provided I was able to find just what I needed. Shimgray's link to OCLC.org I was able to find the summary. Now I will be able to keep our classification consistant and heave others help me do the cataloging. We want the library to be extra user friendly and so we are using a modified DDC. I checked with all our PC monasteries on-line and culled the best possible classification system for our smaller libraries whcih ae also over loaded in the 200's. And, yes, I am at the Poor Clare Monastery in Great Falls, Nunh-huh. I would like to go back and do some adding to the Poor Ladies entry as it is bare bones information. It will have to wait a while as I have my hand full right now.Judith 20:26, 12 April 2006 (UTC)
- Sounds good, and glad you got to a solution. Have a good time organizing, and we await any contributions to the Poor Clare article with patient antici-----pation! (It really is a little pitiful!) - Nunh-huh 23:34, 12 April 2006 (UTC)
- I suppose it depends on how many books you have but I always prefer a more idiosyncratic filing system - largest to smallest, left to right. It makes it more fun looking for something & increases the chance of finding something you never knew you were looking for.AllanHainey 10:04, 13 April 2006 (UTC)
Gasoline and styrofoam[edit]
I've always been told that styrofoam will dissolve in gasoline and therefore you shouldn't store one in the other. Does this happen? And if so, why? Dismas|(talk) 22:17, 11 April 2006 (UTC)
- Yes, gasoline does dissolve styrofoam; I know because I once tried to fashon a funnel from a styrofoam cup, to help get gas into my moped. Gasoline is a non-polar solvent, and it is good at dissolving other non-polar materials, including stryofoam. Some synthetic polymers are much more resistant to this than others. ike9898 22:21, 11 April 2006 (UTC)
- Gasoline is a good solvent. Some idiots apparently use it in the washing machine to clean grease off their clothes, and then get a nice explosion when they put the clothes in the dryer. (The clothes may not have any oil stains, but you will need to pick them out of the debris to find out.) StuRat 22:34, 11 April 2006 (UTC)
- Styrofoam melts easily in any number of solvents, including gasoline, turpentine, paint thinners, etc.. It can also sometimes melt from lemon juice. Ande B 00:48, 12 April 2006 (UTC)
- Dissolving styrofoam and soap in gasoline is a good cheap way to jellify it, creating a napalm. :) --
 Mac Davis] ⌇☢ ญƛ. 04:29, 12 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 04:29, 12 April 2006 (UTC)
Coyote/dog hybrids[edit]
I overheard a man at the pet store the other day say that his dog is half coyote. Are Dog/Coyote hybrids fertile? User:Zoe|(talk) 23:01, 11 April 2006 (UTC)
April 12[edit]
Gettin' burnt...[edit]
i accidentally burnt my finger yesterday with an iron, and little watery poxes appear on my finger, why's that? Thx --203.218.93.206 01:08, 12 April 2006 (UTC)
Need Help on an Article[edit]
Can I get some help with checking the information on the Boiling Constants page and also setting up the data into tables? Ctifumdope 01:33, 12 April 2006 (UTC)
- One comment, the Wikipedia naming convention is to only capitalize the first word in an article name, and make it singular, so you should rename the article accordingly to Boiling constant. StuRat 03:43, 12 April 2006 (UTC)
- Help:Table is a good page that can help you with formatting your information into tables. -- Daverocks (talk) 04:35, 12 April 2006 (UTC)
- I moved the page to List of boiling constants of solvents. --
 Mac Davis] ⌇☢ ญƛ. 05:40, 12 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 05:40, 12 April 2006 (UTC)
Sound and Balloons.[edit]
hi, i would like to know why and how does a exploding balloon produce sound? thankyou —Preceding unsigned comment added by 202.138.102.141 (talk • contribs)
- Sound waves in air are composed of alternating pressure differences. The pressure inside a balloon is higher than outside (or it wouldn't be inflated in the first place). When you prick it, the pressure equalises; because the speed of propagation of pressure differences isn't infinite (it is, of course, the speed of sound), this doesn't happen instantaneously -- an expanding sphere of high pressure radiates out from the balloon, which you hear as a bang when it reaches your ear. --Bth 09:46, 12 April 2006 (UTC)
I was interested in this question, since the process is somewhat identical to earthquakes. Here is a good reference: [[21]] It has nothing to do with the air, but the speed of the fracture propagation, and the whip of the fragmented ends. These form mini sonic booms. You can experiment by interferring with the latex (tape) and showing how it muffles the sound. You can do different things to get a really loud pop. --Zeizmic 14:30, 12 April 2006 (UTC)
ElectroMagnetic Disturbance[edit]
How is it that a nuclear explosion creates an elecromagnetic pulse?How does this harm modern IC's only when they are in use?
- See electromagnetic bomb for the processes involved and how they interfere with electronics. But where do you get the idea that ICs are only vulnerable when in use? Unless they're shielded (by being put in some sort of Faraday cage) they're going to be vulnerable on or off. --Bth 10:56, 12 April 2006 (UTC)
Computing Data Storage DVD[edit]
What is the difference between a DVD-RW DVD-R and DVD+RW DVD+r?
- "R"s can be written to once, then the data's fixed; "RW"s are rewritable (with full erasure first for the -, random access for the +). The +/- thing is a standards mismatch between different manufacturers (like VHS/Betamax for video formats, except that we're still at the stage where the marketplace hasn't chosen a standard). Various arguments are advanced in favour of the two; you can read more at the articles in question. --Bth 10:45, 12 April 2006 (UTC)
Altitude and temperature[edit]
Why does it become cooler as we go higher?As we are nearing sun i suppose it should get hotter.-explain
- Erm, no. For one thing, the heat from the Sun reaches the Earth as radiation, much of which passes through the atmosphere and heats up the ground, but the overall siutation is complicated --there are layers where temperature increases with altitude, but the processes involved are more involved than just "it's nearer the sun". See Earth's atmosphere for more. --Bth 10:35, 12 April 2006 (UTC)
- The higher you go the thinner the atmosphere is, which means fewer particles to transfer the sun's radiation (or heat absorbed from surroundings) to you. Also with more room to move the particles are less energetic. Higher pressure = hotter. Lower pressure = cooler.--Anchoress 11:08, 12 April 2006 (UTC)
- However, in the thermosphere the temperature is controlled by the absorption of solar radiation and the temperatures can get as high as 2000oC. So there the questioner's original assumptions do sort of hold. --Bth 11:11, 12 April 2006 (UTC)
- It's still entirely irrelevant that you're closer to the sun. The difference in solar radiation intensity between the ground and the top of the thermosphere (using 690km) is , which is hardly important. It is true that some high-energy radiation is available there for heating that isn't available on the ground, but it's more like the rest of the atmosphere is in the thermosphere's shadow, rather than that the thermosphere is appreciably physically closer to anything. --Tardis 17:11, 12 April 2006 (UTC)
- However, in the thermosphere the temperature is controlled by the absorption of solar radiation and the temperatures can get as high as 2000oC. So there the questioner's original assumptions do sort of hold. --Bth 11:11, 12 April 2006 (UTC)
- The higher you go the thinner the atmosphere is, which means fewer particles to transfer the sun's radiation (or heat absorbed from surroundings) to you. Also with more room to move the particles are less energetic. Higher pressure = hotter. Lower pressure = cooler.--Anchoress 11:08, 12 April 2006 (UTC)
- Unfortunately your idea happens to be the common misconception. It is totally irrelevant here, as Tardis has said, whether you are close to the sun or not. The fact is that as you go up higher, the atmospheric pressure drops considerably, and with fewer air particles to transmit the sun's radiation or generate energy between themselves by bumping into each other, naturally the air temperature drops. I suppose your closer distance does mean that you would expect to feel the heat of its radiation more strongly, but even without the effects of the atmospheric pressure as I have described, considering the vast cosmic distances between the sun and the earth, even a mountaineer standing at the top of Everest will not be able to feel all that much difference to when he was upon sea- level itself. Luthinya 18:39, 12 April 2006 (UTC)
Vectors in EMW[edit]
Is there an easier practical illustration of the common terms we use in Electromagnetic Theory like Gradient,Curl,Divergence?
- Which illustrations are you already familiar with? In general, they're slightly fiddly to get across in non-mathematical form, hence the amount of handwaving that generally goes on, but they work wonderfully once you get your head round the del operator.
- Having said which, Feynman probably handles them wonderfully in Volume II of the Lectures on Physics; I'll look it up tonight when I get home. --Bth 10:40, 12 April 2006 (UTC)
- In fact, Feynman pretty much takes the same delaying the physical meaning until you've grasped the maths approach that I was suggesting. Rather surprising. --Bth 10:22, 13 April 2006 (UTC)
Marine Engineering[edit]
How is it possible to maintain a watertight seal between the rotating propeller shaft and the hull in a submarine or ship keeping in mind the intense water pressures the submarine will face when submerged?
- I'm sure this question's been asked before, but I can't find it ... --Bth 11:08, 12 April 2006 (UTC)
Stuffing box --Zeizmic 11:45, 12 April 2006 (UTC)
calcium / sugar[edit]
Is it oxalic acid, made from calcium?, that the body uses to digest sugar?? not sure if i spelling right.
m
- Oxalic acid is two COOH groups bolted onto each other; it contains no calcium. Enzymes are what break foods down in digestion -- for the specifics of the digestion of sugar, see glycolysis and for what happens after that, Krebs cycle. Calcium is important in the body as a constituent of bones and teeth. --Bth 12:48, 12 April 2006 (UTC)
- As a note Calcium is involved in Muscle contraction and is involved in carrying the eletrical charge down a Neuron in the Electrical synapse. Wish I had better sources for the neuron thing as it's been 9 years since my animal physiology course.--Tollwutig 13:30, 12 April 2006 (UTC)
- Oxaloacetic acid is an intermediate in the breakdown of most sugars, yes. Neuronal transmission is mostly the affair of sodium and potassium. Physchim62 (talk) 13:41, 12 April 2006 (UTC)
- Though calcium is involved in transmission across chemical synapses, by triggering the release of neurotransmitter vesicles. I'm guessing calcium has only a small role in typical electrical synapses because the intracellular concentration of calcium is so low in most cells. --David Iberri (talk) 22:03, 12 April 2006 (UTC)
- And, yet, if the concentration of calcium ions in the extracellular fluid drops, it produces excessive and painful muscle contractions called hypocalcemic tetany. The concentration may be low, but Ca is certainly not unimportant to neurmuscular transmission. alteripse 00:43, 13 April 2006 (UTC)
- Though calcium is involved in transmission across chemical synapses, by triggering the release of neurotransmitter vesicles. I'm guessing calcium has only a small role in typical electrical synapses because the intracellular concentration of calcium is so low in most cells. --David Iberri (talk) 22:03, 12 April 2006 (UTC)
- Oxaloacetic acid is an intermediate in the breakdown of most sugars, yes. Neuronal transmission is mostly the affair of sodium and potassium. Physchim62 (talk) 13:41, 12 April 2006 (UTC)
MIME[edit]
Can you please explain what a "MIME TYPE" is?and can you list a few MIME types?
- We have a good article on MIME. MIME helps identify what the type of content that is being sent is. Some examples are: text/plain, text/html, image/jpg (I think). -- Daverocks (talk) 12:42, 12 April 2006 (UTC)
- IIRC the last one should be image/jpeg. --cesarb 19:18, 12 April 2006 (UTC)
Time series analysis[edit]
The linear regression model can be used to estimate expected value. When applied to a time series, linear regression may not be useful because it is time-neutral. If I have a scatter chart of a time series, what kind of statistics tool should I use, if I want to:
- Estimate a rope's possibility of breaking over time. The rope is under a significant load, the fibers of the rope could break any minute. So the strength of the rope may suddently decrease over time. To estimate the possibility of breaking at t0, we may forget about past values. Let's say we have a lab and 1000 such ropes and loads.
- Estimate a rich person's tendency to buy gifts over time. Suppose the man is influenced by his ever-changing mood. If he's very happy, he can be buying gifts all the time. We don't know if he's happy at any given moment, but we can increase the weight of t0±Δ to emphasize the influence of his mood.
- Estimate a person's tendency to invest over time. Suppose all that person has is a stock ticker. At the time of decision making, he/she only has past performance. -- Toytoy 12:40, 12 April 2006 (UTC)
- Did you come up with those examples yourself? 'Cos they sound awfully like homework ... --Bth 13:02, 12 April 2006 (UTC)
- Yes, that's homework. And I am my own teacher. @#$% I should have taken some statistics while I was in college but I did not. -- Toytoy 13:13, 12 April 2006 (UTC)
- I'm not sure how a linear regression is relevant here. In the rope example, it sounds like you're trying to statistically describe the lifetimes of ropes under stress. That's just one-dimensional data: a vector of times. You'd just be using standard things like mean, variance, and skewness to analyze that. The other two examples don't have a clear goal to me... if you have data like "on December 11, Roger bought 2 gifts" and "on December 12, Roger bought 0 gifts", all you can usefully do is find times that seem interesting and posit that important events happened then. If you additionally had "on the evening of December 11, Roger's pet mouse caught the flu", then you might want to compute some sort of correlation between the two sets of data. With appropriate quantification of everything and some luck, you might be able to come up with a reasonable model ( or something), and then you could do predictions of one variable (gifts or good news) from the other. But when, say, you were trying to calibrate , you'll just want to use regular statistics, since your model exists outside of time and does not need to be causal. If you do do past-only analysis, it's typically as simple as truncating your data to whatever point in time, fitting some sort of curve to it in the usual ways, then extrapolating beyond it. --Tardis 17:24, 12 April 2006 (UTC)
This is just a shot in the dark as I'm not sure I understand your question fully, but have you considered multiple regression analysis with time being the third variable? It's available on excel. Sorry if this makes no sense, it's been a while since I took stats. Loomis51 00:01, 16 April 2006 (UTC)
A problem about Live Linux CD.[edit]
Hi, just recently i got a softwares dvd, in which Tablix Live Linux CD's ".iso" file was supposed to be there. but actually it was a ".rar" file. so i extracted the whole "rar" file, and then "burned" all the extracted files onto a CD. i am using windowsXP. then i tried to run "tablix" from that cd by making default boot from cd drive, but it didnt work. after that i ran the cd on winXP, even then it didnt start, actually a ".html" file about "tablix" from the cd did start on autorunning, but the OS wouldnt start. now how to do it? even on their website they have not mentioned how to install and use it for windows user. Similarly, on the same DVD, there is an "image" of Cluster Knoppix, but when i see that file's properties, it doesnt show it to be an "iso" file. i tried to burn that knoppix image on to a cd, by using nero 7, but it showed the message that unrecongnisable format, and so i didnt go further. so wats all this going on ? i am a windows user, and if a normal user like me has to go through so much hassles for just trying Linux, is it serving good to the open source community? not at all. so could you please recommend some Live CD OS which can work directly without such hassles. Also , can u tell me any website or organisation , which could send me these Live Linux CD's or other free Linux CD stuff to me. i am from india, and internet speed at my home is not too great, and also too expensive for me to download the ".iso" files of around 600mb size. so if u could recommend some sites which would do so and send me the CD's in india at my place? thank you.
- If you have WinRAR installed on Windows, it makes itself the default application to open ".iso" files. This could possibly be why you think it's a rar file, because WinRAR likes to open iso images. If you're right, though, and the images are really ".rar" files, it would probably be better to put all the extracted files into an iso of its own, rather than burning all the files manually. Also, when you say you tried to run Tablix and it "didn't work", does that mean that Windows started booting and the CD didn't? More details would be appreciated. Also, if you tried to get Nero 7 to burn an image file and it said "unrecognisable format", then what you're trying to burn definitely isn't an ISO. What format is that image in? -- Daverocks (talk) 12:51, 12 April 2006 (UTC)
- More generally, you could try Linux India (that's the WP article, this is their site) or one of their affilliated LUGs (Linux User Groups) for help getting started with Linux -- it seems to be a large part of their mission to help newbies. And if you don't manage to solve your problems, apparently thanks to LI's activities, sites like this one now sell distros on cheap CD to India. --Bth 13:01, 12 April 2006 (UTC)
- I think Ubuntu linux will still mail you free cd's. They send a live CD and an installation CD. And your problems seem to have to do with the configuration of your Windows system, not the linux distrobutions themselves. But I agree getting in touch with the local linux groups would be good, as you'll have lots of things that will be much easier if you have someone to help you with. - Taxman Talk 17:48, 12 April 2006 (UTC)
- Have you set your bios to boot from cd? For great justice. 01:15, 13 April 2006 (UTC)
- Yeah, sorry, I missed that! For great justice. 15:55, 14 April 2006 (UTC)
Why are adults less able than children to tolerate dizziness?[edit]
Most kids could spin in circles for a minute or more with only mild nausea that quickly passes. Many or most adults would feel ill to the point of vomiting from such motion, and are probably more likely to get nauseous from a simple thing like swinging. Why are children generally better able to handle getting dizzy? --Jonathan Kovaciny 12:49, 12 April 2006 (UTC)
- Your assumption does not sound correct to me. People that are highly active can handle motion better than people who rarely move. Children spin, tumble, and run around more than adults. So, a higher percentage of children have a tolerance for that action. Adults who continue to spin, tumble, and run around maintain the tolerance. Also, children who spend all their time sitting in front of the tv do not have a tolerance for motion. --Kainaw (talk) 13:11, 12 April 2006 (UTC)
- I certainly can't go on as many roller-coasters as a twelve-year-old. DJ Clayworth 03:34, 15 April 2006 (UTC)
Need to buy a 'surge protector'[edit]
1:24 PM 4/12/2006 DST Need to buy a 'surge protector'
Hi all, I need to buy a 'surge protector' or I think an 'automatic voltage stabilizer' for my home computer as power cuts are becoming more
frequent. The budget is low and i don't think I need anything as fancy as a Uninterruptible Power Supply; just a Plain Old stabilizer is sufficient? I
don't know much about the impact of a hot boot on the devices in my Willamette processor or the 1.5 GHz board. All I know is there might be some
damage to the hard disk with repeated incidents of power failures. Is there anything I am missing? Thanks for your care and dedication. I have been reading this page for quite some time now and I CTRL+D ed it on my firefox. Yours truly, -- Kushal one 17:38, 12 April 2006 (UTC)
- A surge protector is no use at all in a power cut. It is designed to protect against sudden increases in power ("surges"). In a power cut, the computer switches off, unless you have an Uninterruptible Power Supply. Notinasnaid 18:34, 12 April 2006 (UTC)
Don't know what a stabilizer is, but you need some form of Uninterruptible power supply --Zeizmic 19:55, 12 April 2006 (UTC)
- A UPS can act as a stabilizer, in that it will maintain a steady voltage in the event of power sags or power surges. A surge protector alone might help in eliminating any surge that occurs as power is restored after a power cut. --205.143.37.68 21:25, 12 April 2006 (UTC)
- I think there is a difference between a 'surge' protector and a 'spike' protector. I am not sure if that is relevant to what is being discussed here, but here goes what I think is the difference. A 'surge' protector is for things like electric motors which will overheat if too much current is pushed through them, often they have circuit breakers to protect against that. A 'spike' protector protects against brief overvoltages which often occur when powerlines are near a lightning strike, these are very hard on electronics and can cause progrsssive failure because each spike does some damage to the components.
- Yes. If you are living somewhere with frequent power cuts, you likely have spikes and surges too. Get a UPS. If you can't afford one, a surge protector will help reduce damage from too much electricity, but won't help with not enough. For great justice. 01:08, 13 April 2006 (UTC)
Thanks all of you. I will need to look for a spike protector then? FGJ (if I call you that), I don't think I can afford a UPS but I will look into the prices for it too. My second question stands. I don't know much about the impact of a hot boot on the devices in my Willamette processor or the 1.5 GHz board. All I know is there might be some damage to the hard disk with repeated incidents of power failures. Is there anything I am missing? Sorry for the added trouble.
The colour of water[edit]
I recall reading once a upon a time that the colour white corresponds to the highest frequency point on the Sun's black body curve (somewhere around 6000K). This is not coincidence, but an evolutionary adaptation. Rather than saying "sunlight is white at its brightest" it is probably more accurate to say "our manner of perceiving white has been conditioned by the sun." An animal around a red dwarf might perceive our red as white and our white perhaps might be invisible in the manner of x-rays etc. OK, if that's utterly out to lunch somebody tell me.
So this got me thinking about water the other day. Again, it's obviously not coincidence that our main biotic solvent happens to be transparent. Would it be fair to say "our manner of perceiving transparency has been conditioned by water"? Another hypothetical alien basking in liquid ammonia might view H2O as an opaque poison? Any formula that describes this sort of thing? I suppose the evolutionary mechanism would be "the clearer the water the safer the drinking." Marskell 17:50, 12 April 2006 (UTC)
- As I understand (part of) your question, you are asking if, given different conditions during our evolution, we could ahve evolved in a way that water was not transparent to us, but something else (that is opaque to us) might be transparent to these alterative people. Water is transparent becuase it absorbs little radiation from the 'visible' portion of the spectrum. The radiation absorbed by water wouldn't change, but we could have evolved so that our eyes were attuned to a different part of the spectrum. I believe water is fairly opaque in the UV, so if our eyes saw UV, water would indeed be opaque as well. As to whether there is some solvent that is opaque in the visible but transparent on UV or infrared, there probably is, msaybe someone could provide an example? Chapuisat 18:41, 12 April 2006 (UTC)
- "the colour white corresponds to the highest frequency point on the Sun's black body curve", better to say, not highest frequency, but rather , most abundant. Which is maybe what you mean. Yes, it is natural that we are sensitive to the light frequencies that are most copious in penetrating the atmosphere. To be otherwise would be a missed opportunity. But it doesn't apply to transparency. What is transparent to light is physically determined couldn't be changed subjectively in the viewer. Anyway, why would it matter if water where opaque? What if water were silvery like mercury, why would that matter? Except for minor adjustments, like not diving into water without testing depth etc? I don't see why life couldn't evolve if that where the case. (Water in the eyes, would be a problem; probably a workaround) GangofOne 23:08, 12 April 2006 (UTC)
- Transparency could be changed if we are talking about seeing with a radically different spectrum of "visible light". For example, if infrared was all we could see, we would perceive some things as being transparent which are not transparent to the radiation we call "visible light", and we would perceive some things as opaque which are currently transparent. Transparency is not quite the same sort of thing as color perception but it is not radically far off. But the "color of water" bit is misleading in this regard, I think. --Fastfission 00:02, 13 April 2006 (UTC)
- "Highest" --> "most abundant", yes thank-you, that was poor word choice. So you're saying it is essentially coincidence ("couldn't be subjectively changed in the viewer")? As for why it would matter, I would guess that opacity would be something of a handicap to early marine creatures. Perhaps a transparent solvent is not required for evolution, but it certainly doesn't hurt once you develop eyes. Marskell 08:06, 13 April 2006 (UTC)
- Of course the light we get from the sun is ALSO in the range of energy transitions of many chemical reactions, (many chemical reactions for chemicals our living world is made of, because it evolved under those conditions. note the circularity...) EG photosynthesis rxns, etc. Here is where in the intelligent design advocates can speculate. If the sun gave off microwaves, say, than a planet to form life would have to have a mwave transparent atmosphere, chemistry of life as we know it would be impossible, would have to be different chemistry. etc. Many techincal problems. And if you try to track down solutions, it may be that eventually all alternate scenarios won't work. Then ID might say, constants of physcics are such that nuclear rxns in the sun are such that the radiation it gives off are such that on a particular type of planet with certain gaseous atomosphere at certain temperature could form life rxns that make life possible and allow life to see and cognized that the constants of physics are such that .... (It all fits together) I mean the IDists COULD make such a claim, if they were smart enough. --GangofOne 19:40, 13 April 2006 (UTC)
- If water were opaque with respect to our definition of 'white', then life evolving in oceans would presumably adapt to whatever the most abundant radiation filtering through was, and the surface of the ocean would probably become prime real estate. It could lead to some interesting results, like radically different mechanisms for photosynthesis at small variations in depth. Peter Grey 03:21, 14 April 2006 (UTC)
- Water transparency is vital for terrestrial as well as aquatic organisms. If water were not transparent to humans, critical eye components such as the lens and vitreous humor would be opaque. :furthermore, since vertebrate eyes evolved in ancestors to vertebrates, we inherited our vision from aquatic creatures. — Knowledge Seeker দ 05:40, 14 April 2006 (UTC)
- Even if vision could not be developed for whatever reason, there are other ways to sense one's environment. Echolocation can be an adequate substitute for vision, and an organism living in a sufficiently dense environment (such as underwater) can sense movement around itself via a pressure sense. 84.239.128.9 18:02, 14 April 2006 (UTC)
A problem about GIMP[edit]
Hi, just recently i downloaded GIMP (latest version) from sourceforge.net, and all the help files and also animation package along with the necessary GTK. it was working fine on my laptop, but when i tried to install it on my personal computer, running winXP, during installation it said that some files were already present , so it gave me option of "renaming" them. but i decided not to rename them. so it got installed with no problem.but now whenever i try to run it, a msg appears that "an error has occured and GIMP will shut down". this error just wouldnt go. i re-installed it several times, but it didnt work, now what should i do? GIMP is great , but these problems just make open source a headache. could u help me?
- Why is this an open source problem? As for Gimp, did you remove it first before reinstalling it? --Kainaw (talk) 18:38, 12 April 2006 (UTC)
- yes i did uninstall it before installing it.
- For more specific help here, you'll need to be a bit more specific about the errors during installation, and whether you get the same errors each time. Also, have you tried that project's help forum? --Tardis 20:15, 12 April 2006 (UTC)
Skew Universe[edit]
What exactly are the implications of a skew universe, and what are the possiblities that we may live in one ourselves? Luthinya 18:41, 12 April 2006 (UTC)
- You'll have to give a rigorous definition of "skew universe" first. It doesn't seem to be a well-established scientific term. Perhaps you'd be interested in brane cosmology? Also, skew in other fields is typically a relative description; it means nothing if you only consider one object. If that's the case here, then the question "do we live in a skew universe?" is meaningless, and the "implications of a skew universe" are null. Now, if we somehow found another universe and it was skew to ours... --Tardis 20:34, 12 April 2006 (UTC)
- I think this is a follow up to the question about the perpendicular universe theory that was asked a while back--172.129.106.218 00:10, 13 April 2006 (UTC)
- Unless its the same person, I think he is just asking about parallel universes. Check it out. --
 Mac Davis] ⌇☢ ญƛ. 01:18, 13 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 01:18, 13 April 2006 (UTC)
- I know which section you were talking about vaguely, since I myself have seen it, but I am NOT the same person- since I am obviously female- and as far as I know my problem does not concern parallel universes directly, which I had taken a considerable interest in a few months earlier. My question arose from an article of a scientific journal- whose name I can't recall- discussing the various possibilities of different "models" of the universe arising from modern physics. The article only contained a brief sentence concerning the skew universe so I decided to ask here to satisfy my curiosity. I have had a vague idea now- I've checked for the word skew in a mathematics article and obtained the relevant information. LCS 08:32, 13 April 2006 (UTC)
- Unless its the same person, I think he is just asking about parallel universes. Check it out. --
- I think this is a follow up to the question about the perpendicular universe theory that was asked a while back--172.129.106.218 00:10, 13 April 2006 (UTC)
radiology[edit]
What is the flow of electrons in an x-ray tube?
- See X-ray; in particular, the history section. --Tardis 20:47, 12 April 2006 (UTC)
cells[edit]
Why do lysosomes function best in acidic environments?
- Maybe you should look up lysosome? I'm sure that has the answer to your homework question. --Tardis 20:56, 12 April 2006 (UTC)
- This isn't sufficiently answered on Lysosome. --David Iberri (talk) 21:17, 12 April 2006 (UTC)
- (After an edit conflict, and noting that this didn't immediately strike me as a homework question...)
- It's really the digestive enzymes within the lysosomes that function better in acidic environments. Each of these enzymes (like all enzymes) is a protein whose ability to function is dependent on its ability to assume the proper shape. If the enzyme is in the wrong shape, it won't be able to act efficiently on its substrate. For lysosomal enzymes, having the wrong shape means not being able to perform enzymatic digestion optimally. A protein's shape is governed by its tertiary structure, which is maintained in large part by hydrogen bonds between the protein's amino acids. And hydrogen bonds are exquisitely sensitive to pH. Change the pH enough and the hydrogen bonds will be disrupted, forcing the protein to assume an improper shape (called denaturation) and therefore become less active.
- This provides some measure of protection to the cell. Consider a case in which the lysosome bursts, spilling digestive enzymes into the cytoplasm. The higher pH (less acid) of the cytoplasm would render the lysosomal enzymes less active, protecting the cell from digesting itself. Hope that helps, David Iberri (talk) 21:17, 12 April 2006 (UTC)
Relativity and the speed of light[edit]
My friend posed the following scenario to me earlier: Assume that you have a highly efficient solar sail attached to a ship, and that you set the ship and sail in motion in space. Assuming the ship does not pass near enough to any highly massive objects to greatly affect its speed, and that it receives a steady supply of solar energy, will the ship ever exceed or match the speed of light?
I say no. Mass increase tells us that, even given a steady source of energy, the ship's mass would increase without bound as its velocity increases, and the ship would require infinite energy to meet the speed of light.
However, my friend tells me that, given Newton's F=MA, sufficient energy would allow the sail and ship to meet and exceed the speed of light.
I am quite confident of my own thoughts on the matter. However, I would like concrete mathematical or physical evidence to show my friend the error of his ways.
--Doubleplusungood 22:46, 12 April 2006 (UTC)
- You're entirely correct; in fact, it requires an infinite energy (and hence infinite time) to accelerate an object with non-0 mass to c , which will, of course, never happen. The kinetic energy of the spacecratft would be (see Kinetic_energy#In_relativistic_mechanics). As v approaches c, this number approaches infinity. Recommend a good book on relativity to your friend. --Borbrav 00:26, 13 April 2006 (UTC)
- He's kind of right - sufficient energy will accelerate the ship to the speed of light. It's just that sufficient energy doesn't exist in the universe... For great justice. 01:05, 13 April 2006 (UTC)
- As for concrete physical evidence, particle accelerators verify relativity all the time. You can accelerate a charged particle with changing electric fields, and as it gets closer to the speed of light, its energy grows (and therefore it acts heavier) without limit. No matter how long you keep accelerating it, it will never reach or exceed the speed of light. —Keenan Pepper 08:15, 13 April 2006 (UTC)
Hello! This is doubleplusungood again, with a few additional questions. My aforementioned friend is with me now. Here are his thoughts: 1. In the solar sail example, assume the universe is composed only of a single star and the sail device. The star is thus emitting radiation that continues constantly to hit the sail. The constant addition of energy will never reduce. Due to the absence of gravity (negligible gravity, that is), the sail will never slow down of its own right, and the continual input of energy will continue to accelerate the sail, due to conservation of energy. 2. Since light is affected by gravity (e.g. black holes), and can also be slowed down (by gravity and refraction through everyday objects, like water or diamond). Since Newton declared that only mass is affected by gravity, light must therefore have mass. In accordance with relativity, light thus cannot attain the speed of light. What is the speed of light (as light can slow down), and what is light, since it must have mass and cannot travel at a velocity of "c"? --Doubleplusungood 17:44, 13 April 2006 (UTC)
- There's a very simple explanation you're overlooking: Newton was wrong. Massless particles are affected by gravity. Chuck 20:40, 13 April 2006 (UTC)
- There are two types of particles: those with a rest mass and those without. Those with a rest mass cannot be accelerated to c, regardless of energy input, per above. Those without rest mass (e.g. photons) can only move at the speed of light, and have a "mass" that is related to the energy they carry. --Borbrav 22:10, 13 April 2006 (UTC)
- An even simpler explanation: you have to substitute the relativistic version of F=MA. Peter Grey 03:26, 14 April 2006 (UTC)
- Answering point 1) of Doubleplusungood. Let's simplify the problem: forget relativity, use only newton's mechanics. Let the Star be in point (0,0,0) and the sail moving along axis x. Newton's equation for sail, projected on axis x, will look like this:
- M*ax = -G*M*m/x^2 + C/x^2, where C is a constant
- where ax is projection of sail acceleration on axis x. C/x^2 is force, caused by star's light: as the sail moves from the star, the spacial angle, at which it's seen from the star is reduced as 1/x^2. It's just geometry. (And the light intensity is the same in the same spatial angle.) C is some constant, depending on intensity of Star's shining and surface of the sail. OK? We can rewrite the right hand of equation as Y/x^2, where Y is new constant, positive or negative. This new equation M*ax = Y/x^2, is the same equation, as the equation of a charge in electrical field. So, the answer is this: sail will never gain infinite speed. It will gain some constant speed if Y>0, and will return to Star if Y<0.
- Just, guys. Don't stop thinking and finding new problems. Currently i know better; tomorrow you'll do. :-) ellol 11:30, 14 April 2006 (UTC)
- Also, as the sail approaches c relative to the light source, the light will be redshifted further and further, constantly decreasing the energy available to push the sail, even if the light was in a coherent straight beam. Tzarius 01:36, 15 April 2006 (UTC)
- Another point -- the "slowing down" of light in refraction doesn't really have anything to do with relativity -- it's the result of the electromagnetic wave that makes up the light coupling to the electric fields in the material doing the refracting. The "fundamental speed limit" c, the speed of light in a vacuum, is unchanged. A particle can go faster than the "local" speed of light in a medium as long as it's still going slower than c. This creates the light equivalent of a sonic boom -- Cherenkov radiation. --Bth 05:43, 15 April 2006 (UTC)
Excretion and body temperature[edit]
Is it possible to lower one's body temperature by urinating or defecating? The answer is probably a resounding no, but I want to know for sure. Bhumiya (said/done) 22:47, 12 April 2006 (UTC)
- No. Poop and pee are the same temperature as the human body as long as they're in the human body. So there's no chilling or warming effect by getting rid of either of them. - Nunh-huh 23:30, 12 April 2006 (UTC)
- Not by urinating or defecation but by eating and drinking it is possible because all of that food/water has to be heated. Just look at the energy balance. Assume you take in all food and water at room temp. (25 C) for one day. Assume it comes out of your body at 98.6 F (37 C). Then, 12 C or 12 K is the difference. Now find the specific heat of the composition of urine, probably around water, which is about 4.18 J/(g*C). Approximate urine as water again and at 1 g urine / 1 mL urine
- ..then for every 1 mL of water you consume at room temp you're burning 50 J which is .012 food Calories (kcal). I think this is negligible. Even if you drank 10 liters of water a day that'd only be 120 Calories of heat exiting your body. That's nothing.
- Because I don't have an approximation of the specific heat of feces I don't know how much energy it takes to heat it, but you can bet that based on our water approximation that the energy spent to heat up the food you take in is nothing compared to the Calories in the food (or even if the food has no Calories it would still be so small as to be negligible).
- So no, I don't think it's physically possible to intake so much food and water as to decrease your body temp.
- -Snpoj 02:13, 13 April 2006 (UTC)
- I have no idea why Snpoj completely changed the question. The question was whether you can reduce your body temperature by urinating or defecating, and Snpoj concludes: "So no, I don't think it's physically possible to intake so much food and water as to decrease your body temp." Wha...?
- In any case, I disagree. First, it must be remembered that human body temperature varies depending on the area of the body. The internal organs are generally warmer than the extremeties. For example, the torso is, under most circumstances, warmer than the fingers and toes.
- Thus 98.6F is merely the mean temperature of the healthy human body. The fingers and toes are no doubt lower in temperature, and the innards, for example the bladder, is somewhat higher.
- Since urine is excreted from the bladder, it goes to reason that its temperature is somewhat higher than the mean temperature of the body, and by urinating and eliminating this warmer than average liquid, the mean temperature of the body naturally decreases. (What else would create the "shiver" effect when a guy pees?) ;-) Loomis51 00:34, 16 April 2006 (UTC)
- As a liquid, urine will evaporate, so the truly desperate might try urinating on themselves in order to take advantage of the enthalpy of vaporization. Isopropyl 08:24, 13 April 2006 (UTC)
- Or get into watersports with a friend. JackofOz 00:00, 15 April 2006 (UTC)
April 13[edit]
Perfect Secrecy in a Cryptographic System[edit]
According to Claude Shannon's paper, perfect secrecy is obtained when |P|=|C|=|K|. Is it admissable to have |C|=|P|<|K|? i.e. if I have a cipher where Pr(y|x) = Pr(x) does the size of the key matter as long as it isn't smaller than the C or P space? This is all assuming that the keys are equally possible and unique. Any help would be greatfully appreciated, C.Meyers
- Have a read of one-time pad, and consider what would happen if any part of the key longer then the plaintext is simply discarded by both sender and receiver. --Robert Merkel 07:08, 13 April 2006 (UTC)
- The statement that I have in my lecture notes from the crypto module I took a year ago says that perfect secrecy exists iff , where K, C, and P are the sets of all keys, ciphertexts, and plaintexts, respectively (and |X| is the cardinality of set X.) This is slightly different from the relation you've given, where all three sets have to be the same size. -- AJR | Talk 12:29, 13 April 2006 (UTC)
Cheese![edit]
I assume individually packaged cheese slices solved two problems:
- 1. consumer demand for pre-cut cheese (i.e. not having to cut out a slice from a big block, just like sliced/unsliced bread)
- 2. consumer demand for cheese with a longer expiration date. Individually wrapped cheese will last much longer than cheese in a block which supposedly goes bad after about 5 days because once you open it, you've opened an manufactured air-tight seal.
Question: is assumption 2 correct? (how bout 1?)
-Snpoj 02:25, 13 April 2006 (UTC)
- 1 makes sense, 2 less so. Many of the best types of cheese last a lot longer than a few days (though pre-processed ones may not, of course). In fact, many types of cheese are far better after they've matured for a considerable length of time. Grutness...wha? 03:57, 13 April 2006 (UTC)
- Assumption 2 is incorrect. My friend (who's worked with cheese professionally) tells me that cheese is pretty much good until it molds, and even then those parts can just be cut off. Also, if exposure to air was a problem, then you would want to reduce the surface area exposed. Isopropyl 08:21, 13 April 2006 (UTC)
- I'll chime in with a bit of a note on this. While cheese that is exposed to air doesn't go bad itself, it will dry out. For softer cheeses with higher moisture content, this definitely affects the texture and the quality of the eating experience.
- A minor caveat to the 'just cut off the moldy bits' approach—mold and toxic mold secretory products can travel a short distance into the cheese beyond the extent of visible contamination. Again, this is more of a concern in soft, moist cheeses. The bold are still welcome to cut off the mold and enjoy; just remember that you may want to cut a bit of a margin around the mold.
- Finally, individually wrapped cheese slices do let you avoid the mold problem altogether. Presumably, a person will only open a slice with the intent to eat it (nearly) immediately; there is no time for mold spores to settle and grow. In a sense, I'd say that assumption 2 holds because people want to avoid the perception that their cheese has gone bad, whether or not it is still safe to eat.
- The major problem with the processed, prepackaged, hermetically-sealed cheese slices is that – in my entirely subjective opinion – they don't taste very good. They're also only available in a limited number of flavors. TenOfAllTrades(talk) 12:37, 13 April 2006 (UTC)
- A good cheese will last months, though its appearance will get worse and it might frighten some people. Consider (3) it allows people who don't trust their small children with knives to still have them make a cheese sandwich (4) it provides a controlled and measured amount of cheese, rather than a rather random personal slice (5) it is easier to snack on the pack outside the kitchen. Notinasnaid 09:10, 13 April 2006 (UTC)
cgi script code demands unix?[edit]
I looked at a cgi script and it's got '$mailprog = '/usr/lib/sendmail -i -t';' in it. Does that mean it can't be used on a Windows based server? Can you get unix emulators for windows like you can get Wine for unix/linux...? --Username132 (talk) 05:02, 13 April 2006 (UTC)
- Don't know about the first question but I think this is a popular linux emulator for Windows -Snpoj 05:06, 13 April 2006 (UTC)
- cygwin is not a linux emulator. It does provide parts of POSIX though. -WhiteDragon 18:26, 20 April 2006 (UTC)
- According to [22] there is a version of exim that provides sendmail functionality. --WhiteDragon 18:26, 20 April 2006 (UTC)
- You could rewrite the script so that it uses a mail service on Windows instead (you'd have to check what mail service is available, there is no one standard). Notinasnaid 09:07, 13 April 2006 (UTC)
- Actually, I think you do have MAPI --WhiteDragon 18:26, 20 April 2006 (UTC)
PC monitors on TV[edit]
Why does a monitor on TV (like in the news) have weird bands going down it over and over? --Username132 (talk) 05:24, 13 April 2006 (UTC)
- Cathode ray tubes create pictures by scanning from top to bottom. So if you point a television camera at a CRT screen, each frame [29.97 per second in NTSC, 25 per second in PAL] will see the screen at a different part in the cycle, and a different part of the screen will look bright. -- Filliam H Muffman 06:01, 13 April 2006 (UTC)
- This is an issue that occours whenever a continuous signal is sampled too slowly with inadequate prefiltering (prefiltering visual data before sampling it is very hard because of the sheer bulk of it). A signal that the sampler is too slow to correctly capture (e.g. thats changing faster than half the sample rate) will manifest itself as a slower signal at the difference between the original frequency and the closest multiple of the sample rate. Other common examples of such effects on TV are racing car wheels (they appear to slow down and then stop again as the car accellerates) and helicopter blades (they are invisible to the naked eye but appear as black bands rotating in a semi-random way on TV) Plugwash 23:22, 16 April 2006 (UTC)
- And don't forget to read the article on the sampling theorem. Dr Zak 19:49, 19 April 2006 (UTC)
Can't ping 127.0.0.1[edit]
I run Mandriva Linux version 2006.0 and I've struggled with this problem for quite a while, namely that I can't ping 127.0.0.1 (local loopback), nor can anything make a connection to it. I can use ping to ping everything else (e.g. yahoo.com, wikipedia.org), so ping is not the problem. This is what happens when I try to ping 127.0.0.1:
[root@davidlaptop ~]# ping 127.0.0.1 connect: No buffer space available
Configuration of the loopback interface is fine:
[root@davidlaptop ~]# ifconfig lo
lo Link encap:Local Loopback
inet addr:127.0.0.1 Mask:255.0.0.0
UP LOOPBACK RUNNING MTU:16436 Metric:1
RX packets:9279 errors:0 dropped:0 overruns:0 frame:0
TX packets:9279 errors:0 dropped:0 overruns:0 carrier:0
collisions:0 txqueuelen:0
RX bytes:524961 (512.6 KiB) TX bytes:524961 (512.6 KiB)
Some people have asked me about my /etc/hosts file, but this is also normal:
[root@davidlaptop ~]# cat /etc/hosts 127.0.0.1 davidlaptop localhost
Now here is the strangest part. After bootup, I am able to ping 127.0.0.1 with no problem. However, after about 5-10 minutes, pinging 127.0.0.1 suddenly stops working, again yielding the message "no buffer space available". And although most of the time I am unable to ping 127.0.0.1, sometimes it suddenly starts working for seemingly no reason. Usually it stops working after this as soon as I stop being active on the Internet (my Internet is through interface wlan0). This problem is extremely frustrating as it causes me to be unable to test my Apache server, my FTP server or use Tor at all. Does anyone have any idea why it might be happening? I can provide more details on request. -- Daverocks (talk) 07:02, 13 April 2006 (UTC)
- These links are the closest that I could find to a solution, although there is no confirmation that the solution worked for all involved. [23] Another link is [24] they have an even longer explanation and it says solved, but I am not sure that the problem is the same. Ansell 07:54, 13 April 2006 (UTC)
- Thanks, those links are quite helpful, more relevant than the ones I was able to find. Particularly the first one you provided had helpful information. I'm still not sure what's going on, and I still haven't "definitely" solved the problem. However, something seems to be happening when I disable the lisa service and stop cupsd. Thanks for your input. -- Daverocks (talk) 01:44, 14 April 2006 (UTC)
Black Holes and Gravity[edit]
If Black Holes may possess a gravitational field so great that even light cannot escape it (except under exceptional circumstances such as Quantum tunnelling), then is it implausible to assume that a same type of mass-energy may be generated within the universe that will really allow superluminal velocities? I know that the square root within the transformations of Special Relativity turns imaginary when the velocity exceeds the speed of light, and thus explained away as making no sense, but considering the implications again of a Black Hole- if the gravitational field may be generated so great that even the photon cannot escape it, then this field, being a curvature in spacetime caused by mass-energy of some sort, must similarly lead to the possiblility of an energy level capable of exceeding the speed of light. In that case, maybe the imaginary square root may be taken to mean something even more esoteric. Or is something still missing from the story? All help appreciated. LCS 08:44, 13 April 2006 (UTC)
- is it implausible to assume that a same type of mass-energy may be generated within the universe that will really allow superluminal velocities?
- See tachyon. There's no evidence for them. —Keenan Pepper 11:06, 13 April 2006 (UTC)
- As you probably already know, travelling faster than light means travelling backwards in time. The implications of this for causality are the main reason why faster than light travel is considered implausible. But there are some more Wikipedia articles that deal with this whole subject. Try reading, as already mentioned tachyon, but also wormhole, Faster-than-light and Grandfather paradox. Simon A. 12:38, 20 April 2006 (UTC)
java[edit]
I sort of know that java is a program [to help you?] but do you need it on your computor? I get more updates to install on my M/C than I do from Microsoft and it's very persistant to get me to install them I have XP with service pack 2 installed 60.229.175.88 09:12, 13 April 2006 (UTC)
- It is useful if you want to see Java Applets on webpages, or if you want to run Java based programs on your computer. The Java download here [25] enables this. Ansell 09:32, 13 April 2006 (UTC)
- Java is often used to show you part of a web page. You probably have it already. Bear in mind that Microsoft offer you updates because you already have an older version of the software and if they are security fixes it may be very important to install them. Notinasnaid 09:33, 13 April 2006 (UTC)
- Never ever accept Microsoft Updates. You'll just be putting a lot of hard-working hackers out of business. Please, someone, think of all the poor hackers. --Kainaw (talk) 12:02, 13 April 2006 (UTC)
- Untrue, Kainaw : the old game betwen fortress and cannon must go on. If flaws are not corrected, then poo' hacker is unemployed. --DLL 18:01, 14 April 2006 (UTC)
snake[edit]
My daughter wants to know from which part of snake's body its eggs come out ? Not the process of mating or reproduction but somthing like from where exactly the baby comes out.
Thanks
- At the risk of stating the obvious, the vagina. (Google produces some fascinating results, but you may not want your daughter to see them). HenryFlower 09:34, 13 April 2006 (UTC)
- The Snake article has a reproduction section that doesn't exactly entail where the eggs come out, however, it does explain a bit about some snakes which do not lay eggs, rather keeping them inside them and/or giving birth to live young. I assume the position that they lay eggs from would be about 2/3rds of the way down the body, however I can't seem to find any sources for that right now. Ansell 09:40, 13 April 2006 (UTC)
- This page claims a video, which I have not watched: http://www.arkive.org/species/ARK/reptiles/Coronella_austriaca/more_moving_images.html. Notinasnaid 09:49, 13 April 2006 (UTC)
- You can't see the whole body of the snake in the video, so it's hard to judge, but I'd say it's about 3/4 of the way down. On the bottom, of course. Interestingly our vagina article mentions that the vagina leads to the cloaca 'in some reptiles', but doesn't say which reptiles. HenryFlower 09:59, 13 April 2006 (UTC)
The same question was asked and better answered at "Ask A Scientist" - Zoology Archive - Snake Delivery - 1/19/2004:
- "most snakes lay eggs (oviparous) some of them deliver a baby, outside the eggshell ready to live (viviparous). The reproductive system of the reptiles including the snakes are simpler than the one of the mamals and resembles the birds. There are both in males and females an opening to the exterior called "cloaca". The cloaca is the passage from a internal chamber into which the digestive, urinary and reproductive systems empty. So both the baby snake or the non-hatched egg go to the exterior through the cloaca. Incidentaly, inside the female snake body there is a tract called oviduct provided with conditions for egg fertilization and embryo formation." [26] WAS 4.250 11:32, 13 April 2006 (UTC)
The human brain[edit]
I’ve was told that researches were made about our brain’s function that found that when we see things and when we imagine things, the brain uses the same parts. Meaning that our brain cannot differ between what it sees to what it remembers/imagines. Can you please guide me to those researches? Thank you
- There was an article all about this in the February/March Scientific American Mind pages 18-23. Called "Picture This." by Thomas Grueter. It refrences:
- Image and Brain: The Resolution of the Imagery Debate. Stephen M. Kosslyn. MIT Press. 1996.
- Return of the Mental Image: Are There Really Pictures in the Brain? Zenon Pylyshyn in Trends in Cognitive Sciences Vol. 7. No. 3, pages 113-118; 2003.
If you can't find it on the internet, I can scan in the article for you without any trouble. See my talk page User_talk:Mac_Davis. Here is a search for zenon pylyshyn cognitive [27].
--Mac Davis] ⌇☢ ญƛ. 10:22, 13 April 2006 (UTC)
A myth or a true story?[edit]
I was told that the Indians couldn’t see Columbus’s ships arriving because they didn’t have that image in the image bank in their brain. Is it a myth or a true story?
- Hahahahah! It is a myth. Just because you haven't seen anything before doesn't mean you can't see it! How do you think you see things that you haven't seen before? --
 Mac Davis] ⌇☢ ญƛ. 10:13, 13 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 10:13, 13 April 2006 (UTC)
- It's true. I kept telling 16 year old girls that I cannot see their nakedness because I do not have that image in the image bank in my brain and so if they take off all their clothes then all I can see is a blank spot where their nakedness is. Ohanian 10:33, 13 April 2006 (UTC)
- Do they believe you? I would think not, but then again people may be more guillible than I think. --Bowlhover 01:53, 15 April 2006 (UTC)
- I heard that story. I believe it was from a documentary on Amerigo Vespucci. In his novel about the New World, the native women were beautiful, big breasted, and extremely dumb. The men were stupid and lazy and handed over tons of gold and silver to the white men to have sex with their women for them. His story was, obviously, not written from real accounts. --Kainaw (talk) 12:00, 13 April 2006 (UTC)
- Definitely a myth. This story has been spread a bit by the movie What The Bleep Do We Know!?, a "documentary" which is not at all scientifically accurate. rspeer / ɹəədsɹ 15:35, 13 April 2006 (UTC)
- I believe "What the BLeep" got the idea from some anthropologist's writing. I am trying to track down whose. Any body know? I KNOW this story was out there before that movie came along. Plus, the movie explains it badly and exaggeratedly. --GangofOne 19:06, 13 April 2006 (UTC)
- I'm not entirely sure about the truthfulness of What the bleep do we know, but a similar thing I heard about when going through the topic of evolution comes to mind, I'll try to retell the story briefly:
- An explorer went to live with a jungle-dwelling people, who obviously had never left the jungle, he befriended them and decided to take one of their people to his world. On their way to a city, they crossed a savanna, the short jungle-dweller (being short is a nifty adaptation for jungle life) spotted a couple of grazing animals in the distance, he recognized them as flies, and tried to wave them away with his hand.
- The reason for this is that the jungle-dwelling man had never ever seen anything so far away, and his concept of distance was limited to perhaps five metres away at most, as jungles don't really have that much open space. In a sense, he could not possibly imagine something that big being visible from that distance, and could thus not know that things look smaller with distance. -Obli (Talk)? 21:38, 13 April 2006 (UTC)
- I'd be suspicious of that one too. Most stories about the silly things "savages" do are false at best and insidious at worst. --Fastfission 01:36, 14 April 2006 (UTC)
- There are many reports of pygmy tribes from the forest who have difficulty working with directions that go over long distances and gawking at wide-open plains. It is really no different than Americans failing to comprehend a kilometer and gawking at fields covered with green instead of cement. --Kainaw (talk) 12:50, 14 April 2006 (UTC)
Well, it's definitely false. Of course you can see things you've never seen before. When you were born, you didn't have any images in the "image bank" of your brain. Does that mean you're going to be blind for life? --Bowlhover 18:34, 14 April 2006 (UTC)
- Cognitively blind. If you don't have a concept for something, you don't register it. Babies are such, until they are taught, (by which I mean interactions with reality, not in school). Concepts, that is to say, bundles of recurrent sensations that have been reified, are then defined in terms of other concepts available to the developing conscious being. However, if you have bad conceptual scheme through which you, as adult, perceive everything, then you are also insulated from the new; this is also a tragedy, for which the cure is claimed to be various spiritual/psychological processes that are available for a reasonable fee (classes now forming), but generally just substitute one conceptual scheme for another. Anyway, there are many examples from psychology experiments. They flash a playing card for a fraction of second, to be identified. Well, the trick is they make a black King of Hearts, say, but the experimentees CAN'T SEE IT, and say King of Hearts , or King of Spades , and NOT WHAT IT IS. That black K of H is like the ships of the Europeans to the indigious; doesn't fit into their conceptual schema, at first. This general state of affairs is definitely NOT a myth. Read The Social Construction of Reality for starters. --GangofOne 22:05, 14 April 2006 (UTC)
- Hmm, not entirely comfortable with that exposition. Babies may be said to be cognitively blind, but only because it takes many months before "the penny drops" and the baby suddenly understands that he/she is separate from "other things". Until then, to the child there is only one all-encompassing "thing". That's not to say they can't see and desire to touch physical objects, but they still don't make any cognitive distinction between themself and the object. But when a Spanish galleon appears in the harbour, the 15th century American native definitely sees it and definitely knows it is a strange object external to themself. Whatever name they give it would simply reflect their lack of a frame of reference at that time. We refer to UFOs as "flying saucers" too, but I'd bet that's not what they are. JackofOz 23:52, 14 April 2006 (UTC)
- Gangofone, are there any psychology experiments in which participants are shown something bizarre, something they haven't seen before, and the person conducting the experiment asks the participants if they can see the object? The experiment you gave as an example is irrelevant--the participants can't see the card very well if it's only flashed across their eyes, so they take whatever they managed to see and compare it with what they know about poker cards, to find the card that matches best. If the participants were given a chance to carefully look at the cards, I'm sure they could see that the king of hearts is black (for example). --Bowlhover 01:53, 15 April 2006 (UTC)
I've never seen a black king of hearts in my entire life. Does that mean that if I was shown one it would be invisible to me? Before I saw Star Wars for the first time, I had never seen a wookie before. Why wasn't Chewbacca invisible to me? The whole idea is definitely a myth, and not even a very believable one at that. Loomis51 00:55, 16 April 2006 (UTC)
- "I've never seen a black king of hearts in my entire life. Does that mean that if I was shown one it would be invisible to me?" A rather strange argument, especially for a lawyer. I don't have all the details of the card experiment from Psychology 101, probably could find it somewhere. The experimentees who don't see the black king of hearts, are not asked "Did you see that?", they are asked "What did you see?" They have no difficulty identifying the red K of H or other regular cards. If they are allowed to examine the deck, and are thus clued in, THEN they have no difficulty recognizing the black K of H etc. So it's not the breifness of the exposure that is preventing them from seeing the reality, because they see reality otherwise just fine; the problem is in their concepts of reality. The story is not about optics, it's about concepts (preconceptions) and perception. --GangofOne 07:00, 16 April 2006 (UTC)
- I've never read the original paper before, but here it is: http://psychclassics.yorku.ca/Bruner/Cards/ Classics in the History of Psychology "On the Perception of Incongruity: A Paradigm", Jerome S. Bruner and Leo Postman (1949) Harvard University. First published in Journal of Personality, 18, 206-223. --GangofOne 07:10, 16 April 2006 (UTC)
- I see this experiment is also mentioned on p62 and 112, Kuhn ,THe Structure of Scientific Revolutions, 1962 . "Until taught by prolonged exposure that the universe contained anomalous cards, they sww only the type of cards for which previous experience had equipped them... What a man sees depends both upon what he looks at and also upon what his previous visual-conceptual experience has taught him to see..." Also mentioned in this CIA textbook for analysts: http://www.cia.gov/csi/books/19104/art5.html Chapt 2 "Perception: Why Can't We See What Is There To Be Seen?", mentions Jerome S. Bruner and Leo Postman, "On the Perception of Incongruity: A Paradigm," in Jerome S. Bruner and David Kraut, eds., Perception and Personality: A Symposium (New York: Greenwood Press, 1968).
- I believe it was Columbus who wrote in his log that he saw mermaids, but they weren't as beautiful as expected. Some say he maybe saw manatee, but maybe he really saw a swimming wookie. --GangofOne 10:12, 16 April 2006 (UTC)
The original question asked was if it was true that the Indians could not see Columbus' ships arriving as they had never seen large ships before. The question seems to me to clearly have implied that the Indians saw nothing, i.e. the ships were invisible. However, if the question is whether or not the Indians recognized these large objects that they did in fact see to be ships, then the answer would possibly be no. But that doesn't seem to be what the questioner was asking. The question was whether the Indians were able to see Columbus' ships at all, which clearly they were. Loomis51 13:37, 16 April 2006 (UTC)
- I concur. If you have never seen a key before and someone showed you a brass one, you would say that you saw a brass object, but you would definitely not say you saw a key, because the concept in words did not exist for you and you don't have the word in your vocabulary anyway. Luthinya 10:08, 17 April 2006 (UTC)
Computer Network Tockin Ring[edit]
Dear All,
I have some confussion related to Computer Networking below theories/terminology, If anyone can clear me below points. than I highly be thankfull.
- 01: Why saying? "Token Ring" is Local Area Network Technology
- 02: Why "Token Ring" is not inlist in Channel Access Methods?
- 03: Please tell me difference between "Network technology, Access Method and Network Protocol"
Thanks.
M.Sadiq Qadri [enough with the personal info]
- Why do you care whether a "token ring" is a LAN or not. As long as it's implemented correctly it will work. The only time, I care about the definition of "token ring" is when I need to hand in my computer science assignment at University. Ohanian 10:29, 13 April 2006 (UTC)
- Token ring is it's own access method. Think about it, terminals can *only* speak when they have the token. The Token Ring article does quite a good job at describing it. --Jmeden2000 13:16, 13 April 2006 (UTC)
- Token Ring is a LAN technology, it just uses a different structure for determining which system is transmitting data. In fact modern Token Ring systems use the same physical infrastructure as Ethernet. The reason Token Ring is not commonly used is 1) Originally IBM propriatary technology. 2) More expensive due to 1. You may want to look up Local Area Network, Token Ring. If you want more detailed information on Networking I would suggest you by a used copy (new is $65+) of a Comptia Network+ exam. As these guides cover the basics of Networking and a lot of the terminology of Networking.--Tollwutig 15:00, 13 April 2006 (UTC)
Fundamental forces, "weak charge"[edit]
Electromagnetism is the coupling of photons to electric charge. Gravity is the coupling of hypothetical gravitons to all energy. The strong force is the coupling of gluons to color charge. The weak force the coupling of W and Z bosons to... what? My physics book mentions "weak charge", but doesn't go into any detail and doesn't say what kinds of particles have it. I notice we don't have an article on it either. Is there really such a thing as "weak charge"? —Keenan Pepper 11:21, 13 April 2006 (UTC)
No fundamental weak charge exists. "Originally, weak interactions were thought to be separate from electromagnetic forces. Eventually, three fairly massive particles were discovered: W+, W-, Z. The weak force is an electromagnetic interaction producing one of these particles. The particle travels then is absorbed through electromagnetic interactions with another particle. Although the Z-particle has no electric charge, it does have a spin. It can interact with magnetic force. Standard electromagnetic force is transmitted by photons of light. Weak force is transmitted by these "weak" particles. Still the actual interactions are the based on the same force." [28] WAS 4.250 11:47, 13 April 2006 (UTC)
- All leptons and quarks with left-handed chirality are susceptible to the weak force. Notably, as a result it's the only force to affect neutrinos, and the only force that can cause flavour changing processes (thus it's responsible for beta decay, though note that the Z boson cannot cause flavour changing -- there are no flavour changing neutral currents). See weak interaction for lots more. But as per WAS's quote there isn't an identifiable "weak charge" as such. --Bth 12:00, 13 April 2006 (UTC)
- See Weak hypercharge. I'm guessing that that corresponds to the weak coupling constant, or maybe it's weak isospin. Cedders 00:30, 14 April 2006 (UTC)
- Well, I still don't understand it, so I guess I'll just have to wait until I take... Particle physics? Quantum field theory? What? —Keenan Pepper 01:04, 14 April 2006 (UTC)
- The thing is that the weak force comes out the other side of the spontaneous symmetry breaking of the electroweak interaction. At "high" energies, the electroweak force couples to weak hypercharge. At low energies, the EM force couples to electric charge and the weak interaction is the mess that's left over when you separate that out from the electroweak interaction. As such the thing it couples to is rather complicated and messy (it doesn't couple to weak isospin, really: weak isospin is a symmetry of the weak interaction the way isospin is for the strong). We could really do with a page on Wikipedia that serves as an introduction to the all the gauge stuff, though -- you could argue that all these articles need more context for the non-specialist than they currently have.
- Just for the record, though, I took a few particle physics courses, and I never really got a good sense of the weak force coupling to some specific property (though maybe I wasn't paying enough attention); it was always taught primarily in terms of what it did. --Bth 09:30, 14 April 2006 (UTC)
CDMA 2000[edit]
Qualcomm says CDMA 2000 provides an always-on Internet connection. What does it mean and how does it work? How is always-on different from other type, that is not always one in previous cell phone technologies?
- I presume it means it is always connected, like broadband, rather than only connected when you connect it, like dial-up. For great justice. 15:40, 13 April 2006 (UTC)
- Some unconventional ISPs may claim they are "always-on" when in fact they only connect when information is transferred. Normal ISPs can't claim that because idling would be impossible and they'd get a lot of pissed off customers, but that kind of service is very plausible for cell phone technology.
[edit]
What cultural bias exists towards western economic philosophies as thsy relate to the IT and Software industry in India?
- Did the homework question also misspell the word they? hydnjo talk 14:56, 13 April 2006 (UTC)
- The instructions above for answering questions specifically states to be nice. Spelling flames and assumptions about the origin of the question (without even answering the question) isn't very nice. As to the question, googling '"western economic philosophy" india software' got nothing, but '"western economic" india outsourcing' got lots of interesting hits, including:
and lots of others. I didn't find anything on Wikipedia, but maybe someone else will.--Anchoress 21:24, 13 April 2006 (UTC)
Width of the United States[edit]
How many miles wide is the United States? I could not find this statistic in United States nor Geography of the United States. Thanks. -- Reinyday, 17:35, 13 April 2006 (UTC)
- This question is not very well-defined. First we must specify what portion of the Earth we designate as "the United States". But it's not very interesting to say that the US is typified by the distance between Hawaii and Maine, as that's certainly not representative. So let's suppose you mean the contiguous United States. Obviously even that is not a rectangle (even disregarding the curvature of the Earth), and so does not have a single "width" or "height". But presuming that by "width" we mean "east-west extent", we can talk about the lengths of various lines of latitude running through the country (these will be of many different lengths). As a very rough approximation, using the Extreme points of the United States article, I see Lubec, Maine at 66°59′5″ W, and Cape Alava at 124°43′59″ W. They're not at the same latitude -- about 3° different -- but it's close. The portion of the latitude line midway between theirs (about 46°25′ N) between their longitude lines is (using the quadratic mean radius of )
- A similar expression using San Diego, California and Miami, Florida (though they are 7° apart in latitude, so this is a poorer approximation) gives 2237.4 mi. The best one can really say from these figures is that the "width of the US" is . (Remember that these are not great-circle distances; the shortest route from (40°N,90°W) to (40°N,80°W) is not to go due east. But the differences at these latitudes are on the order of a percent or so; the errors in averaging the latitudes are larger.) --Tardis 18:20, 13 April 2006 (UTC)
If you define the US in terms of effective control, we control land and water all across the globe, and indeed into space. If you define US as legal sovereign teritory by law, our embasseys are technically our sovereign territory, and again we are global. If you define US as States and territories we entend from at least Maine to Guam and probably further, but I'm not gonna look up every island the UN assigns us control over. The distance of the two points in Alaska that are furthest from each other is interesting and similar to Canada and Russia useless distances. It is all about definition. Something to ponder: When you find the right way to ask a question, the question is the answer to itself. WAS 4.250 20:43, 13 April 2006 (UTC)
- Or you could go from Hawaii to Florida, or Hawaii to Alaska, or Florida to Alaska for even longer distances. --
 Mac Davis] ⌇☢ ญƛ. 23:58, 13 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 23:58, 13 April 2006 (UTC)
This is the cousin of a question I asked about a year ago and never got much of a response. It's about crows flying within the borders of a country. How would one go about accurately determining the greatest "straight-line" distance between any two points within a contiguous area? E.g. a glance at a map suggests the longest line within the 48 contiguous US states might be from somewhere on the west coast near Seattle to somewhere on the east coast in Florida, but that's very rough. Is there a more accurate measurement? Does a list of longest crow-flights by country exist anywhere? JackofOz 23:22, 14 April 2006 (UTC)
- You basically want to know the diameter of the circumscribing circle for whatever area (great-circle diameter for a circle on the surface of the earth, of course). (Note, however, that if the area is concave, the longest line may leave the area, which you might not want; it hardly counts as a long line in the U.S. if it goes through Mexico!) Certainly such a determination is possible, and the answer is well-defined up to such annoyances as erosion and altitude, but I know of no "easy" algorithms to do it. I think if you treat the area as a planar polygon (which might be okay for small areas on the earth), there's some sort of linear algebra approach. --Tardis 20:01, 19 April 2006 (UTC)
Fractal Structures in natural landscapes may also cause you necessary problems. Luthinya 12:06, 18 April 2006 (UTC)
Protiens and Sugars[edit]
Where might I find a list of the important protiens and sugars that build the human body? Specifically, something that will help me in understanding how humans decompose at teh chemical level. 64.198.112.210 20:20, 13 April 2006 (UTC)
List of proteins WAS 4.250 20:30, 13 April 2006 (UTC)
- A list of proteins and sugars won't give you what you need. Try decomposition, though it's not too detailed, it may get you started. There was a book called something like Corpse that discussed this, too, but I can't quite think of the exact title. - Nunh-huh 20:57, 13 April 2006 (UTC)
- I agree that a list won't be much help. What happens chemically will depend on what's causing the decomposition - bacteria or microorganisms will be quite different to what happens in cremation. You might take a look at Amino acid, Denaturation_(biochemistry), Glycoprotein, and Chemical_makeup_of_the_human_body. There's also decomposition Putrefaction and rancidification. Looking from the point of view of preventing decomposition there's the Mummy article. Richard Taylor 04:13, 15 April 2006 (UTC)
- Thank you very much! 64.198.112.210 19:20, 19 April 2006 (UTC)
Bomb defusal[edit]
I was watching a certain movie over the weekend, and it contained a scene where a bomb is being defused Hollywood-style: a guy with wirecutters clipping the red wire. Does this have any factual basis? Is there a history of bombs being defused in this fashion? Isopropyl 22:50, 13 April 2006 (UTC)
- It's the correct way to defuse a Hollywood-style bomb: one with a half-dozen sticks of dynamite wired to an alarm clock. Our bomb disposal article covers how real-world bombs are taken care of. --Serie 23:55, 13 April 2006 (UTC)
- Thanks! Isopropyl 02:42, 14 April 2006 (UTC)
I don't know, but I'd like to add a similar question. What is the big, red LED readout for on such Hollywood-style bombs? Is it there for the convenience of those who just may be standing by the bomb, so they'll know when they'll be blown up? If so, that's very considerate of the bomb designer, don't you think? Erik the Rude 23:20, 13 April 2006 (UTC)
- I have another question: why do bombs on TV often beep (they also have a red LED that flashes) before exploding? I'm guessing that it's for the same reason as what Erik the Rude said--so that people who happen to be near the bomb have time to escape. --Bowlhover 17:41, 14 April 2006 (UTC)
- Tradition. The first design for a clock-based time bomb consisted of a mechanical alarm clock with a percussion cap or other impact-based explosive attached to one of the bells as a detonator. Because of this, if the detonator took a few hits to trigger, you'd hear the alarm ringing before the bomb went off. --Serie 20:16, 14 April 2006 (UTC)
April 14[edit]
Ramjet[edit]
I was wondering if anyone could give me a few really nice links on how to build Ramjets? Patrick Kreidt
Dermatographic urticaria[edit]
Is this disorder genetically inherited? --218.102.207.71 00:55, 14 April 2006 (UTC)
- In general, Urticaria (also known as hives) is not something that can be passed to others, be it either genetically or through contact. However, if there is something causing this reaction, that stimulus may be transmissible. For the most part, hives are caused by allergic reactions, and the propensity for allergies can be passed on genetically. Therefore, in a situation where two individuals both are allergic to a substance, if that substance is transfered via physical contact from one to the other it may appear that the urticaria was transferred.
There is another type of urticaria that is not a syptom of an allergic reaction. These hives can be brought on by periods of extreme emotions. This type of urticaria tends to be more prevalent in certain populations, suggesting that it can be passed on genetically. Emotionally induced urticaria, however, usually do not last more than a few minutes and very rarely require treatment of any kind, prophylactic or otherwise.Tuckerekcut 03:12, 14 April 2006 (UTC)
- I don't think anyone knows for certain if dermatographic urticaria is genetically inherited. It may be due to the development of one's particular immune system which is a product of one's environment and genetics. - Cybergoth 04:45, 14 April 2006 (UTC)
Combining CC-BY-SA[edit]
I wanted to combine a CC-BY-SA 2.0 and CC-BY-SA-2.5 image. Is there any changes between the two licenses that would make this impossible? I assume this is doable, but I wanted to make sure, as not to create a copyright violation. Thanks in advance, Linuxerist L/T 01:14, 14 April 2006 (UTC)
- CC-BY-SA 2.0 says that you can use later versions of the same license (CC-BY-SA), so it shouldn't be a problem as long as you license the resulting image as CC-BY-SA 2.5. --Fastfission 04:11, 15 April 2006 (UTC)
Darn search engines[edit]
What is the name of the theory that the act of observing changes the behaviour of the subject being observed? -- Francs2000 ![]() 01:27, 14 April 2006 (UTC)
01:27, 14 April 2006 (UTC)
- I think this is what you mean: Uncertainty_principle but according to that page "The uncertainty principle is frequently, but incorrectly, confused with the "observer effect", wherein the observation of an event changes the event. The observer effect is an important effect in many fields, from electronics to psychology and social science.". Not sure if I completely agree with that statement but there you have it. -Snpoj 01:46, 14 April 2006 (UTC)
- Thanks for that. What I was actually looking for was the Hawthorne effect because it applies to social sciences, but your link led me to it. -- Francs2000
 01:51, 14 April 2006 (UTC)
01:51, 14 April 2006 (UTC)
- Thanks for that. What I was actually looking for was the Hawthorne effect because it applies to social sciences, but your link led me to it. -- Francs2000
male midwives[edit]
how many men are there in midwifery in UK? thanks.robyn
- This doesn't look like an especially reliable source, but according to menstuff.org there were 87 male midwives out of 35,000 or so practicing midwives. That's data from ten years ago, though. Isopropyl 03:00, 14 April 2006 (UTC)
- Hmm, this site says 10.73% in 2005. Isopropyl 03:02, 14 April 2006 (UTC)
- How many men in midwifery are there in the other countries of Europe except in UK?thanks a lot.robyn
- How many men are there in midwifery in some other european countries (except in UK).thanks.robyn
- How many men are there in midwifery in some european countries, except in UK?thank a lot.robyn
- How many men are there in midwifery in Finland?in Netherlands? thanks a lot.rob
- I'm afraid I know nothing about the subject, but I'd just like to say that 'midwifery' is one of my favourite words. Thank you. Phileas 05:43, 14 April 2006 (UTC)
Invision power board[edit]
Is it possible to export/move an invision power board to another host, either by condensing it to a core file somehow, or using some sort of util? Any response is appreciated. -Tim Rhymeless (Er...let's shimmy) 05:27, 14 April 2006 (UTC)
Please could you clarify your question? It is possible to export the post data from an Invision Power Board to another forum (say phpBB for instance) But to point you towards the appropriate utility you would need to say what forum software you are hoping to resolve your data onto. --Limeyuk 19:43, 14 April 2006 (UTC)
- Well, I've been looking at either setting up a new Invision Power Board, or going to phpBB. There's a bit of a constraint on time. I don't know what I'm doing, but I can relay any complicated info onto people that do. -Tim Rhymeless (Er...let's shimmy) 05:02, 15 April 2006 (UTC)
Is this a hoax?[edit]
Has anyone heard of the term isodiasphere (according to the article, describing two nuclides with the same neutron excess) before?
I came across the article while rooting through Wikipedia:Cleanup and am tempted to prod it as an OR neologism (or a subtle hoax, assuming less good faith). The phenomenon it describes seems real if obvious enough, but the term doesn't appear anywhere I can find except Wikipedia and its mirrors. It's not known to Google Scholar, it appears nowhere in the nuclear physics preprint servers, it's not in the index of Krane (ISBN 047180553X), and it doesn't even appear on the site listed as an "external source" by the article.
It's not linked anywhere in Wikipedia except from Chart of Nuclides, and that link was added by the originator of the article (User:Esotericv2), who has no other contributions. But I just thought I'd check with all the knowledgeable people here first. --Bth 07:57, 14 April 2006 (UTC)
- I did a search for this term in Chemical Abstracts which contains the abstracts of nearly every publication in the world covering chemistry and the major journals covering physics. It did not locate any mention of that term. --Ed (Edgar181) 12:48, 14 April 2006 (UTC)
- Based upon the link for this, I expect it would not be a chemistry term, but one for Nuclear Physics. I did find this link
http://sun.folk.en.ogarnij.info/en/Isodiasphere
- Chem Abstracts covers the major journals in the area of nuclear physics, so that shouldn't be an issue. The link you provide is also just a copy of the English Wikipedia article. So still, as far as I can tell, there is no use of this term apart from the article here. --Ed (Edgar181) 14:46, 14 April 2006 (UTC)
- I nominated this for deletion. I think it's nonsense. --BluePlatypus 17:42, 14 April 2006 (UTC)
Space-Time Curvature under General Relativity[edit]

This is a question about how non-scientific explanations of the Theory of General Relativity are supposed to explain gravitation. Layman explanations of space-time curvature usually have a diagram (apparently always the same one) which shows a two-dimensional surface streched (downwards) into a third dimension at the location of a body having mass. The story goes that a second body going by with no acceleration will follow a curved path because space-time is curved by the first body.
What I don't understand is why this would cause one body to curve towards another rather than away. When I try to figure out what the curved path will actually be (from the diagram), it looks like the it should curve away. Apparently, it is implied that the second body would fall into the 'depression' in the original two-dimensional surface because of.... what? the influence of gravity? It's apparently using gravity to explain gravitation. How is this explanation supposed to work? The Wikipedia article hints it's not a simplistic as the picture, but doesn't seem to go beyond that.
- If someone is able to post a complete and thorough reply to this question, please forward it to the Nobel Prize committee. Seriously, though, the curved sheet model just takes advantage of the fact that objects rolling around on a big sheet in a 1 G field happen to move in a way similar to what general relativity predicts for objects moving past planets and the like. It's meant to show you how things move, not why things move. To really understand why things move, you will have to understand nasty things like metric tensors and the Einstein field equations. -- Filliam H Muffman 07:24, 7 April 2006 (UTC)
- Well.. I don't quite see why you'd think it would curve away. If you have a downward "dimple" in a tablecloth or similar and roll a ball towards it, moving in a straight line, it will go around the rim in a curved path once it hits the dimple and continue away in a straight line in a different direction, having been deflected somewhat inwards. That analogy is the point of the picture. If the dimple was raised instead of lowered, then it would be deflected in the opposite, outwards, direction. But gravity doesn't act in that direction, which is why you've got a lowered dimple and not a raised one in the picture. --BluePlatypus 07:54, 7 April 2006 (UTC)
- The diagram also gives a nice visual analogy for the inverse square law, since the gradiant of the curve is much steeper near the object than far away. But like the others have said, this is a way of visualizing how the objects would move so, not why. — Asbestos | Talk (RFC) 13:36, 7 April 2006 (UTC)
- In other words, it's just a cool picture? It's describing the exactly the same behaviour as Newtonian gravitation. Peter Grey
- Pretty much. It's also meant to give an idea that general relativity deals in spacetime curvature, which, among other things, predicts that light is also affected by gravitation. That makes gravitational lensing possible. Newtonian gravity claimed that light doesn't bend because it's massless. -- Filliam H Muffman 03:03, 10 April 2006 (UTC)
- One of the byproducts of Special Relativity has been the famous equation, E= mc², that first stated the fact of mass and energy being only two sides of the same coin, instead of what previous physicists have conceived. While Newton had previously thought light to be massless and thus insupsceptible to gravitation, nonetheless certain details within the mathematical framework he had produced for gravity as a force did predict a certain amount of bending occuring when light approaches gravitational bodies (a third less than what Einstein later proposed), which, although he knew was true, was never able to explain away with his theory. The new definition in modern physics, now, for the word massless, also meant something different. It refers only to those particles with no rest mass, but not necessarily no remaining kinetic energy whatsoever. Due to Einstein's equation, as previously indicated, you may say that light does have mass if you wish to, since its energy could be easily converted to mass via the constant of c². However, the energy of the photon is constantly changing during its flight, so the records for mass you will be able to obtain will never be invariant, thus referred to as the relativistic masss. In modern physics, however, it is no longer considered appropriate to define the particle on terms of its relativistic mass for obvious reasons, and thus it suffices to say that light is massless in the fact that it has no rest mass. Nonetheless, as long as the photon still possessed the kinetic energy/relativistic mass necessary for its existence, then it shall be supsceptible to curvatures in space- time like other particles with rest mass. Luthinya 10:21, 10 April 2006 (UTC)
- Erm, where have you got that "third less" from? The Newtonian prediction for the deflection of light around a point mass is half the value given by GR. --Bth 10:34, 10 April 2006 (UTC)
Sorry, got my figures wrong. Thanks for correction. Luthinya 10:45, 10 April 2006 (UTC)
- This kind of picture is indeed confusing, but there is another reason for it that hasn't been addressed fully above. The key is the mechanism by which "curved spacetime" affects the motion of objects (particles, satellites, light, whatever). General relativity says that an object moving only under the influence of gravity follows a geodesic in spacetime, which is the analogue of a straight line in Euclidean geometry. Since the spacetime itself is curved, these "straight lines" have properties we do not expect, but they are, in a precise sense, the "least curved" lines you can have within curved spacetime.
- Roughly speaking (see below), one of the important properties they share with Euclidean straight lines is that they are the shortest distance between two points. This means you can picture a geodesic in the following way. Take a curved surface, such as the dimpled fabric surrounding the ball in the relativity diagram asked about above, and fix two points, one to be the "pitcher" and one to be the "catcher." We then want to draw the geodesic on the curved surface connecting the two points, representing the path of an object from the pitcher to the catcher. We do this by using a rubber band, stretched taut: this automatically follows the shortest path between the points.
- Now suppose we look at the result as viewed from above. If there were no curvature, the rubber band would be a straight line between the two points. Since the ball is there, dimpling the surface downward, the rubber band will not take a path that appears straight as viewed from above, since that path will be rather long due to the dimpling. Instead, the rubber band will curve around the ball slightly, to avoid the trip into the depths of the dimple. When seen from above, it appears that the ball is affecting the path of the object with some "force", when in fact the object is trying to follow the best approximation to a "straight line" that it can in the circumstances.
- All that is rather hard to say without additional pictures, hope it comes across. Anyway, very little of that is usually included with the usual picture, which is why it is easy to come to very inaccurate conclusions about what the picture is trying to say. If you want a better version of all this, look at Taylor and Wheeler, Spacetime Physics.
- Note for experts: in spacetime, with its Lorentz metric, geodesics are actually local maximizers of proper time; but part of the point of the kind of picture we're discussing is to give a Riemannian picture. --Spireguy 20:12, 11 April 2006 (UTC)
- Still, for most layemen beginners, it does offer a much more approcheable beginning for the subject, even if much of the important details have been left out. As far as the geodesic problem is concerned, since we as humans possess four dimensions (including Time), and yet space- time may curve in a way that is impossible for us to conceive or imagine- only "talk about", it immediately shows that space- time curves into at least one more dimension than that to which we are accustomed of in our daily life, i.e. the 4D bodies. And just as the 2D figures upon cardboard cannot stand up vertically upon it, since they have no motion or conception of 'depth', we as 4D people cannot cross the barriers of dimension and traverse into the 5th or other higher dimension freely, as we now have the ability to in our 4D world. We therefore have to kind of traverse with the curvatures of the surface of higher- dimensional space- time, which is to us expressed in a 4D fashion like differently laid card boards are to the 2D beings. Thus, for us, the easiest way between two places in space- time may not necessarily be a straight line, but more usually a curved 4D geodesic adapted from the curvatures of space- time from gravity. Luthinya 20:34, 11 April 2006 (UTC)
- That's what I'm getting at. Isn't a path curving away from the point mass shorter? What we would need to visualize would be a contraction of space, not a stretching, right? Peter Grey 04:25, 12 April 2006 (UTC)
- Bear in mind that we're measuring "shortest" in the coordinate system defined by the grid drawn on the sheet. The deformations of the sheet deform the coordinate system itself -- that's the whole point of the analogy. What looks longer to us from outside the sheet is shorter, when measured in a system where the side of a "square" on the sheet is a constant however stretched it looks to us. --Bth 11:12, 12 April 2006 (UTC)
- The shortest path "in the coordinate system" is the straight line. Or is something still missing in the story? Peter Grey 16:13, 12 April 2006 (UTC)
- Um, no. In order to walk the "straight line" in which you are depicting, we must abandon the 4th dimensioned (including Time) curvature of the "surface" of the grid (space- time), and seek to traverse into extra dimensions in order to ignore the curved influences of gravitational objects. However as 4D beings this is pratically impossible for us, so instead of this the shortest route for us will have to be the the curved surface of the grid, which at least is 4D and possible to traverse. Therefore, instead of what Newton has previously proposed, the shortest route between two things is not a 'straight line' in the usual sense of the word, but a geodesic varying according to the shape of surrounding space-time influenced by gravitational fields. At least a longer way is shorter than the impossible, so to speak. The thing is that on the diagram you cannot see the grid curving into extra dimensions the way it should, which is what makes the analogy slightly hard to come to mind. Remember the analogy is only a start to understanding; try eventually to draw away from the picture and just let the ideas flow accross your head- aided by some mathematics, you'll find this much easier.
PS May I add that my above language is extremely inaccurate in depiction, especially without the mathematics to compensate for it. Hope it comes through anyway. Luthinya 18:17, 12 April 2006 (UTC)
- The point I'm trying to underline is that, unless gravity is assumed (as part of the illustration of gravity), then there is no 'up' or 'down', and the diagram should demonstrate the same behaviour whether the deformation is 'down' or 'up'. Peter Grey
- I'm afraid I cannot quite catch your point. What exactly do you mean by down or up? The deformation is curved into extra dimensions for which we may only talk of, yet never be able to obtain direct conceptions of for which we will understand properly since, as I said before, we cannot cross the barriers of dimensions. One should also be careful between the shortest path and the easiest path. Traditionally, we tend to think that the shortest path between two things, usually a "straight line", is also the easiest path to traverse between the two objects in question. However, because of this simultaneous involvement in meaning our brain has got accustomed to thinking that the shortest journey must necessarily be the easiest one to achieve, taking seemingly the smallest period of Time (if you can put it like that). Unfortunately, this stops being true as soon as we step out of the world of the Greek Geometres- the flat world of 2Ds where the curvatures made we can entirely ignore, since we are one dimension (in Space) higher. Already in the 3D world we are encountering trouble. When a tourguide shows you the fastest way to cross a mountain, he shows you the curved path just by the foot of the mountain and fitted to its topological curvatures. However, who would not agree that the shortest way is actually to tunnel through the mountain centre itself, and come upon the other side? True, this may be the shortest way, but it is by no means the easiest. Far easier it is to adapt to the geodesic of the landscape than to delve in it.
Space- time itself possesses more dimensions than our race, so delving becomes completely impossible. All we can do now is follow, once again, the geodesics of the landscape, and trust them to be our easiest way. (or trust maths- whichever)There will be no talk of picking or choosing either- all objects follow intuitively the easiest way to traverse between two points. When there is a stone that curves a rubber sheet downwards the ant naturally walk as close to the stone as possible in getting its way round to the other side, not even waste time climbing or delving into the stone. The same it is for us- we naturally follow the curvatures already made in the fabric of space- time. When a gigantic gravitational body curves space-time into extra dimensions unreacheable, the easiest way to cross to the other side is to the follow the geodesics and walk as close to the body as possible, giving the effect of being "gravitated towards it", rather than away from it where the journey will naturally be harder. Luthinya 09:55, 14 April 2006 (UTC)
- There seem to be two different answers emerging:
- The picture is simply hinting at the connection with geometry. The illustration assumes a vertical force of gravity, and shows geometry causing the attraction to a point mass. That's interesting because it is bridging our intuition about gravity (basically one-dimensional) and attraction to a point mass in two dimensions, but it's also 'cheating' since one force of gravity is being explained using another force of gravity.
- The more sophisticated version is that the curvature causes acceleration of a point moving along a geodesic. However, if this is a behaviour due only to the geometry, and there is no assumption of Newtonian gravitation, then there is no up or down, and it's not immediately obvious if a particle in motion would be deflected away from the point mass, or towards it. Peter Grey 19:48, 14 April 2006 (UTC)
- It seems that we're all talking at cross purposes to one another. Gravity is not explaining gravity; it's illustrating it. These are two very different things, at least to me. -- Filliam H Muffman 00:17, 15 April 2006 (UTC)
- I think you may need to clarify what you mean by explaining gravity. The above image shows a simplified visual model of gravitational acts, supplemented to which will be a corresponding mathematical model, along with the verbal analysis itself. These together form collectively what we term as a model of gravitation according to Einstein's Relativity (universally acknowledged as one of the most beautiful models on the subject ever), where instead of understanding it as a force, Einstein chose to interpret it as a curvature in spacetime. In short, as a model was always supposed to be, it is an aid to us in interpreting the results physicists discover throughout their observations in spacetime, and in a way of fitting this into a general system of theories, whereby we have kind of explained why things work the way they do, and how they work the way they do, if you follow me.
- But if you wish to explain gravitation in the terms of how it existed "behind the scenes", then I'm afraid not a single human being in the history of creation will ever be able to help you. For all we know, gravitation may just as well not exist behind the scenes, because in being a model of the Nature which physicists try ever so hard to understand, it cannot replace nature for what it actually is. It is only a symbol of Nature, and symbols are different from real existence, merely being a re-presentation of it. Gravitation has been a useful model in attempting to interpret the universe, and by its own terms we have found a suitable fashion of "explaining it" in such a way as we can, yet we can never establish a concept that may capture it as it is "behind the scenes", as it is that -which- is; because, like David Bohm said, no idea can ever capture reality in the sense of that- which- is. It'll be a bit like trying to describe the Tao in Chinese Taoism- utterly impossible except through experience, and that does not describe. After all, reality is not cogi, ego sum, for the simple reason that the concepts and models so important to the human mind are our own hazards and guesses based on what nature would give, but never ultimately nature herself.
- So if your question was concerning how one could explain gravitation "behind the scenes", then a thousand Nobel laureates will not be able to help you, even if they all had IQ 180. Einstein once said that the universe was like an unopenable watch, and we who gaze in wonder at its workmanship will begin ourselves to haphazard our way through how it might have worked. But, he concluded, we know that whatever path we may cleave may certainly be dramatically different from how the watch really worked, nor can we even imagine the consequences of such a comparison. Luthinya 10:29, 17 April 2006 (UTC)
- This is not asking for the ultimate theory of gravitation, but for the justification of the typical picture in non-technical descriptions. "Explanation" is perhaps a poor choice of words. The question is: is the picture assuming a linear force of gravity, and if so, does it have any merit as an illustration of gravity? Peter Grey 15:24, 18 April 2006 (UTC)
- Pedantic correction: in Riemannian manifolds (positive definite metric tensor), a geodesic is the shortest curve between any two sufficiently nearby points on the curve. In pseudo-Riemannian manifolds (indefinite but nondegenerate metric tensor), a timelike geodesic arc is the longest timelike curve between two sufficently nearby events on the curve. (Oops, just saw that Spireguy already said that.)
- Luthinya wrote May I add that my above language is extremely inaccurate in depiction, especially without the mathematics to compensate for it. Agree with that, unfortunately I must dash what he said next: Hope it comes through anyway. Don't worry, Luthinya, your heart was in the right place.
- Peter, I consider your summary inaccurate, except for The picture is simply hinting at the connection with geometry.
- I have to say I found this discussion almost incomprehensible, no doubt because precision of expression is so important in a subtle topic and because those who have learned a good deal of gtr share a common background which involves not only mastery of specific mathematical topics like tensor fields but also various examples illustrating crucial distinctions which untrained persons are unlikely to spot, like intrinsic versus extrinsic curvature (failure to distinguish between these is clearly evident in some of the confused discussion above). If I might recommend a nontechnical book: see Geroch, General relativity from A to B. Don't be discouraged by the (not inaccurate) title: if you study this, you will learn a heck of a lot.
- One last point: the goal of gravitation physics is not to explain gravitation in the sense many laypeople appear to have in mind; rather physicists hope to model gravitation, even under extreme circumstances. See for example Theories as models. This is why physicists are happy to use Newtonian gravitation unless conditions are so extreme (or measurements so sensitive) that general relativity is required. At some point, everyone expects the advent of well defined quantum theories of gravitation, and these may allow us to model gravity accurately under conditions even more extreme than gtr can handle. We can expect that such a theory will be hard to use, and that it will continue to make sense to use gtr unless we really really need the new theory. Since the way that one thinks of gravitation in Newton's theory, Einstein's theory, and very possibly in yet unknown quantum theories of gravitation are completely different, this clearly shows that physicists are much more pragmatic than laypersons tend to recognize. In popular books, many authors to tend to pander to rather than correct the idea that theories can be simply right or wrong. The truth is far more complex and to my mind far more interesting than that. HTH ---CH 05:28, 21 April 2006 (UTC)
Excel problem[edit]
The default settings for the general format has changed into a percentage. When I start a new excel spreadsheet and type a number (eg 5), and press enter, the number appears as '0.05'. When I go to format cells, it says that the catergory is 'General'. Any idea on how to fix it? It's Office 2003. Thanks all. - Akamad 12:19, 14 April 2006 (UTC)
- Go to Tools/Options, Edit tab. Uncheck the "Fixed Decimal" box. --LarryMac 15:15, 14 April 2006 (UTC)
- Thank you very much. - Akamad 23:54, 14 April 2006 (UTC)
pepper pad[edit]
Have anyone used the pepper pad? (no, I am not going to buy it, but just want to know about it) I just want to know whether we must have to use the scroll button most often to move left and right? because the screen is just 6 inches wide? or is it enough since we keep the screen horizontally and read? Is the font size small and is a small font size enough since we keep it near our eyes compared to computer monitor?
Life expectancy of LCD vs Plasma HDTV operating 24 hours a day[edit]
I'm looking to upgrade a large scale video surveillance setup with a high definition video wall consisting of 12 - 50" monitors capable of displaying any number of cameras simultaneously, yet have the ability to display one camera streatched across all 12 monitors if I so choose. I cannot justify spending large chunks of budget money replacing LCD or plasma screen TV's every 6 months because either they can't handle being on 24 hours a day, or images have been burned into the phosphorous (LCD) thereby ghosting out the display.
I can hold out for the next generation if need be but I'd buy them now if they could last 2 years (17,500 hours) in that type of environment. I cannot seem to find a spec sheet outlining continuous use...I hope you can
- [32] seems to say the industry promises around 20,000 hours of life out of a plasma screen. But it also says that burn in may be an issue. Anecdotally, at work we have LCD screens that are on 24/7 I know of one that has been in place for two and a half years will no ill affects. The image changes regularly though. --Chapuisat 19:25, 14 April 2006 (UTC)
Tree Height - Why Is the Sky Not the Limit?[edit]
Given species and environment, trees reach a peek height. What is it that prevents trees to grow ever taller?
- The same reason why humans dont go on growing tall forever... The height depends on the strenght of the tree and a tall tree is no use if it cant stand on its own properly. Jayant,17 Years, India • contribs 16:40, 14 April 2006 (UTC)
No that's not it. Humans are genetically designed to grow up to a certain height, and stop growing at a certain age of maturity. Trees don't work that way at all. A tree tends to continue growing until it dies, which leads to another interesting question: Do trees have lifespans? or do they only die of disease or other pathologies?Loomis51 16:40, 16 April 2006 (UTC)
- The main reason is not structural consideration but water. You have to get water to the top of the tree. See the cohesion-tension theory. I notice that this article is not that great in wikipedia so you should probably do a google search. In summary, there are a couple of factors that limit the ability of a tree to pull water from the soil to the top. 1) The strength of xylem vessels to with stand the negative pressure creating by "sucking" the water up. 2) the amount of force available from transpiration to pull water to the top of a tree. This is limited by the maximum tension that can be exerted on the water column (determined by the cohesion between water molecules and the adhesion between the water molecules and the inside of the xylem vessel).David D. (Talk) 16:43, 14 April 2006 (UTC)
- This is all interesting, and admittedly, much of it I don't understand. Nonetheless, the explanation seems lacking in one main respect: scale. It would seem to me, the larger the tree, the stronger the xylem vessels would be, the stronger the force available from transpiration etc... The only answer I can think of as to why trees don't continue growing forever is that the eventually die, which leads me back to my above question concerning whether or not trees have natural lifespans. Loomis51 16:40, 16 April 2006 (UTC)
- Why would it not be a structural consideration as well? (in the evolutionary context) A tall tree has a higher center of gravity and may topple more easily. I don't think there are a lot of evolutionary advantages to being extremely tall. --BluePlatypus 17:33, 14 April 2006 (UTC)
- Once again, this argument ignores the relevance of scale. Of course the taller the tree, the higher the center of gravity, but then again, the taller and older a tree is, the thicker its trunk is, thus compensating for the higher center of gravity.Loomis51 16:46, 16 April 2006 (UTC)
- Evolutionary advantages 1) Tall trees get access to unlimited sun. 2) Shade out the competition. The reason it is not a structural issue is that the load bearing forces on the wood of trees could with stand much taller trees. Clearly there are structural limitations but in most cases it is the transport of water that is the limiting factor with respect to height. Obviously we could also discuss root architecture (shallow vs deep) and susceptability to wind since this would be more important for some species. Another issue might be if a tree is in a forest or standing alone and soil conditions could make a difference too but i think this is beyond the scope of the original question. David D. (Talk) 18:02, 14 April 2006 (UTC)
Water transport can be accomplished by active molecular pumping at the level of individual cells. If need be, there could be chains of cells passing the water from cell to cell all the way up a tree. Plants do not need to depend on long single columns of water from roots to leaves. However, transport of molecules requires energy. When a tree gets very tall, a large percentage of its energy will be used for transport of molecules. Growth will slow and eventually something will destroy the tree before it reaches "the sky".....structural weakness if nothing else. --JWSchmidt 16:56, 16 April 2006 (UTC)
- I'm not sure this is feasible even if there was unlimited energy. This requires diffusion of water through cells and would be too slow. Bulk flow is necessary to move water any distance in a timely manner. Also what active transport mechanisms are available for the movement of water? I thought aquaporins work passively? Are you thinking of some other transporter? David D. (Talk) 20:13, 17 April 2006 (UTC)
With respect to User:Loomis51 argument of scale. Consider if you have to pull water up a xylem and that the weight of the water column increases as the tree gets taller. This is the scale issue where the bioological system breaks down. More weight means more pull is required. More pull means a greater negative pressue in the xylem especially atthe top of the tree. At some point the xylem cannot withstand the negative pressure and air will enter the xylem tube. This is called cavitation and the air will expand massively in the negative presure since there is no cohesion for air molecules. Thus, that particular xylem becomes 'blocked' with air and no more water can move up that vessel. So there is a scale related limitation at the level of the integrity of the xylem vessels. David D. (Talk) 20:26, 17 April 2006 (UTC)
- Fair enough, but now I'm curious about my follow-up question. Do trees (or other plants) have natural lifespans? Is it theoretically possible to prune a tree in such a way so that it can live indefinitely or is there some finite limit to how long a tree can live? Loomis51 04:26, 18 April 2006 (UTC)
- I think a well managed tree, also known as a hedge, can easily be encouraged to live for a long time. It could subcome to disease but other than that it should be able to keep growing due to the indeterminate nature of meristems and the abundance of meristems (aka lateral meristems). Some bristle cone pines (?) in CA are over 4000 years old. David D. (Talk) 04:48, 18 April 2006 (UTC)
- Fair enough, but now I'm curious about my follow-up question. Do trees (or other plants) have natural lifespans? Is it theoretically possible to prune a tree in such a way so that it can live indefinitely or is there some finite limit to how long a tree can live? Loomis51 04:26, 18 April 2006 (UTC)
- While a single tree may be kept alive through stemming and cloning, any organism that wants to survive the evolutionary race eventually needs to sexually reproduce and then die at some point in time, at least according to the Selfish Gene theory. László 07:52, 21 April 2006 (UTC)
Telephone problems[edit]
There are 2 telephones using my phone line. About 13 hours ago, I picked up the telephone in my room, and instead of hearing the dial tone, I heard "Please hang up immediately and try your call again. This is a recording." I hung up the phone, waited for a few seconds, and picked it up again. I heard the hang-up beeps. I again hung up, waited for several seconds, and picked the phone up. I heard loud beeps, the same beeps that are heard when you don't hang up but the person on the other end did a long time ago.
It seems that a telephone using my phone line is not hanging up. But there are only 2 telephones connected to the phone line, and nobody was using the telephone not in my room.
As of now, when I pick up either phone, there is no dial tone but only a low buzzing noise. This noise is similar, though a bit louder, than the sound you hear during a successful call but when nobody is speaking.
What is likely the cause of these problems? Why did another phone line, used by the family sharing my house, fail too? I'm guessing that the problem is with the place where all the phone lines meet (in a "control panel" in the basement), since both lines failed nearly simultaneously (but the other one works now--my line still doesn't). --Bowlhover 18:17, 14 April 2006 (UTC)
- I suggest there may be a problem with one of the phones. Disconnect them both, take a nap then connect one and see if it works. If it does, connect the other one and see if both work now. Your comment that the other phone line also failed suggests the problem may be the control panel in the basement like you thought.
- Oh, Bowlhover, what have you been doing to draw the CIA's attention to yourself? (heads off in search of tinfoil hat) --Bth 18:41, 14 April 2006 (UTC)
- LOL, I also suspected the problems might be because of a wiretap. But which wiretapper leaves their device on for hours, preventing their "victim" from making phone calls? Anyways, my phone line works now--I don't know why, and I don't know when it started working. --Bowlhover 23:42, 14 April 2006 (UTC)
- This is not very common with phones. It is an old modem problem - the modems can connect, but they fail to disconnect. By chance, do you have a computer also plugged into the line? As for the sounds, they are common. The first message is a pre-recorded message that tells you to hang up. The next is a loud beep to get your attention. Then, the line is disconnected at the phone company's end and you get nothing by static. --Kainaw (talk) 18:48, 14 April 2006 (UTC)
- No, none of the computers in my house are connected to a phone line. Also, it's not a loud beep I hear after the recorded message--it's a series of beeps that you get every time the person on the other end hangs up, followed by another louder series of beeps telling you to hang up immediately. --Bowlhover 23:42, 14 April 2006 (UTC)
- Your lines are buggered. Time to haul out the old phone and call the phone company. I had a problem with my phone broadband. Turns out that when the house was renovated, the bell wires had been folded over from one end of the house to the other. We had to put in a new straight line, since DSL hates folds.--Zeizmic 13:15, 15 April 2006 (UTC)
- Something was obviously holding the line open, could have been a line fault, could have been one of the phones, could have been a poor attempt at a wiretap as has been suggested. If it happens again try the phones seperately. If that fails borrow another phone to try and if that fails its time to look at the wiring. Plugwash 00:35, 20 April 2006 (UTC)
Is Oxygen poisonous?[edit]
O^2, more precisely. I've been told that it is actually poisonous and the information about oxygen poisoning here on Wikipedia doesn't explain how it occurs very well so I'm rather confused about it. Also, is it possible to live on pure oxygen or are the other elements of air necessary for a human to respirate?
- Everything is poisonous in large enough quantities. The concept of 'poison' is basically to do with the quantity. People need it, but can have too much of it. Do you have some specific questions about the oxygen poisoning article? For great justice. 20:48, 14 April 2006 (UTC)
- You can live in an atmosphere of pure oxygen, but bad things can happen -- Apollo 1
- The 'poison' concept means that free oxygen is poisonous within the body. That is why great pains are taken to encapsulate oxygen with iron - Hemoglobin. --Zeizmic 20:52, 14 April 2006 (UTC)
- "Poison" means "too much" more than "this is poison and that isn't". Vitamin A in tiny quantities will kill you and in even tinier quanties is necessary. Everything if taken in sufficient quantity is poisonous.
- At low pressure, like is used at high altitudes and in space, 100% oxygen is fine. No other gas is needed by the body
- The higher the pressure (the deeper in the ocean of air or water you are) the less time one can breath a given percent of oxygen. To not be poisoned by oxygen when deep sea diving the time must be short or the oxygen reduced in percentage. WAS 4.250 21:15, 14 April 2006 (UTC)
- Oxygen is poisonous to certain bacteria that evolved before there was oxygen in the atmosphere. This group of bacteria is called, for obvious reasons, "anaerobic bacteria". They only live in closed spaces, like in deep wounds. For them, hydorgen peroxide, which releases oxygen, is poison, ie a "disinfectant". So what is poisonous to a given species , like possible extraterrestial species, depends on the paritcular biochemistry of that species. GangofOne 21:26, 14 April 2006 (UTC)
- My understanding is a small quantity of carbon dioxide is necessary to keep a person breathing. Buildup of CO2 is what triggers respiration. In an atmosphere of pure oxygen this does not happen and a person will pass out.
- No, the body creates it own CO2. WAS 4.250 21:33, 14 April 2006 (UTC)
- My understanding was the CO2 in the body would be liberated in a non-CO2 atmosphere so fast the respiration would slow down and thus, less CO2 would be generated. This would result in a vicious circle sort of situation.
- CO2 in the atmosphere (compared to your lungs) is already very low, removing it completely would not increase the concentration gradient by that much. Also, the CO2 is a result of metabolism in the cells so its production would not slow down if there was less CO2 in the lungs.David D. (Talk) 22:16, 14 April 2006 (UTC)
- My understanding was the CO2 in the body would be liberated in a non-CO2 atmosphere so fast the respiration would slow down and thus, less CO2 would be generated. This would result in a vicious circle sort of situation.
Breathing oxygen can be poisonous to humans.
This is probably caused by the excess production of superoxide, a highly reactive molecule with a free electron which can interact with other cellular molecules and distort or destroy them. One site where these superoxides are formed is in the mitochondria, the power production plants of eukaryotic cells. It is estimated that, in normal cicumstances, up to 5% of oxygen flowing through a mitochondrion can end up as superoxide, instead of being incorporated into the the normal end products of glucose metabolism, carbon dioxide and water (aerobic cellular respiration).
Superoxide combines with other cellular molecules to form reactive oxygen species or free radicals (these two are not necessarily the same), which are also highly reactive.
The problem with free radicals is that they typically cause chain reactions, i.e. superoxide damages molecule A, which becomes a free radical and damages molecule B, which becomes a free radical and damages molecule C, and so on. Fortunately, these harmful free radicals would normally be inactivated by enzymes such as superoxide dismutase and catalase, and any damage done is usually repaired before the body suffers. They are never the less implicated in the process of ageing.
In the case of oxygen toxicity, or poisoning, an increased flow of oxygen through the mitochondria leads to an increase in superoxide. A high enough increase for long enough, can cause superoxide levels to become so high that the free radicals and the resulting chain reactions cannot be held in check by the normal cellular countermeasures. The proteins, fats and even the genetic molecules (DNA and RNA) of the cell are damaged, and the cell loses its normal function, or even dies.
Two effects are most prominent:
1. The lungs become stiff, congested with fluid and inflammatory exudate, and eventually scarred, so that it becomes difficult to breathe and oxygen cannot get through to the to the blood in the pulmonary capillaries (respiratory distress syndrome). This is caused by direct cell damage and cell death, as well as changes in production of surfactant. It can be permanent and cause death.
2. The person develops fits, identical to grand mal epileptic fits. This could be due to damage to cells as such, with swelling and generalised disfunction, or to alterations in nerve transmission – either changes in neurotransmitter release or changes in the receptors for those substances. As in epilepsy, fits can cause further brain damage, physical injuries or breathing problems associated with loss of consciousness.
In humans, symptoms and signs of oxygen toxicity usually occur only after breathing oxygen with a partial pressure of greater than 60 kPa for 24 hours or longer (lower concentration are probably safe for very long periods). The higher the partial pressure, and the longer the exposure, the more the damage done.
Damage to the lungs can occur with ordinary supplementary oxygen use in hospitals, when more than 60% concentration is used for extended periods, while fits are more commonly seen under hyperbaric (high pressure) conditions, such as is encountered in deep diving or in hyperbaric chambers.
In premature babies, high oxygen concentrations are associated with damage to the eyes (retinopathy of prematurity), which can lead to blindness. There are probably more risks to the babies’ health, such as protracted lung problems and even an increased incidence of leukemia, but these have to date not been precisely defined.
Note that free radicals and reactive oxygen species are essential for normal functions. They are for instance used by the body to destroy bacteria, or for cellular signalling, so that they are in themselves not “bad”. It is the excess that harms.
Humans do not need any gas other than ogygen to respirate. Specifically, we do not need to inhale any carbon dioxide. However, if one breathes completely dry gas, especially if the gas bypasses the nose, respiratory secretions tend to dry out and thicken, increasing the risk of infection or plugging of airways in the lungs.
Seejyb 20:00, 15 April 2006 (UTC)
April 15[edit]
Animals and Babies[edit]
Do animals think like we do? Is it possible that back long ago animals used to talk? Do babies think like we do? I mean like if they are eating bananas at the age of one and don't know how to talk might they be thinking about how they hate the banana?
- Category:Animal communication? Melchoir 02:00, 15 April 2006 (UTC)
- Oh, and Category:Developmental psychology. Melchoir 02:07, 15 April 2006 (UTC)
Humans don't even think like one another. WAS 4.250 03:13, 15 April 2006 (UTC)
- Most animals would definitely not think like we do. In fact it was recently proven lobsters are not evolutionarily developed enough to nocicept. However, animal lovers are frequently completely sure that their dog is feeling lonely, happy, or confused. It is a common tendency for people to think of inanimate objects as having human-like characteristics, in fact it is inherent, and one of the most basic ways of learning. A baby copies what its parents do. I can't find the link, but there is an article on here somewhere about different levels of emotion. Primary emotions are like fear, and contentness, the most basic. Secondary emotions are more complicated, and include confusion, and depression. --
 Mac Davis] ⌇☢ ญƛ. 05:11, 15 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 05:11, 15 April 2006 (UTC)
- I sort of feel compelled to point out that animals aren't "inanimate objects", and that it's well-known that animals have emotions. It's also quite clear that lobsters are capable of nociception: the question that remains open to debate is to what extent that nociception is accompanied by emotional distress. The idea that humans have emotions and animals don't is, frankly, rather silly from an evolutionary standpoint. Our emotions didn't spring from nowhere, and emotions have been quite clearly described in various animals. - Nunh-huh 09:07, 15 April 2006 (UTC)
I think the word 'emotion' is not helping us here: to me it implies things like love, or anger, which I very much doubt (other) animals experience. If we can agree that my cat is not as emotionally developed as me, but that it does have some form of consciousness, then the only uncertainty is as to the degree of difference. My best guess is that my cat can experience distress, contentment and pleasure, all of which I think are induced by physical stimuli of one sort or another, rather by feelings of love for me or hate for the neighbour's cat. I doubt that animals get much more emotionally developed than that, though I haven't met many gorillas or whales. I'm quite sure that some animals are less developed. HenryFlower 11:22, 15 April 2006 (UTC)
Linguists don't have a definite consensus, but one theory holds that the language faculty is principal difference between humans and other animals, and language is the fundamental medium of human thought. Animals, even those with communication, do not have the language faculty, and infants less than two years have only a rudimentary language ability. So although they might learn an association between a food and negative emotions, they could not be thinking about how they hate in the normal sense of 'thinking'. Peter Grey 11:39, 15 April 2006 (UTC)
- Francis Galton once wrote an article about why dogs were so great as pets -- they seem to have very similar emotional faculties as humans, and as such we can understand them pretty easily (it is easy to tell when a dog is happy, sad, angry, excited, hungry, bored), and they can understand us pretty easily (they understand tone of voice, facial expressions, they can tell when we are unhappy, etc.). He then, quite humorously, alluded to the fact at how rare this is, with a line something along the lines of "Who ever imagined shooing away a mosquito with a disapproving look, or pacifying an angry wasp with a winning smile?" In any case, with emotions in man and aninmals, I don't think one can draw too many really hard-and-fast difference between the two as categories go, with the only potential instance being language, but even then I am not sure the verdict is in on whether or not certain forms of communication amongst animals don't involve some sort of language faculty or not. --Fastfission 18:30, 15 April 2006 (UTC)
I would say that some animals exhibit all the emotional range of humans, with other primates being the closest. Even some single celled animals appear to exhibit fear and aggression. However, symbolic logic seems to be almost exclusively human. That is, the ability to think in words is not shared by animals. Some people with delayed language skills can likely recall what it's like to think without words. A series of pictures is sometimes how it's described. For example, Helen Keller didn't learn to speak sign language until later in childhood so would have recalled thinking without words, as animals do. StuRat 22:01, 15 April 2006 (UTC)\
- I agree with that. The ability to describe or express a feeling is what turns a feeling into an emotion. I would say that only humans have emotions, because only humans have language, but most sensate beings (including plants) are known to have feelings (which we tend to refer as 'instincts' in the case of animals). JackofOz 02:11, 16 April 2006 (UTC)
- I disagree. According to your def, Helen Keller had no emotions until she learned how to sign. I also disagree that plants have feelings. StuRat 09:01, 20 April 2006 (UTC)
- OK. Scrub the "plants have feelings" bit for now (although I'm amazed we don't have an article on Cleve Backster - he's certainly become notorious in the scientific world, which in my view qualifies as notable).
- But yes, Helen Keller did not have emotions until she learned to sign. Of course she had feelings, and of course she knew what those feelings meant to her even if she couldn't articulate them to another person (or even to herself). But that, in my understanding of the words, is what differentiates a feeling from an emotion. People tend to use the words interchangeably, but I think this is a mistake. JackofOz 10:24, 20 April 2006 (UTC)
- Do you have a source for your def: "The ability to describe or express a feeling is what turns a feeling into an emotion" ? StuRat 00:29, 22 April 2006 (UTC)
Drug Ratings[edit]
I work at a pharmacy and sometimes when I read pharmacutical magazines, I notice it say "AB rated" on some of the drug advertisements. I think there are other ratings also, but I can't remember. What does it mean? —Preceding unsigned comment added by 68.171.53.125 (talk • contribs)
- Those are Therapeutic Equivalent Evaluation Codes that are used by the FDA to denote therapeutic equivalence to other pharmaceutically equivalent drug products. A drug which is AA-rated or AB-rated to another indicated that they are considered interchangeable by FDA standards and can be substituted unless the physician designates otherwise. Sometimes a number is appended (AB1, AB2, AB3, etc). Another designation, BN-rated, means "not equivatlent". see [33] - Nunh-huh 04:54, 15 April 2006 (UTC)
Organ[edit]
Which of our organ, distributes the nutrients which we (humans) gain from the digested food, to different parts of our body according to their needs?
- The majority of digestion and absorption takes place in the small intestine - where the acidic broth from stomach is completely digested. Polymers such as proteins, polysaccharides, nucleic acids, and lipids are broken down into their monomer components by hydrolytic enzymes. These monomers are then absorbed through the lining of the small intestine into capillaries. These capillaries drain into the hepatic portal vessel, which drains into the liver. The liver has the metabolic versatility to interconvert various organic molecules, so the blood that leaves the liver has a very different composition than the blood that enters the liver. Wapatista 08:52, 16 April 2006 (UTC)
- But the thing about the question that struck me was that the questioner seems to think there might be an organ overseeing appropriate distribution of nutrients, and there isn't. To some extent, the body has an ability to preferentially "feed" the brain on the basis of producing fuel preferentially used by the brain, but otherwise it's pretty much a case of every organ for itself. - Nunh-huh 09:01, 15 April 2006 (UTC)
- Per Dalembert's comment below, the body does have at least some ability to regulate the delivery of nutrients and oxygen to different tissues and organs through manipulation of the cardiovascular system. Certain triggers (a shortage of oxygen, a spurt of epinephrine (adrenaline), heat or cold, etc.) can trigger the constriction or relaxation of blood vessels and changes in heart rate, regulating the amount of blood reaching particular parts of the body. There's no single organ that controls the cardiovascular system, however; it's a complex system that responds to a lot of different inputs. To name a couple, blood pressure is regulated by baroreceptors mostly in the aorta and the carotid arteries; epineprine comes from the adrenal glands that sit on top of the kidneys. TenOfAllTrades(talk) 15:57, 15 April 2006 (UTC)
- So the body parts control delivery of nutrients by consensus :) WAS 4.250 16:22, 15 April 2006 (UTC)
- In a sense it is the liver, as it alters blood composition to what it is standard and therefore utilised in the body. It is also the circulatory system, as this distributes nutrients throughout the body. And in some sense it is the various organs of the endocrine system, as this releases hormones throughout the body responsible for regulating metabolism and uptake of materials by cells. Wapatista 08:52, 16 April 2006 (UTC)
The literal answer to the question, though, is simple: it is the cardiovascular system that distributes nutrients to all the parts of the body. Dalembert 14:26, 15 April 2006 (UTC)
People, look at the question. Which organ distributes nutrients to different parts of the body? Not "allocate" or "regulate" or "parse out", but simply "distribute". No debate here. Nutrients get from one organ to another in the blood, propelled by the heart. Period. Cardiovascular system is the only, literal, single correct answer. As much as I respect the liver, brain, and endocrine system, the blood flows for a purpose, and transport of nutrients, gases, and signals is that purpose. alteripse 12:16, 16 April 2006 (UTC)
- "According to their needs" - the cardiovascular system isn't involved in regulation, so it's worth mentioning the other organs that do this, and also helpful to build up the "bigger picture". Although you are right. Wapatista 13:07, 16 April 2006 (UTC)
Physiological differences between white and red muscle[edit]
What are the differences between white muscle, for example, fish and bird muscle, and red muscle, for example mammal muscle? i've never come across a satisfactory answer for this question. My basic assumption is that it has something to do with blood supply to the muscles, and if so, how does this relate to function of the muscle? Wapatista 07:38, 15 April 2006 (UTC)
- Muscle fibers come in different varieties. One, called the fast fibre, fast twitch fiber, Type II fiber, fast-twitch glycolytic fiber, or white muscle fiber, contracts quickly after nerve stimulation. They result in powerful contractions, but they fatigue rapidly because they contain few mitochondria. Another, called the slow fibre, Type I ffiber, slow-twitch glycolytic fibre, or red muscle fiber, contracts three times more slowly, but they fatigue less easily because they contain more plentiful mitochondria, and extensive capillary supply, and myoglobin.
- Most muscle contain both types of fiber, but they are in different proportions. In animals that have "white meat", the type I fiber predominates; in those that have "red meat", the type II fibers do. - Nunh-huh 08:57, 15 April 2006 (UTC)
- The article corresponding to what Nunh-huh is saying is muscle fiber.-gadfium 09:13, 15 April 2006 (UTC)
- Thanks a lot. Wapatista 08:54, 16 April 2006 (UTC)
Properties of PVC[edit]
I am looking for a (simple) table/explenation of the physical properties of PVC (PolyvinylChloride). Thanks. —The preceding unsigned comment was added by 203.122.88.32 (talk • contribs) .
- Polyvinyl chloride. You see, there's this search box on the page, and there's somewhere else on the page that says ... oh, forget it. Confusing Manifestation 11:01, 15 April 2006 (UTC)
- Maybe someone could make a template to automatically suggest a search so it doesn't need to be typed it out manually in the future? --Username132 (talk) 01:53, 16 April 2006 (UTC)
embedded systems[edit]
what is the ato —The preceding unsigned comment was added by 202.65.149.74 (talk • contribs) .
- Possibly the Australian Taxation Office? Or the Agricultural Trade Office? In Japanese the ato is the time after now! freshgavinΓΛĿЌ 06:22, 17 April 2006 (UTC)
Which tablet PC?[edit]
I am buying a tablet PC. I use the computer all the time to surf the net and also read news. I prefer to use the tablet like a slate and read because that is comfortable for me compared to using it like a laptop. What size tablet should I buy? If I buy a 10.4" tablet, will it be too small to browse the web? (Note here that I plan to use it in the slate format and not like a laptop). Or will a 10.4" be good enough size to browse websites? Should I scroll frequently in a 10.4" tablet to read pages? Or should I try 12 inches?
- I would say you need excellent eyesight to make out a reasonable amount of text at 10.4 inches. I suggest you test it out on a few of your favorite websites before buying it. StuRat 19:12, 15 April 2006 (UTC)
Convert HTML to JPEG or GIF[edit]
Can I Change The Whole Internet Page (HTML) To Picture Format (JPEG or GIF ) ?
- Well, you can certainly take a screenshot or your browser window. I suppose, if the page is too long, you can scroll down and take more screenshots and stitch them together in a paint program. There may well be programs that can automate this process, but if you only want to do this for one or two pages, this might be the simplest solution. —Ilmari Karonen (talk) 20:01, 15 April 2006 (UTC)
- Another solution might be to print the page to a file, open the file in a paint program, and save it in whatever format you like. Of course, this assumes your paint program can read whatever format the "print to file" option produces on your computer; if you're lucky, it's PostScript, if you're not it's some proprietary printer-specific format. —Ilmari Karonen (talk) 20:05, 15 April 2006 (UTC)
- You can do this easily with Ulead Photoimpact. Just open the page and then export it to an image file. -Halidecyphon 20:49, 16 April 2006 (UTC)
One may also simply print/export as a pdf file and open in photoshop or photoshop elements. 70.183.234.163 17:38, 20 April 2006 (UTC)Thisdude415
C++ cin stream failure[edit]
Imagine I write the following as part of main():
int n = 0; cout << "enter a number please: "; cin >> n;
And compile and run the program. Everything works great unless the user types in a letter, in which case the cin stream fails and the program ignores any further attempt to use cin.
How do I get around this problem? Thanks, JD --128.248.77.71 21:14, 15 April 2006 (UTC)
- There are several things you can do, depending on whether you just want to detect the condition or recover from it. Take a look at [[34]].-gadfium 00:19, 16 April 2006 (UTC)
cinis anistream, it is reading input up until white space. Once it reads a letter, it is in an error state; you clear it withcin.clear(). But what you probably need is more sophisticated input parsing. Peter Grey 12:17, 16 April 2006 (UTC)
- Simply using
cin.clear()is not enough, as the letter typed is still in the input buffer and the problem just reoccurs with the next attempt to read a number. You therefore need to both clear the error condition and read the unwanted characters into a throwaway buffer, or else usecin.ignore(...)to get rid of the letter(s). The link I gave above explains this much more clearly than I could do.-gadfium 20:20, 16 April 2006 (UTC)
- Simply using
eMule causes DNS failure[edit]
Can anyone tell me any hint of why this is happening? It only happens in Windows, in Linux it's fine.
- Specifically, I bet you have a Netgear ADSL router. I've seen several cases where eMule seems to "fill up" the DNS tables inside a Netgear router, which can only be fixed by rebooting the router. -- Finlay McWalter | Talk 23:59, 15 April 2006 (UTC)
Thanks ;)
- If thats the problem then bypassing the routers dns server and setting your machine to talk to your isps servers directly may bring a fix. The dns proxies in home routers seem to be fairly poor in general. Plugwash 01:01, 22 April 2006 (UTC)
learning Computer language[edit]
I am not trolling. I need to learn a 'computer langage' by August or I am vegetable. Please help me!
Situation: I have no previous experience with computer language. (And pleae don't tell me to learn QBasic, my sister does not count it as a computer language.) I need to learn a new language from scratch and I have heard about Python, Perl, FORTRAN (my dad used it but I don't want to nag him all the time) but I have not used any of these. Please also recommend me some free good pdf books online. Thank you.
Yours sincerely, Kushal Hada [Four tildes] Kushal one 23:41, 15 April 2006 (UTC)
- Bruce Eckel has freely downloadable books teaching programming in C++, Java and Python. His books on the first two are probably not suitable for someone who has absolutely no programming experience, but I haven't looked at the last. Your choice of language should depend on what you need to use it for; if you explain why you need to learn one we may be able to help more.-gadfium 00:29, 16 April 2006 (UTC)
- This issue has been discussed here before, see Wikipedia:Reference_desk_archive/Science/March_2006#Learning Computer Programming. I might add, that despite what your sister might say, QBasic is a completely valid programming language. In fact, any program that can be written in a particular language, can also be written in any other language, as long as they're Turing complete (most are, including QBasic). In fact, I would recommend starting with QBasic or C, then move on to an object-oriented language, such as C++ or Java. Of course, you can also choose the path of functional programming, and learn Scheme. It really depends on what you need to use the language for, if you're going to be writing simple things for an embedded system that you created, you might as well just start with an Assembly language, and not bother with any higher-level ones. --Aramգուտանգ 00:37, 16 April 2006 (UTC)
- A pretty simple language which is also quite powerful is PHP. You can learn the basics of it in a few hours if you run through a few tutorials. It is pretty straightforward as far as scripting languages go. --Fastfission 17:09, 16 April 2006 (UTC)
Start with Pascal programming language. It was designed as the language to use to learn how to program. After you read the just mentioned wikipedia article on Pascal, read [35] and use [36]. WAS 4.250 17:21, 16 April 2006 (UTC)
Java 2 is currently one of the most powerful languages available on computer programming. It is not too difficult as long as one is willing to think. I'll recommend the book I used :Java 2 In Easy Steps by Mike McGarth from the "In Easty Steps" series. Luthinya 10:34, 17 April 2006 (UTC)
- I can program in Perl, PHP, Python, Bash, Javascript, and Ruby, yet I have tried repeatedly to learn Java without any success. While it may be easy for some, it's certainly not for all. If I was to recommend a programming language for someone just starting to learn, without all the weightiness of other languages, it would definitely be Ruby. --Kickstart70-T-C 22:46, 21 April 2006 (UTC)
- Afaict there are 3 basic approaches to learning programming all with thier pros and cons.
- 1: start simple, e.g. start with a language like basic or one of the scripting languages. Easy to get started but the habbits you pick up may set you in bad stead when you move onto more serious languages. (the poster above seems to be an example of this, he's picked up a load of scripting languages but is having trouble moving on from there to more serious stuff)
- 2: start well structured, e.g. with a language like pascal or possiblly java (starting with java creates gc addicts though which is not something i wan't to encourage). Such languages force you into learning good structural habbits early but may have a steep learning curve.
- 3: start low level. e.g. start with assembler (NOT i386 assembler though if you value your sanity). The big advantage with this approach is that you learn what can be done in few instructions and what can't. This will stand you in good stead to write well performing code as you move on. If your going this route i'd reccomend getting a pic18f452 board to experiment with. Plugwash 01:09, 22 April 2006 (UTC)
April 16[edit]
Ice age[edit]
Here it says how the three main causes of ice ages are: The earth's orbit around the sun changing; the atmosphere changing; and the "arrangment of the continents". I heard how scientists say there will be another ice age in about five million years from now. How do they know that? Jonathan talk ![]() 00:40, 16 April 2006 (UTC)
00:40, 16 April 2006 (UTC)
- They are estimating based on the past. They find evidence of changes in Ice cores and from those changes they try to predict what is likely to happen. Admittedly, the "arrangement of the continents" reason sounds suspicious. I will have a look at that one. The orbit around the sun can be predicted with a very high degree of accuracy, and its effect on the amount of heat the earth receives, and the rates at which it receives the heat will affect when the next ice age will occur. Ansell 00:58, 16 April 2006 (UTC)
- The arrangement of the continents is very important. The arrangement of land is relevant as it affects the circulation of the oceans, which has a strong modifying effect on climate - ice ages are far more likely in some continental arrangements than others. It only sounds like an odd reason because we normally think of "The Ice ages" as occurring only in the last few million years, during which time the continents have been roughly in the same places - but they've actually been going on intermittently for hundreds of millions of years. Sometimes there are long periods with no ice ages or long periods with many ice ages - the reason for these two distinct climatic histories is the positions of the land masses. Grutness...wha? 01:32, 16 April 2006 (UTC)
While I have no doubt that a significant change in the Earth's orbit could cause an ice age, what makes them think the Earth's orbit has ever changed significantly ? StuRat 23:36, 17 April 2006 (UTC)
Thanks for all the answers. (Does anyone think my signature is ugly?) Jonathan talk ![]() 14:23, 18 April 2006 (UTC)
14:23, 18 April 2006 (UTC)
- I do (at least the name part, the Canadian flag is OK). StuRat 09:11, 20 April 2006 (UTC)
black people[edit]
Do they tan or is their skin always the same color? A Clown in the Dark 01:47, 16 April 2006 (UTC)
- Sun tanning is simply the skin activating chemicals that are already in our skin. People who have those compounds activated because of their genes, may get slightly more tanned, however, I am not sure it would be easy to tell the difference as their extra level of activation may be very minor. Ansell 02:48, 16 April 2006 (UTC)
Yes, black people tan. WAS 4.250 17:32, 16 April 2006 (UTC)
They are susceptible to tanning and burning, and the effect can be just as dramatic as in whites. Bhumiya (said/done) 21:22, 16 April 2006 (UTC)
- Though, as one would expect, it's much easier to notice the difference in a 1% shade to 11% shade change than a 30% shade to 40% shade change. In other words, your big fat white Uncle Al would look quite a bit more rediculous with a light burn than your dark muscular Aunt Franco. freshgavinΓΛĿЌ 05:20, 17 April 2006 (UTC)
Yes, they do tan, though the effect is considerably much less noticeable because of the high melanin level found naturally in their skin in the firs place. Luthinya 10:36, 17 April 2006 (UTC)
Drive Arrays In Proliant 3000[edit]
Since linux doesn't treat my HD very separately and treats them as one big 'hda', what does that mean if one drive fails on start up?
Similarly, if I erase my server and treat the drives as one big "logical drive" does that enhance risk of data loss in the event that one drive fails? Would they be better all as separate logical drives? Also, what is the meaning of a an array - why are my logical drives arranged under the umbrella of an 'array'?
I would like to make use of the maximum amount of space with the minimum risk of failure which I think is RAID 0, all HDs treated separately. Also, why if one drive fails, should the server demand that it is replaced before starting up? Even if it is replaced, the data isn't going to be there...? --Username132 (talk) 01:50, 16 April 2006 (UTC)
- Maximum space doesn't go with minimum failure. You choose which one you are more worried about and go with that. I would personally choose RAID 0 for speed, but with the knowledge that because it is one logical drive, if one fails, there is no backup. However, if you want minimum failure, look at RAID 1, where you get everything put down twice on separate hard drives so if one fails you can still use the other one.
- Linux only treats them as one big hda if they are all put together in RAID, or if you are using Logical Volume Management, or any of the alternatives. If you put two non-RAID hard drives in Linux is likely to assign one the hda and one the hdb, unless they are SCSI in which case they will be sda and sdb.
- If one drive in a RAID 1 array fails on startup nothing bad happens, your computer will boot as normal. However, if you have RAID 0, you have just lost half of the data on your computer. Ansell 02:44, 16 April 2006 (UTC)
- Well I want to use 8 (18.2 GB) drives. I figure I could split this into two logical arrays - one consisting of a single HD for the OS(es) and another for storage. But if 7 drives are in one logical drive does that mean that they're all one partition? I the logical drive entry doesn't explain what would happen if one drive failed? Would I be better off having 8 logical drives or the two like I just mentioned?
- If you have a large number of drives your best bet is RAID 5 - this will strip data across multiple drives, but will add a parity drive so that you have one level of redundancy - a failure in any single drive will result in no loss of data. As you add drives your chances of having a failure go up - with seven drives you are definitely in a high-risk position with RAID 0. With RAID 0 your space available is the sum of all drives. With RAID 1 it is the size of one drive. With RAID 5 it is the sum of all drives minus 1. Performance of RAID 5 is less than RAID 0 or 1, but better than single drive performance. Raid can be implemented both in hardware (which is common on server-class hardware like a Proliant), or by software (LVM, etc). Rich0 12:37, 23 April 2006 (UTC)
When did RAID come into existence? The computer I'm referring to is about 8 years old and uses hot-pluggable SCSI drives in their own little caddies. When I use Compaq SmartStart to configure 'the array', it invariably wants to put them all into one big logical drive and the "array condiguration utility" gives me a graphic kind of like this;
Controller
|
Array
|
Logical drive 1
Logical drive 2
What's this array nonsense? I don't want any arrays... :( --Username132 (talk) 03:43, 16 April 2006 (UTC)
- See Redundant array of independent disks for history and more. Servers such as yours have had RAID controllers for long, it seems, but common PCs/Desktops generally only for the past 3-4 years. Max space / Min failure would seem at it simplest to correspond to a RAID 1 setup, 2 logical drives of 4 identical physical disks each = 100% duplication. --Seejyb 14:06, 16 April 2006 (UTC)
P2P Malware[edit]
If you download a linux distro from a p2p network, couldn't someone have altered the code to add some malware and control your computer remotely? --Username132 (talk) 02:16, 16 April 2006 (UTC)
Depends on where you got the source from. If you get the link from the distro's website, then no it's fine.
- You can run md5sum on the downloaded file and compare this to the sum that is given on the distro's website to confirm that your copy has not been tampered with. They may even have digitally signed the file and you can confirm it that way as well. Ansell 02:38, 16 April 2006 (UTC)
- Thanks :) --Username132 (talk) 03:28, 16 April 2006 (UTC)
Forum on IMDB working?[edit]
Here is a link to the [Chinatown (1974) entry on IMDB. The forum links aren't working for me. How are they for you? -Username132 (talk) 03:44, 16 April 2006 (UTC)
- Did you register? Going to the message board came up with a "Free Registration" window for me, but I don't see the need to give away my details, so i didn't proceed. The user comments were working for me. Is that what you meant? Ansell 04:47, 16 April 2006 (UTC)
- Yeah, I'm registered. It even gives my login name in the top right, but when I click on the link to go to a forum (not comments), the browser load bar appears and then makes no progress... indefinately. Thanks for trying anyway. --Username132 (talk) 04:57, 16 April 2006 (UTC)
- I'm having no trouble with the link. I'm registered with IMDb, and I went to the main movie page, the main forums page for the movie, and the top topic in the forums list with no problems. Was it perhaps a particular thread that you were having trouble with?--Anchoress 11:38, 16 April 2006 (UTC)
- Yeah, I'm registered. It even gives my login name in the top right, but when I click on the link to go to a forum (not comments), the browser load bar appears and then makes no progress... indefinately. Thanks for trying anyway. --Username132 (talk) 04:57, 16 April 2006 (UTC)
- It was a whole forum that wouldn't work. Couldn't get to the forum, or the selected threads on the main movie page. It's spontaneously resolved now, but I don't know why. --Username132 (talk) 18:29, 16 April 2006 (UTC)
Statistics - type 2 error (oh noes!)[edit]
According to false negative;
"In statistics, a false negative, also called a Type II error or miss, exists when a test incorrectly reports that a result was not detected, when it was really present. (Alternatively, a Type 2 error can be thought of as a failure by accepting the alternative hypothesis when the null hypothesis was truly false.)"
So what it says at first is that a type 2 error is accepting the null hypothesis when the alternative hypothesis was correct? And then it contradicts itself by saying alternatively, a type 2 error is accepting the alternative hypothesis EVEN THOUGH the null hypothesis is actually wrong? Putting the word 'truly' before false is also stupid. I'm changing it to 'actually'... -Username132 (talk) 04:54, 16 April 2006 (UTC)
- The "clarification" inside parentheses is wrong! A type 2 error is an erroneous rejection of the alternative hypothesis. (In other word, the null hypothesis is false but one fails to reject it.) --68.238.254.236 15:20, 16 April 2006 (UTC)
How long before a feral animal becomes a native?[edit]
Hey people. I was wondering if there was a hard and fast rule in place about how long a species of feral animal has existed before it is referred to as a native. For example, the dingo was introduced to Australia about 15000 - 30000 years ago and today is generally considered a native animal to Australia. Meanwhile, the feral cats that one sees in outback Australia are as different to domesticated cats that one would consider them a different species (my mother, who used to work in rural Australia, saw examples of feral cats that looked twice as big as a domesticated cat and whose faces looked considerably different). Some of these cats can trace their ancestory in Australia back 400 or so years, leaving one to ask the question, "how long until feral cats are considered native to Australia?"
Cheers --Roisterer 06:12, 16 April 2006 (UTC)
- I'm not sure that dingoes can be considered native to Australia. See introduced species for clarification; since the feral cats were introduced, by definition they cannot be native. As far as species is concerned, if these feral cats can still breed with their domestic counterparts and produce fertile offspring, they'll probably be considered the same species. Hope this helps. Isopropyl 06:32, 16 April 2006 (UTC)
You realize that you are asking a question about semantics, not natural fact? Native is an especially slippery word, but in this type of context native usually means only that things or people of its kind were already present in that place when people speaking a European language first arrived. Much evidence suggests that nearly all people and animals and plants have not existed forever in a particular place but arrived at that place at some time in the past. There is no precise answer to "how long should x have existed there before we can call it "native". alteripse 12:09, 16 April 2006 (UTC)
Eyes and sunlight[edit]
Why are eyes so heavily affected by sunlight? All it is is just a bunch of photons, it shouldn't be all that harmful.
- Gamma radiation is "just a bunch of photons", too. --BluePlatypus 06:34, 16 April 2006 (UTC)
- See Sun#Sun observation and eye damage. —Keenan Pepper 07:45, 16 April 2006 (UTC)
- The problem is the photons are at too high of an intensity in number that the eye is built to handle... not that the eye was built. --
 Mac Davis] ⌇☢ ญƛ. 11:29, 16 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 11:29, 16 April 2006 (UTC)
A photon is pure energy. The eye is a delicate instrument that aquires, measures, and organizes information on that pure energy. If that pure energy is too much energy, then it damages the sensitive parts of that delicate instrumnet. WAS 4.250 17:38, 16 April 2006 (UTC)
- Its just like cooking something....as long as the flame is under control the food gets cooked properly....but if you increase the flame or the heat given to it, it gets over cooked and in extreme cases, burnt... our eye is like the food....we dont want it to get burnt..... Jayant,17 Years, India • contribs 17:51, 16 April 2006 (UTC)
- As our article says our eyes have evolved to stand directly looking at the sun by accident with little chance of permanent damage. Light concentrating optics and partial solar eclipses are where the real danger lies. The former can hardly be called part of our natural environment and the later is a pretty rare event. Plugwash 17:59, 16 April 2006 (UTC)
The human brain is also probably part of the reason. Just like pain receptors reacting though no damage is being done, the brain has probably evolved to make it seem that our eyes are very sensitive to the sun, in order to teach our less intelligent ancestors a lesson before they started making hobbies of looking at bright yellow balls. freshgavinΓΛĿЌ 05:12, 17 April 2006 (UTC)
Photons are massless particles, therefore may be said as pure energy. Too much energy in the eyes- ouch! Luthinya 10:37, 17 April 2006 (UTC)
Squiggly shapes in the eyes[edit]
In certain lighting conditions I can discern small transparent shapes in my eyes. i guess they are suspended in the sclera, as any attempt to look directly at them results in them moving away in relation to the movement of the eye (a rather comical situation). The shapes are varied, but range from small spots to squiggly-amoeboid shapes, all very small.
Other people seem to have them as well. I looked but couldn't find what I was looking for under disorders of the eye.
What are they, are they bad, and is there any way to remove them? Cheers, Wapatista 09:01, 16 April 2006 (UTC)
- See Floater. — Knowledge Seeker দ 09:08, 16 April 2006 (UTC)
- They are normal, don't worry about it. Also see Phosphene --
 Mac Davis] ⌇☢ ญƛ. 11:28, 16 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 11:28, 16 April 2006 (UTC)
- I've been seeing them for more than 10 years now... if they are really bugging you, try not to concentrate on them and look at some dark area... Jayant,17 Years, India • contribs 14:36, 16 April 2006 (UTC)
Also, if you wear contact lenses, they may just need cleaning or replacing. Little spots on them can behave the same way. StuRat 21:56, 17 April 2006 (UTC)
Then again, if you don't wear contacts, try removing your corneas and giving them a good cleaning, LOL. StuRat 00:35, 22 April 2006 (UTC)
Center of Mass & Center of Gravity[edit]
I have a conceptual question about a roll of toilet tissue. Are its center of mass and center of gravity the same? Also, could they located in the hollow inside of the roll?Patchouli 14:16, 16 April 2006 (UTC)
- Assuming that the roll is in a uniform gravitational field, the center of mass equals the center of gravity, and they are at the center of the roll, in the hollow inside of it. --Andreas Rejbrand 14:22, 16 April 2006 (UTC)
- Wow, that center of gravity article is a travesty. We've gotta fix that. Melchoir 06:22, 17 April 2006 (UTC)
Evolution-based explanation for physical differences among races[edit]
Native inhabitants of difference parts of the world have different physical features. For example, Europeans tend to have lighter skin/eye/hair colors and "pointier" nose shapes etc. Africans, on the other hand, tend to have darker skin/eye/hair colors, bigger and rounder noses, and hair with very small curls. From the standpoint of evolution, these differences should be explainable in terms of adaptations to the environment and evolutionary advantages. I've tried to come up with an evolution-based explanation for the differences in nose shapes and hair texture, but I couldn't tie these differences to evolutionary advantages. Is there a consensus among scientists as to how nose shapes and hair texture affect survival in different geographic regions?
- There is a process called genetic drift by which traits can become common in a population by "chance" without there being a "reason". You can speculate about "reasons" for traits that might lead to active selection, but it is often hard to know if you have found the real cause of a trait being selected in a population. --JWSchmidt 17:09, 16 April 2006 (UTC)
There is no consensus. There are a lot of "just-so" stories ;some are nonsense; some are probably true. Current genetic studies are being done that are beginning to provide scientific evidence that address these issues. We are a long way from having it all figured out. WAS 4.250 17:46, 16 April 2006 (UTC)
- Sunlight is used by the skin to synthesize vitamin D. However, too much sunlight causes skin damage. So, in the tropics, where sunlight is abundant, melanin is needed in the skin to absorb most of the sunlight, while in temperate zones, light skin is needed to let the lower quantity of light in. Large nostrils are helpful for breathing, especially when hot. However, large nostrils in cold areas would allow too much cold air in and damage air passages. Straight hair is better at retaining heat and curly hair is better at cooling. StuRat 23:23, 17 April 2006 (UTC)
- Soft fatty waist-lines are ideal for balancing opened cans of beer in a sitting position. freshgavinΓΛĿЌ 07:00, 18 April 2006 (UTC)
EMF (or WMF) to SVG converter[edit]
Does anyone know any free software able to convert EMF (or WMF) images to SVG files? --Andreas Rejbrand 14:17, 16 April 2006 (UTC)
- You mean, apart from the programs listed by typing "wmf to svg converter" into Google? --Heron 16:32, 16 April 2006 (UTC)
- Well, I thought most of them weren't free (it isn't the first time I'm looking for a free converter app.), but I'll have a more carefull look at them. --Andreas Rejbrand 17:01, 16 April 2006 (UTC)
JPEG thumbnail[edit]
After installing some software, my computer (Windows ME) no longer shows a thumbnails for jpegs in Explorer. Is there a registry class I should edit to bring it back again.--Bjwebb (talk) 14:37, 16 April 2006 (UTC)
That happens when the ShellEx settings go wonky. Admittedly my WinME install, in Virtual PC is very old, but if you put the following into a .reg file it should, hopefully, do the trick. I take no responsibility if this causes the heat death of the universe. --Blowdart 08:23, 18 April 2006 (UTC)
- Do you mean .reg? --WhiteDragon 19:00, 20 April 2006 (UTC)
- Err yes :)N --Blowdart 03:21, 22 April 2006 (UTC)
REGEDIT4
[HKEY_CLASSES_ROOT\.jpg\ShellEx]
[HKEY_CLASSES_ROOT\.jpg\ShellEx\{BB2E617C-0920-11d1-9A0B-00C04FC2D6C1}]
@="{7376D660-C583-11d0-A3A5-00C04FD706EC}"
Rapyrox[edit]
In a book I was reading, a old lady held an injection of 'rapyrox' against some guy's leg and held him hostage that way. What is rapyrox? I've done google searches, and I get nothing. Recon0. (talk) 14:47, 16 April 2006 (UTC)
- I have tried to search for 'rapyrox' using several search engines but havn't found anything. Hence, it is most likely that the word has no real meaning, but is only a "invention" of the author. --Andreas Rejbrand 14:52, 16 April 2006 (UTC)
An Unusual Error in Matlab Program.[edit]
Hi, i was trying to create a program to find out the sum of the digits of a given number until the sum was a single digit. For example, if i find sum of digits of the number "15", then it would be 1+5=6. But for "47", it should be 4+7=11 and 1+1=2. so i just wanted to write such a program. First i tried in C, but there handling of floating point variables would have posed problems, so i tried in Matlab. The following is the code which i wrote in Matlab. Please note that in the following code, "type" is the variable whose sum of digits i want to find out. And the variable "sumfinal" stores the final value of sum of digits.
THERE IS A PROBLEM WITH WIKIPEDIA'S CODE DISPLAYING METHODS, I HAVE MODIFIED THIS CODE ON THIS PAGE SO THAT IT SEEMS EXACTLY AS IT IS, BUT WIKIPEDIA ITSELF CREATES NEW INDENTS AND MAKES IT QUITE CLUMSY.
%%%%%%%%%%%%%%BEGINNING OF CODE%%%%%%%%%%%%%%%%
clear
format long
sum=0;
temp=0;
num=2222222222222222222; % this is the number
while temp==0
while num~=0
digit=mod(num,10);
sum=sum+digit;
num=(num-digit)/10;
sum
end
if sum>9
num=sum;
sum=0;
else
break
end
end
sumfinal=sum;
sumfinal
%%%%%%%%%%%%%%%%%%%% END OF CODE %%%%%%%%%%%%%%%%%%%%%
Now, the problem is that i tried out various combinations of numbers and all of them worked out well, but suddenly i found out that whenever i was entering numbers in multiples of "19", the results were not correct. For example, when i entered "1" nineteen times, the correct result displayed by "sumfinal" should be "1", but it shows "9". So i tried it with "2" nineteen times, even then the result was "9", though the correct result should be "2". And surprisingly for "3" nineteen times the result was "-456". So could anyone please help me with this problem. And could you also provide with the bug-free code for this problem of summation of digits. PS: I have been programming in matlab since last 8 months or so, so i am quite experienced in that. secondly, if anyone says that "2" repeated nineteen times is large number , and will not be handled correctly by matlab, then its incorrect to say so, because for simple calculations also, extremely large numbers beyond imagination can be handled. So the "range" is not a problem. I use Matlab 7.
- To show code in html enclose it within <pre>...</pre> elements. Can't help you with matlab, but here's some C code that i think does what you want.
void add_digit(char* a,int c) {
if(!*a) *a = '0';
c += *a - '0';
*a = '0' + c % 10;
c = (c - (c%10))/10;
if(c>0) add_digit(++a,c);
}
void sum_digits(char* a) {
char* sum = a++;
while(*a) {
int c = *a - '0';
*a++ = '\0';
add_digit(sum,c);
}
}
int main() {
char num[256] = "2222222222222222222";
do {
sum_digits(num);
printf("sum digits: %s\n",num);
}while(num[1]);
return 0;
}
- This works, as long as you don't mind reading the interim sums right to left.EricR 17:23, 16 April 2006 (UTC)
- Good evening! I happen to believe that the sum of the digits of any number (base 10) is mod(num,9) where mod is the modulo function. If you have it, use it. --DLL 18:49, 16 April 2006 (UTC)
- A simple one-line solution would be: "while(number > 9) { number = (number % 10) + (number / 10) }" This assume integer division on "number / 10". If you don't have that, use "floor(number/10)". In the end, "number" will be the one-digit sum. I didn't check this with a lot of numbers, but it worked for a handfull of them. --Kainaw (talk) 19:11, 16 April 2006 (UTC)
- Well, if you've got arbitrary sized integers that works great. Try a few > 2^32 or 2^64. EricR 20:12, 16 April 2006 (UTC)
- culd store them in a string as well and split it up etc --24.193.235.188 07:04, 17 April 2006 (UTC)
Face-to-face intercourse in animals[edit]
What animals, if any, engage in sexual intercourse face-to-face other than humans? (Subquestion: Does it even make sense to consider non-mammals?) Off the top of my head, it seems like most mammals go with a rear mounting, 'doggy style' if you will. Seems like whales and dolphins would have to go face-to-face, or belly-to-belly as it were. Seems like the other primates might be the only other animals capable of face-to-face intercourse, but I think they don't. Any information out there? --Ananda
- Bonobos face each other when copulating and are rather famous for it. --Fastfission 17:08, 16 April 2006 (UTC)
- From Missionary position, there's also armadillos. I could swear there was also an insect that uses the position; I thought it was aphids but Google isn't helping me there. Melchoir 04:31, 17 April 2006 (UTC)
- Worms don't have faces, but they screw belly up front. freshgavinΓΛĿЌ 06:56, 18 April 2006 (UTC)
- You may be interested in Non-human_animal_sexuality and Canidae#Canine_copulation. Black Carrot 02:56, 19 April 2006 (UTC)
- I don't have a reference, but I believe that somebody did a study on the origins of the spread of genital diseases to the mouth to figure out when the switchover occurred for humans. Rich0 12:41, 23 April 2006 (UTC)
SSL CRT[edit]
What is the difference between a CRT that I generate using Cpanel and one that I can buy from Verisign (and others) and later insert into Cpanel? — Ilyanep (Talk) 16:45, 16 April 2006 (UTC)
- A certificate Cpanel generates is a self-signed certificate, which means a client program connecting to a server using such a certificate has absolutely no assurance the server is not an impostor (sure, I'm amazon.com, trust me, I say so myself, now, what was your credit card number?). Verisign is one of a number of certificate authorities that issue X.509 certificates. To get a certificate authority to issue a certificate you have to prove you're the owner of the IP address the server will run on, so if you trust the CA you can trust that you're not talking to an impostor - for more on this please see the articles on X.509 certificates and certificate authorities. -- Rick Block (talk) 22:56, 16 April 2006 (UTC)
Simple Javascript question[edit]
I'm trying to do some simple Javascript and I seem to have forgotten how to do use the DOM correctly. All I want is a function which will cycle through all of the checkbox elements with an id of "record" and set their "checked" property to "true". What's the best way to cycle through them all? I'm frustrated and not finding easy answers Googling around. --Fastfission 16:54, 16 April 2006 (UTC)
- Could you just traverse the DOM tree?
function topDownTraverse(n) {
// do something w/ the node
for(var m = n.firstChild; m != null; m = m.nextSibling) {
topDownTraverse(m);
}
}
- EricR 22:16, 16 April 2006 (UTC)
- I'm not sure what you're referring to with the traversing, but I know that checkbox elements have a property called "checked", which it shouldn't be too difficult to set to true. You can use the "forms" array builtin to the DOM to cycle through the forms, and if there is more than one element "record", it will be an array and a property of forms[]. Try something like:
function checkallrecord() {
i = 0;
j = 0;
while (document.forms[i]) {
while (document.forms[i].record[j]) {
document.forms[i].record[j].checked = true;
j++;
}
i++;
}
}
- Note that this won't work with only 1 checkbox called "record". For anything more than 1, it should be fine. It also doesn't work if the checkboxes aren't encased in 1 or more <form> tags. Hope this helps. -- Daverocks (talk) 11:38, 17 April 2006 (UTC)
- A tree traversal algorithm can be used to visit each node of a tree (data structure). The DOM represents parsed documents as trees. During the traversal it's easy to check the node type, name and id, then set any properties you like. EricR 17:25, 17 April 2006 (UTC)
Solar Power[edit]
I'm wondering if anybody out there is very experienced with solar power.
I'm not very familiar with the terms involved with electricity and magnetism, so please bear with with me.
Approximately how large would a solar panel have to be, given the approximate average amount of sunlight available in North America, to keep a recharchable battery powerful enough to power a five watt light bulb, sufficiently charged to constantly have power available to turn the light on whenever needed?
If my question is confusing, if I'm using the term "watt" incorrectly or if I'm leaving out some other variable I'll gladly clarify it to the best of my ability. Thanks to anyone who can help me with this. Loomis51 17:06, 16 April 2006 (UTC)
- Your question is perfectly alright...:-D ....and from the solar cell article:
- When exposed to direct sunlight, a 6-centimeter diameter silicon cell can produce a current of about 0.5 ampere at 0.5 volt (equivalent to about 90 W/m² average, range is usually between 50-150 W/m², depending on sun brightness and solar cell efficiency).
- So..i'd guess that'd give you a good idea of how big the panel has to be... Jayant,17 Years, India • contribs 17:45, 16 April 2006 (UTC)
Thanks for the link, but as I said, my knowledge of electricity and magnetism is very poor, so I can't really make sense of all the terminology. Could you tell me how all of that translates into the actual size necessary for the panel? And if I can ask a further question, would you have a rough estimate of what the cost of the panel and the battery would be? (Nevermind the bulb) Thanks! Loomis51 18:13, 16 April 2006 (UTC)
- You are welcome... The terminology (90W/m^2) means that for every square meter of a silicon solar cell it produces about 90 W.... so..if you want to recharge a 5 watt bulb..... the area should be 0.055 square meters..... so the area should be around 60-100 square centimeters.... and as for the cost, i dont know about the price in North America since i dont live there...i'll try to search for the price on the internet and put it over here... Jayant,17 Years, India • contribs 21:09, 16 April 2006 (UTC)
- Yeah...here we go... that site gives a lot of information on solar panels...and gives you some models for sale..... hope you find them useful...try calling up some of the phone numbers on the website and talk to them...... if you want more information, just ask...:-D ..Jayant,17 Years, India • contribs 21:18, 16 April 2006 (UTC)
Thanks Jayant, you've been a great help! If only all Wikipedians were as courteous and helpful as you. I'm sure I'll have a few more questions in the near future, but for now I'll check out the sites you mentioned. Thanks again! Loomis51 00:22, 17 April 2006 (UTC)
This is a very easy question. All you have to do is think in terms of ENERGY.
A 5 watt light bulb comsumes 5 joules of energy per second. Assume that you need the bulb to be on 24 hours per day. 1 day = 24 * 60 * 60 seconds or 86400 seconds.
Therefore a 5 watt bulb requires 5 * 86400 or 432000 joules per day. So all you need to do is to calculate the (area) amount of solar panels to generate as least 432000 joules of electrical energy per day.
Solar panels tells you how much power (in watts) they generate in the "best case senario" so you need to account for the "worse case senario". Also remember that there is no sun light at night. So multiple the "watts" of the solar panel by the amount of sunlight in seconds and make sure you includes some redundancy for cloudy periods to get the amount of energy stored per day.Ohanian 04:43, 17 April 2006 (UTC)
- Well, you cannot really charge up a light. What you can do is charge up a battery which can run the light. A standard rechargable battery (type D, C, AA or AAA) can be charged up using three of the above solar cells in series to deliver 1.5 volts. Make sure you leave it in strong sunlight, and only use a light bulb intended for 1.5 volts. You might also want to put a rectifier in the circuit to make sure it does not discharge itself when it is dark. One other problem is the battery will tend to lose its ability to discharge if left charged up for a long time. Have you heard about the Faraday Flashlight?
I have got one for emergencies, not a bad deal.
Which Test To Find P-value And How Many Freedom Degrees?[edit]
I've done an experiment where we took ONE SAMPLE of neurones, extracted something from them, split it into two samples and measured the quantity of a chemical in the solution, comparing it to a control set of neurones which were treated more nicely during incubation. Do I have one or zero degrees freedom? Although a repeat was performed, it was using the same sample extracted from one set of neurones? How do I know which is the appropriate statistical test to find a p-value? -Username132 (talk) 17:54, 16 April 2006 (UTC)
- It sounds like there is one degree of freedom, whether it was treated "nicely", or not, as you put it. StuRat 23:03, 17 April 2006 (UTC)
- What I meant was that there were some control neurones which were incubated with nothing special added to them, and some others (the mistreated ones) where we added NMDA. Here's how the experiment went down;
One control sample > split into two > chemical measured > average taken One NMDA sample > split into two > chemical measured > average taken
- The two averages were then compared. Since we only took one sample of neurones for each the control and NMDA treated neurones (i.e. one rat) and split the sample into two, I think because there was only one rat involved (out of the entire global population), there are zero degress of freedom. You understand what I'm saying?
- Either way though, what is the test used to compare to values in this way? --Username132 (talk) 00:31, 18 April 2006 (UTC)
- I don't believe any statistical significance test can function with a sample size of 2 (assuming "split into two" means they're meant to function as seperate samples). It seems like they generally need at least a dozen. Without that, there's no way to guess at how wide the standard deviation is. If I understand degrees of freedom right (a big if), I think you have one. I think there are two variables being measured (one categorical and one numerical), and that only the dependent variable (the numerical one) is counted as a degree of freedom. If you get a bigger sample size, the t-test is what you want. Whether it should be paired-sample or not is a bit trickier. All the samples come from one rat, which means they're related, but within that rat the neurons are (I assume) randomly chosen. You'd use an independent sample test (testing the relationship between two well-distributed, unconnected sets of samples) and then, to take into account the effects of the single rat, state in your conclusion that your results only apply to the rat tested and can not necessarily be extended to the general population of rats. If you don't want to go back and get a larger sample, you could of course fake your results (not recommended), use the test anyway (equivalent to faking results, but without as much of a stigma attached), or just subtract one average from the other and mention in your conclusion that the results may or may not be representative. Black Carrot 02:27, 19 April 2006 (UTC)
- One further thought: I'm not actually at all sure you did choose the neurons randomly. You certainly wouldn't have gotten a randomly distributed sample from throughout the brain; I'd expect you to just cut out a small section that looks similar to the rest. That means that the results would apply only to whatever particular types of cells made it into the sample. Also, there's an important difference between simply cutting that sample into pieces and mixing it up, then seperating it into equal amounts. The second would be random (but might damage the cells), and the first would not. Unless you can ensure that the cells in the sample are identical (well, that the inevitable differences between them are negligible), which would be difficult, no statistical test can be really reliably performed. Black Carrot 16:07, 19 April 2006 (UTC)
- Thanks! The neurone sampling was done by the technicians (I actually recon there are no neurones involved and they just did it with chemicals - a lot more predictable, cheaper and more suitable for a university experiment). If the results are insufficient to perform a statistical test, I'll just include this in my critical assesment of the experiment which is a lot easier for me!
- When you mentioned result fudging (my result-fudging days are over I hope!) did you mean to infer that it goes on in the real world by actual research groups? If so, how frequently does this occur? And what are the motivations, aside from having an investment in the company selling a certain product?
- The biggest fudge I ever did was to draw the graph I wanted, plot points around the line, take those points as my averages and make up three repeats that gave that mean average!! Those were the days... -Username132 (talk) 08:09, 23 April 2006 (UTC)
Chronology / Inability to locate events in time or to remember dates[edit]
I stumbled across an interesting phenomenon: While I was trying to locate a painting to a particular epoch, I asked a friend. This friend told me that she cannot remember dates and does not have the ability to juxtapose events in for example political history with events in art history. In other words, a poem written in 1917 in England would not remind her of WWI and a possible connection, although she has had several curriculi in which WWI was a topic. She coined it - amusingly - date dyslexia. Now I am asking myself if there is an official expression for this "condition". Please do not propose laziness. Thank you! Don420 19:10, 16 April 2006 (UTC)
- I don't know, I am inclined to think laziness, or at least an inattentiveness to context. ;-) --Fastfission 21:35, 16 April 2006 (UTC)
- I'm a little confused. Would she be able to recognize a painting as one of WWI, from the style and content (no dates involved)? freshgavinΓΛĿЌ 05:02, 17 April 2006 (UTC)
- Good point. Your question with regard to WWI may be a difficult one since the telltale signs will be predominantly technical - trenches, weapons, radios, gas-masks, tanks, etc. These are elements generally not of interest to her. During my initial surprise at the phenomenon, I asked "when did Picasso live and when was his influential work done?" - having had art history during her education as a photographer and graphic designer - she was not able to say. For the exams she took, she had to learn the dates by heart. At the moment she is working on a Jugendstil design, taking inspiration and researching in numerous books. When asked about the time Jugendstil was active, she could not say except early 20th century. Louis XIV, Biedermeier or Manerism - no clue as to the dates, but she may be able to recognize the styles without the ability to place them in a chronology. Don420 09:58, 17 April 2006 (UTC)
- Then it sounds like she just has a serious problem remembering dates, which seems plausible enough. I still don't understand why she didn't get the poem was about WWI, if it included WWI details. The date 1917 doesn't really seem to relate. If you read me a poem written in 1917, I wouldn't be able to tell you when it was written either! freshgavinΓΛĿЌ 06:54, 18 April 2006 (UTC)
- Good point. Your question with regard to WWI may be a difficult one since the telltale signs will be predominantly technical - trenches, weapons, radios, gas-masks, tanks, etc. These are elements generally not of interest to her. During my initial surprise at the phenomenon, I asked "when did Picasso live and when was his influential work done?" - having had art history during her education as a photographer and graphic designer - she was not able to say. For the exams she took, she had to learn the dates by heart. At the moment she is working on a Jugendstil design, taking inspiration and researching in numerous books. When asked about the time Jugendstil was active, she could not say except early 20th century. Louis XIV, Biedermeier or Manerism - no clue as to the dates, but she may be able to recognize the styles without the ability to place them in a chronology. Don420 09:58, 17 April 2006 (UTC)
- I'm a little confused. Would she be able to recognize a painting as one of WWI, from the style and content (no dates involved)? freshgavinΓΛĿЌ 05:02, 17 April 2006 (UTC)
- It is probably just this simple, then... Thank you both for your time and help! Don420 09:13, 18 April 2006 (UTC)
One Line C Program Signatures[edit]
Hey There
I've seen around a lot some seriously funky 1 line C/C++ programs that are commonly found in signatures that when compiled produce a really nice pattern or shape, such as:
main(c,r){for(r=32;r;) printf(++c>31?c=!r--,"\n":c<r?" ":~c&r?" `":" #");}
Is there a name for these, and if so, does Wikipedia have an article on them, or does anyone know of a place that lists them? I'd really like to know as i find them extremely interesting and astonishing how one loop can produce such amazing results. Thanks :) -Benbread 21:46, 16 April 2006 (UTC)
- Yes. That's mainly due to the language. In this particular case the ?: operator plays a vital role as it allows a single expression to replace a set of much verboser if-else. Also, implicit comparison with zero and operators precedence play a (minor) role. Just to give you an idea, a spelled out version of the program could be:
#include <stdio.h>
int main(){
int c = 0;
int r = 32;
while (r != 0) {
c = c + 1;
if (c > 31) {
c = !r; /* same as c = 0 */
r = r - 1;
printf("\n");
}
else {
if (c < r) {
printf(" ");
}
else {
if ((~c & r) != 0) {
printf(" `");
}
else {
printf(" #");
}
}
}
}
}
- --Gennaro Prota 13:03, 17 April 2006 (UTC)
- These are called signature programs, and there is no Wikipedia article on them (yet). One place with a list is http://www.iwriteiam.nl/SigProg.html. -- Rick Block (talk) 22:36, 16 April 2006 (UTC)
- What a headbangingly obvious name. Thanks very much - I think i'll start working on that wikipedia article tomorrow (well, today, after i go to bed) -Benbread 23:13, 16 April 2006 (UTC)
- Please, keep in mind however that most of these are pre-standard programs, more than often relying on the particular and possibly undocumented behavior of a particular compiler (usually gcc at the time of writing the program). In the best of the hypotheses they lack the necessary includes, but usually it's worse than that. The example above, besides lacking #include <stdio.h>, doesn't specify the return type in the declaration of main, has an absolutely non-standard "main(c,r)" incipit and other subtleties. And it would require a quite careful analysis to see if it doesn't invoke undefined behavior (note in particular the increment of c without initialization).
- P.S.: I was almost forgetting... if you write an article on this, please, never write "C/C++". That expression makes many C and C++ programmers literally fly off the handle :)
- --Gennaro Prota 12:40, 17 April 2006 (UTC)
- Tech savvy teenagers! What would Wikipedia be without you? :-) See, it's this sort of stuff that makes it interesting to come back to the reference desk over and over and over! --HappyCamper 17:42, 17 April 2006 (UTC)
- BTW, it could be easily generalized. And a couple of changes would make it strictly portable (such as replacing
~c&rwith(31-c)&ror in general(h-1-c)&r; note that I declared c as int and left~cin my code: it was meant to be a faithful "rewording" of the original, not a fix). In any case I guess the intent was for r to start from 31; when it starts from 32 it will just produce an additional "all-space row" at the beginning.
- BTW, it could be easily generalized. And a couple of changes would make it strictly portable (such as replacing
- P.S. for HappyCamper: if you liked this you might enjoy googling for "IOCCC" :)
- Cheers. --Gennaro Prota 21:17, 17 April 2006 (UTC)
- Excellent answers thanks - About the whole C/C++ thing, sorry about that, in my spare time i do write a little C++, but wasn't aware it annoyed many people to see the usage of the above, but I can see why. Thanks again, everyone, particually Gennaro Prota :) -Benbread 23:50, 17 April 2006 (UTC)
- You are welcome :) About "C/C++" no need to apologise, I was just pointing out the mainstream attitude, so to speak, which you can read about at http://www.faqs.org/faqs/C-faq/learn/, under "What is C/C++?". In any case the best answer is that by Rick Block, I just went off at a tangent :) We could clean this up a bit for the archive. I was thinking to insert a brief introduction to Sierpinski triangles (or references, preferably internal to Wikipedia) and explain how the expression
~c&roriginates, from a mathematical point of view. --Gennaro Prota 11:41, 18 April 2006 (UTC)
- You are welcome :) About "C/C++" no need to apologise, I was just pointing out the mainstream attitude, so to speak, which you can read about at http://www.faqs.org/faqs/C-faq/learn/, under "What is C/C++?". In any case the best answer is that by Rick Block, I just went off at a tangent :) We could clean this up a bit for the archive. I was thinking to insert a brief introduction to Sierpinski triangles (or references, preferably internal to Wikipedia) and explain how the expression
- I've created the starting point of a Signature program article. Many thanks to everyone whos information has helped to make it. I've linked to this question in the talk page to show credit where it's due, as well as thanking some members personally. If you oppose to me rewriting some of your information (but it's public domain anyway) feel free to remove my information and repost it as your own. Thanks again for helping me and making wikipedia a better place :) -Benbread 16:41, 18 April 2006 (UTC)
- I had a quick look. The overall style and prose is very good to me. Some details are wrong (for instance, such programs are rarely "optimized"; the example above for instance, outputs all the leading space of a line one after another, even if a single string of appropriate lenght could be printf'd in one shot); they are just "terse" (you could say they optimize (minimize) the number of keystrokes :)). But don't worry, I'll fix it in the next days. What matters is the overall structure which is IMHO very good. As to the example program I don't know if it is copyrighted or not and considered that it has some errors (such as not printing the last line of the triangle) I think I'll replace it with a correct version. If one doesn't care having a spurious space after the '#' that terminates each line it could be quickly fixed as here:
/* Pass a power of 4 as argument for h */
void print_it (unsigned h)
{
const unsigned n=-1;
unsigned c = n;
signed r = h-1;
while (r>=0) printf(++c>=h?c=n,--r,"\n":c<r?" ":~c&r?"` ":"# ");
}
- but of course it can still be improved. I'll try writing a version of my own without looking at external sources, so that we can't have copyright problems. Ah, I also moved the article, to conform to our usual naming convention ("Signature program", with a lowercase 'p'). And thanks to *you* for your good job :) --Gennaro Prota 18:22, 18 April 2006 (UTC)
Microsoft Excel[edit]
Is there a way so that I can write something in a cell without it continuing into next cell. It just stays in the cell you are typing in.
- I don't have a copy of Excel available to me right now as I'm on a Linux system, but you should be able to choose Cell properties->Text format (or text alignment?)->wrap text, or something very similar, and any text which is too long for the cell will display over multiple lines. If the row height is standard, then you won't be able to see these lines, but you can adjust the row height should you wish. In OpenOffice.org, it's Format cells->alignment->Wrap text automatically, and you also have to choose Format cells->alignment->Vertical->top to get exactly the same result.
- An alternative way of stopping long text in one cell from extending over the next (blank) one is to put a space in the next cell.
- Is this what you wanted, or am I misunderstanding the question?-gadfium 23:20, 16 April 2006 (UTC)
- It's under 'format cells', 'alignment' to wrap text. The other suggestion, putting invisible content in subsequent cells, will also work but is not recommended (most notably because excel deems any edited cell to be part of the: print area, edited area (for when you use the home/end keys to navigate, etc) and also for formula-related issues.--Anchoress 05:11, 17 April 2006 (UTC)
- Thanks, I learned something too!-gadfium 05:30, 17 April 2006 (UTC)
- No problem! If you ever have a short Excel spreadsheet that outputs pages of blank cells on printing, you may infer that at some point cells elsewhere in the sheet have been edited. Using the keystroke, 'CTRL+END' will take you to the bottom-right-most edited cell, which will give you an idea of where the extra edits might have occurred.--Anchoress 06:34, 17 April 2006 (UTC)
- Thanks, I learned something too!-gadfium 05:30, 17 April 2006 (UTC)
- It's under 'format cells', 'alignment' to wrap text. The other suggestion, putting invisible content in subsequent cells, will also work but is not recommended (most notably because excel deems any edited cell to be part of the: print area, edited area (for when you use the home/end keys to navigate, etc) and also for formula-related issues.--Anchoress 05:11, 17 April 2006 (UTC)
April 17[edit]
Crucial Gizmo Overdrive[edit]
Does anyone know if a Crucial Gizmo Overdrive USB key will fit into the front USB port on a Dell 'monoblock'-type desktop? Cynical 15:35, 24 April 2006 (UTC)
Ramjet Project[edit]
I have been for three days attempting to build and test a stationary ramjet with no luck, I have read the ramjet article twice but I am still confused on the construction of the ramjet. So I was wondering if anyone could point me in the right direction or give me a link that explains the construction of a ramjet? I am looking for things like types of fuel ignition methods and just bassically anything on ramjets. Thank You. Patrick Kreidt
- For one thing, I thought "stationary ramjet" was an oxymoron. The design requires it to be moving very fast through some medium. —Keenan Pepper 00:43, 17 April 2006 (UTC)
- I agree with Keenan Pepper. A ramjet is probably not what you want. The external links on pulse jet engines might be useful to you.-gadfium 01:52, 17 April 2006 (UTC)
Well it can be stationary if you have a constant airflow, but that isnt my problem.
- Not to be useless, but I'd imagine it would be pretty difficult to find any specific instructions for building a ramjet, simply because it's not the kind of thing people build from kits and most of the plans would be either highly top-secret or simply confidential. freshgavinΓΛĿЌ 04:54, 17 April 2006 (UTC)
- Ramjet engines are not that hard to build and not top secret. Keenan is right in that it needs a flow of air to work. All you need for a stationary one is a large pipe with a one way valve at one end. Have some sort of pump to deliver a find mist of fuel inside, a fan to keep air pressure flowing through the valve and a sparker (like a spark plug) to ignite it. Don't blow yourself up.
- I could be wrong, but I wasn't aware that companies like Boeing, NASA, etc., reveal the entire technicalities of their prototype designs. freshgavinΓΛĿЌ 06:50, 18 April 2006 (UTC)
Thanks
Can a substance conduct heat well but not eletricity (and vice versa)[edit]
Title says it all, but if it is true could you explain how. It's not hw and any help would be apprecaited.
I believe epoxy was designed for just that. I just can't remember whether it's excellent at conducting heat but not electricity or the other way around. Loomis51 01:24, 17 April 2006 (UTC)
- A material is electrically conductive if it contains charged particles that are free to move around, like electrons in a metal or ions in a salt solution. A material is thermally conductive if mechanical vibrations (heat) are quickly transferred. Diamond and deionized water are both thermal conductors but electrical insulators, because their particles are strongly attracted to each other and spread vibrations quickly, but there are no mobile charged particles to conduct electricity. I can't think of a good electrical conductor that is also a thermal insulator, but I can't say for sure there aren't any. It might be because the charged carriers also conduct heat... —Keenan Pepper 01:29, 17 April 2006 (UTC)
Thanks.
Possible. One of the best examples would be the mica used as electrical insulator but thermal conductor in the electric irons - Wikicheng 06:11, 17 April 2006 (UTC)
- Good thermal conductivity without electrical conductivity is somewhat unusual. Try http://en.wikipedia.org/wiki/Beryllium, it talks about Berylium Oxide as having this property, but the Berylium Oxide article leaves it out.
Diamond is the prototypical example of a good thermal conductor (possibly the best thermal conductor known) but a bad electrical conductor, i.e. an insulator. Heat in materials is transferred in two ways, either by electrons or phonons. Phonons are essentially vibrations of the crystalline lattice, and heat in diamonds is transferred purely by phonons. But, because electrons conduct both heat and electricity, it is impossible for a material to conduct electricity and not heat. If it is electrically conductive, electrons must exist and be mobile within the material, and would therefore be capable of transferring heat.
Physics Problem[edit]
You place a 7.50 kg television set on a spring scale. if the scale read 78.4 N, what ist he acceleration of gravity at that location? Seems easy enough, I get an answer butr that isn't what the answer key "says". I went F = ma, F/m = a.... a = 10.45m/s/s.
The answer I'm given is 9.973m/s/s, and I have no idea how that's derived. Anyone know if I missed something or if I'm right? Thanks.
00:58, 17 April 2006 (UTC)
- Perhaps a dyslexic teacher? As far as I can tell if the answer is 9.973 the scale should say 74.8N. -- Rick Block (talk) 01:22, 17 April 2006 (UTC)
- Haha very nice. I have another question where I inexplicably get 312 instead of 321.....I'll assume it's right. Thanks.
01:30, 17 April 2006 (UTC)
Your answer is correct for the given problem. If m = 7.50 kg and F = 78.4 N the acceleration due to gravity is a = F / m = 10.45 ms^(-2) Cedars 10:22, 17 April 2006 (UTC)
- I think that you must make the below experiment in order to define the acceleration of gravity.
Get one string with the length is about 4m, tie one end of the string at a ceiling, another is free and tie one ion piece with the weight is about 100gram,you have a pendulum ,you can mesure the cycle of the pendulum (it is T),(the angle of pendulum swing is about 10 degree)You can mesure the lenght of string from a ceiling to ion piece, it is l, T = 1/2pi*l/g , you can extract g . I think Rick Block is right . Ngocthuan 06 01:36, 24 April 2006 (UTC)
Electromagnetic spectrum upside down?[edit]
Usually on a vertical scale the smallest number is on the bottom and the largest number is on the top or if the scale is horizontal the smallest number is on the left and the largest is on the right. The electromagnetic spectrum is always shown with the longest wavelengths (long electric waves, radio waves) on the bottom/left and the shortest wavelengths (X-rays, Gamma rays) on the top/right. I am wondering what is the scientific reason for doing this. Llarsson 01:20, 17 April 2006 (UTC)
- I think it's probably due to increasing frequency (and therefore, energy) as you go up the scale. — Knowledge Seeker দ 01:24, 17 April 2006 (UTC)
- It really doesn't matter. Frequency goes down as wavelength goes up, so either way you put it, one of the scales would go the "wrong" way. —Keenan Pepper 01:31, 17 April 2006 (UTC)
I understand that as wavelength increases, frequency decreases so one scale would go the "wrong" way. It seems to me though that since the electromagnetic spectrum shows waves and not frequency that one would lay out the scale to show wavelength increasing. Since it does not I wonder if it is more than just a 50-50 chance that it is laid out that way. Llarsson 01:50, 17 April 2006 (UTC)
- As the whole spectrum is continuous, there's no real right or wrong way to view it. Luthinya 10:40, 17 April 2006 (UTC)
BitTorrent[edit]
I need help with BitTorrent.
I downloaded a file from a search and then downloaded BitTorrent and opened the file with the program. It's finished downloading and I don't know where to go now so that I can view the video.
Thanks.
- When you opened the Torrent file, you told it where to download the file to. Remember where you saved it, and use that location to open the file. Simple. Here7ic 04:16, 17 April 2006 (UTC)
- Never lose porno, especially not when it is on a computer that other people also use. Open your downloads window and click the button that reveals the file location that you downloaded. Assuming that you haven't cleared the list, or do not use internet explorer. --
 Mac Davis] ⌇☢ ญƛ. 06:11, 17 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 06:11, 17 April 2006 (UTC)
is there a name for the tendency to see patterns?[edit]
I seem to remember at some point learning a name for the principle that humans tend to see patterns in random data where there are no patterns. Constellations, inkblot tests, that sort of thing. I seem to think there is someone's name associated to it, like "someone's law" or "someone's principle". Searching on the internet for a while provided nothing, and I'm starting to think that maybe I'm imagining it. Is there such a name? -lethe talk + 03:35, 17 April 2006 (UTC)
- Gestalt psychology? —Keenan Pepper 03:57, 17 April 2006 (UTC)
- pareidolia --Femto 13:51, 17 April 2006 (UTC)
Also, the specific case where patterns of human behaviour are seen where none actually exist, is called anthropomorphization. That's the longest word I commonly use, BTW. StuRat 21:41, 17 April 2006 (UTC)
The question used the interesting phrase "where there are no patterns". Who says there are no patterns? Nobody has ever suggested that the Southern Cross was consciously designed to appear this way to people in the southern hemisphere of planet Earth, so it's just a random occurrence. But there's still a pattern there, because my brain says there is. To someone who can't discern the cross (and I know some people who just can't see it), there is no pattern. So is there a pattern there or not? It seems to depend on the viewer's perception. JackofOz 02:40, 18 April 2006 (UTC)
- I would say the pattern is all in your mind. It's interesting how culture shapes the perception of patterns, as well. For example, the Big Dipper constellation looks like different things to different cultures. StuRat 02:00, 19 April 2006 (UTC)
- All patterns are "all in the mind" of the observer, as your Big Dipper example demonstrates. All so-called reality is an illusion (albeit, as Einstein said, a very persistent one). JackofOz 02:28, 19 April 2006 (UTC)
- I disagree. When looking at an actual large ladle, you really are looking at a "big dipper", not just imagining one (as when you look at collections of randomly located stars). StuRat 09:18, 20 April 2006 (UTC)
- It doesn't matter what the constellation is called from culture to culture, or what individuals imagine it looks like. In the context of this question, all that matters is that humans tend to notice patterns in such things as random collection of stars. I'm not imputing anything divine or mystic in that, but to say "where there are no patterns" is to deny the experience of billions of humans over a vast period of time. And from a mathematical point of view, to suggest there are no patterns in a bunch of millions of randomly scattered stars in a particular field of view would be utterly absurd. It's a wonder humans haven't noticed far more patterns in the sky than they actually have; they're all there just waiting to be noticed. JackofOz 11:03, 21 April 2006 (UTC)
- I disagree. When looking at an actual large ladle, you really are looking at a "big dipper", not just imagining one (as when you look at collections of randomly located stars). StuRat 09:18, 20 April 2006 (UTC)
- And there is a name for the tendency not to see patterns : atheism, or Darwinism. Also, written patterns are hard to decipher, so I'll add :) --DLL 19:40, 18 April 2006 (UTC)
- I don't think there's a specific term for this other than pattern matching or pattern recognition. The human brain is hardwired to see patterns all over the place, it is a decent evolutionary advantage though it sometimes goes pretty haywire. --Fastfission 02:33, 20 April 2006 (UTC)
- We're particularly good at seeing faces - so much so that we even once thought there may be a face on Mars. Some
idiotspeople still think so. — QuantumEleven | (talk) 11:54, 20 April 2006 (UTC)
- We're particularly good at seeing faces - so much so that we even once thought there may be a face on Mars. Some
It wasn't anything like that, it was actually a pirated motion picture.
I seem to recall learning in a psychology class about a study where subjects listened to a steady beats of a certain sound. They naturally heard them as organized into groups of two or three even though the tones were in fact all at regular intervals. Can't seem to find mention of it on the internet. Will continue searching. -Wiccan Quagga 00:37, 23 April 2006 (UTC)
Temperature of innerstellar or innerplanetary space.[edit]
I'm probably missing something here, but I can't find the temperature of either spaces. Help? Here7ic 04:14, 17 April 2006 (UTC)
- The space itself has no temperature, though you can measure it by placing objects into the space. Google says that the temperature of a perfectly conducting object at the distance of Earth from the sun will reach about 280K/7C, and you can expect objects at interstellar distances to experience near zero-K temperatures, though I'm not sure how near. freshgavinΓΛĿЌ 04:45, 17 April 2006 (UTC)
- Edit: Google also says that an object very far from galaxies and stars will probably reach a temperature of about 2.7K. Cold! freshgavinΓΛĿЌ 04:46, 17 April 2006 (UTC)
- The thing about temperature is that there has to be some matter for which you're measuring the temperature of. Temperature, at the molecular level, is basically the speed at which the molecules within matter move. Space, by definition, is a vacuum. It's devoid of matter. Therefore, no matter = no temperature. It's true, some will tell you that there may be fine dust particles in space, but then you'd be measuring the temperature of the dust particles, not the space itself. Others will say that the temperature of space is absolute zero, absolute zero (theoretically) being the state where the molecules within matter are absolutely motionless. But still, there must be matter to be measured. Space being a vacuum, there is no matter within which molecules are motionless, so absolute zero is not the answer either. Bottom line: difficult as it may be to explain or comprehend, space has no temperature. Loomis51 05:08, 17 April 2006 (UTC)
- Have to disagree about the neccessity for the presence of matter. What you say seems plausible, but not true. Even matterless space is filled with electromagnetic radiation that has a characteristic spectrum, (Black body spectrum). This is what's left of the big bang, when things were hotter and closer together. After expansion, the temperature of space is now, as was said above, 2.7K. It is literally, "the echo of the Big Bang". The discoverers of this b.b. radiation got the Nobel prize. It shows up most prominently in the microwave frequecies. B.B. radiation is a fetish object for cosmologists, a lot can be determined cosmologically from studing it; the exact spectrum is known to be identical to a standard black body spectrum. Some recent satellites that study it are COBE and WMAP. If the space in question HAS a gas in it as well, the gas molecules will have a range of velocites in a certain distribution. It is computed that the e.m. radiation (better spoken of as photons) with a black body spectrum would hit gas molecules in just the right proportions to give them the velocity distribution that they indeed have. If you like, you can think of so-called "empty space" as a "photon gas"; this is not an eccentric concept in physics terminology, but a conventional one. The explanation of b.b. radiation started with Max Planck, and was the start of quantum theory, so it's a big deal, not a minor detail. (Of course if you happen to be near a star, then the photon stream is larger and you have a higher T, as Google says.) GangofOne 05:41, 17 April 2006 (UTC)
- You're wrong. The definition of temperature is the average molecular kinetic energy of the matter within a given volume. No matter means no temperature, by definition.
- Nor, for the record, has anyone pointed out the fact that space is not, in fact, a pure vacuum; space is actually a rather bad vacuum - there's all kinds of particulate matter there. (See here for the various measures of the density of space). We can produce much better vacuums on earth with relatively simple vacuum pumps. If space were a perfect vacuum, space-craft would not need heating systems (because there would be nothing to conduct heat from the aircraft into space). So, getting back to the question at hand, the 2.7 degree kelvin measurement is almost certainly a reference to the temperature of this interspacial matter. Raul654 06:08, 17 April 2006 (UTC)
- (two edit conflicts) Temperature can be defined for lots of systems, including the electromagnetic field. Thermodynamics has come a long way since the ideal gas law. And in a perfect vacuum, you still radiate. Melchoir 06:17, 17 April 2006 (UTC)
- Have to disagree about the neccessity for the presence of matter. What you say seems plausible, but not true. Even matterless space is filled with electromagnetic radiation that has a characteristic spectrum, (Black body spectrum). This is what's left of the big bang, when things were hotter and closer together. After expansion, the temperature of space is now, as was said above, 2.7K. It is literally, "the echo of the Big Bang". The discoverers of this b.b. radiation got the Nobel prize. It shows up most prominently in the microwave frequecies. B.B. radiation is a fetish object for cosmologists, a lot can be determined cosmologically from studing it; the exact spectrum is known to be identical to a standard black body spectrum. Some recent satellites that study it are COBE and WMAP. If the space in question HAS a gas in it as well, the gas molecules will have a range of velocites in a certain distribution. It is computed that the e.m. radiation (better spoken of as photons) with a black body spectrum would hit gas molecules in just the right proportions to give them the velocity distribution that they indeed have. If you like, you can think of so-called "empty space" as a "photon gas"; this is not an eccentric concept in physics terminology, but a conventional one. The explanation of b.b. radiation started with Max Planck, and was the start of quantum theory, so it's a big deal, not a minor detail. (Of course if you happen to be near a star, then the photon stream is larger and you have a higher T, as Google says.) GangofOne 05:41, 17 April 2006 (UTC)
- The thing about temperature is that there has to be some matter for which you're measuring the temperature of. Temperature, at the molecular level, is basically the speed at which the molecules within matter move. Space, by definition, is a vacuum. It's devoid of matter. Therefore, no matter = no temperature. It's true, some will tell you that there may be fine dust particles in space, but then you'd be measuring the temperature of the dust particles, not the space itself. Others will say that the temperature of space is absolute zero, absolute zero (theoretically) being the state where the molecules within matter are absolutely motionless. But still, there must be matter to be measured. Space being a vacuum, there is no matter within which molecules are motionless, so absolute zero is not the answer either. Bottom line: difficult as it may be to explain or comprehend, space has no temperature. Loomis51 05:08, 17 April 2006 (UTC)
- No, Gang of One is right. The real definition of temperature has to do with the rate of change of entropy when you increase or decrease the internal energy; see thermodynamic temperature.
- Spacecraft do not lose heat primarily by convection, but by radiation; there doesn't have to be anything touching them for them to get cold. See black body radiation. The 2.7 kelvin temperature refers to temperature of the black body background radiation of the universe, not to the interstellar medium per se. --Trovatore 06:17, 17 April 2006 (UTC)
- Dang, it's a game of seconds out here! Melchoir 06:19, 17 April 2006 (UTC)
- [Edit conflict, didn't read Raul's comment] You obviously know a lot more about what we're talking about than I do, so I'm sticking my foot out by saying that I find it hard to agree with you. I'm getting everything except the fact that you say the radiation left over from the big bang makes space itself 2.7K. It may be a semantic argument ... to experience or measure temperature (sense heat) requires absorption (loss) of heat in some way, and since space doesn't absorb heat or energy (as far as I know), I find it difficult to think that it could possibly have any, as you imply when you say the temperature of space is 2.7K. I'm having trouble finding the words but I feel like I'm dealing with a tree falling in the woods with nobody there to hear it. freshgavinΓΛĿЌ 06:16, 17 April 2006 (UTC)
- So I guess, in a sense, the vacuum does "absorb heat"; that is, you can add heat to the vacuum by increasing its content of photons in a distribution corresponding to the black-body law. See how much energy it takes to add those photons corresponding to a given increase in temperature, and you have a reasonable definition of the heat capacity of the vacuum. I think this is a standard notion but I'm a little out of my depth here; maybe Melchoir could explain it better. --Trovatore 06:28, 17 April 2006 (UTC)
Well... if you want to talk about the energy contributed by a new photon, that sounds like a chemical potential.(eh, I misread you) The volumetric heat capacity is pretty simple to define, though; it's just the temperature to the third power, modulo factors of c and hbar. Melchoir 06:55, 17 April 2006 (UTC)
- So I guess, in a sense, the vacuum does "absorb heat"; that is, you can add heat to the vacuum by increasing its content of photons in a distribution corresponding to the black-body law. See how much energy it takes to add those photons corresponding to a given increase in temperature, and you have a reasonable definition of the heat capacity of the vacuum. I think this is a standard notion but I'm a little out of my depth here; maybe Melchoir could explain it better. --Trovatore 06:28, 17 April 2006 (UTC)
- [Edit conflict, didn't read Raul's comment] You obviously know a lot more about what we're talking about than I do, so I'm sticking my foot out by saying that I find it hard to agree with you. I'm getting everything except the fact that you say the radiation left over from the big bang makes space itself 2.7K. It may be a semantic argument ... to experience or measure temperature (sense heat) requires absorption (loss) of heat in some way, and since space doesn't absorb heat or energy (as far as I know), I find it difficult to think that it could possibly have any, as you imply when you say the temperature of space is 2.7K. I'm having trouble finding the words but I feel like I'm dealing with a tree falling in the woods with nobody there to hear it. freshgavinΓΛĿЌ 06:16, 17 April 2006 (UTC)
- Here is a link [38]. The temperatures of space differ greatly. Space near a star is a lot hotter than space far away from any galaxy. --
 Mac Davis] ⌇☢ ญƛ. 06:15, 17 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 06:15, 17 April 2006 (UTC)
Melchoir, I did specifically refer to the fact that within what we normally term "space" there are may indeed be particles. I suppose it's a matter of semantics. If you define space, as I do, as the absence of matter, then by definition, it's a vacuum. If there are stray particles, we're not strictly speaking about space anymore. Of course those particles have temperature. When I speak of "space", I speak of any area devoid of matter. In other words, Space=Vacuum, and Vacuum=no temperature. Loomis51 06:31, 17 April 2006 (UTC)
- Actually, I have no problem with what you wrote. There's a bit of ambiguity about whether you're considering photons as matter, but we probably don't have to get into that. Melchoir 07:00, 17 April 2006 (UTC)
Okay, allow me to elaborate on my question to clear things up. What would the average surface temperature of the Earth be if it had no atmosphere and the sun was a white dwarf of, oh, .6 solar masses? Here7ic 06:49, 17 April 2006 (UTC)
- I assume you'll also want to paint the Earth black and shut down its radioactivity? (Not that I feel like doing the work on this question, just saying.) Melchoir 07:03, 17 April 2006 (UTC)
- Raul654 mentions the particles in space and says man-made vacuums are superior, which is wrong, terrestial vacuums are no where NEAR as good as space, as the link he provides even says: "Space is a nearly perfect vacuum, even better than the best ones made in labs on earth," (One complication that wasn't mentioned was that the black body spectrum refers to "thermodymanic equilibrium", the state without macroscopic changes. There are cosmic rays and stuff moving through space that are NOT in equilibrium. Just mentioning that in passing.) The link from the straightdope that MacDavis provided is excellent. And now, an experiment. A thought experiment. Let's consider a region of space that is a perfect vacuum that is in a chamber. That is, an enclosed space that is a vacuum. The walls are obviously made of matter; but the matter is AT SOME TEMPERATURE. Let's say we have a regular themometer, made of materials suitable for the temperatures we are about to measure. The bulb is painted black. The thermometer extends into the chamber, and it's readings can be recorded somehow. So, heat up the box to 2000 deg. C so that the walls of the chamber are at 2000degC. Remember, it's a perfect vacuum. (And the themometer is isulated from the sides of the chamber.) What temperature does the thermometer read? Absolute 0, -273degC, ie no temperature? As you change the T of the walls of the chamber, from the lowest to the highest, what temperature does the thermometer read? Is it plausible that it is absolute zero? --GangofOne 07:22, 17 April 2006 (UTC)
- freshgavin says "...and since space doesn't absorb heat or energy (as far as I know)..." Well, let's say you shine a flashlight toward the Big Dipper. Energy came out of the flashlight, and it went somewhere. It went into space. So space absorbed it. The sun shines. The light that came off the sun isn't on the sun. It's in space now. So space "absorbed" it. So at the big bang, when the universe was as small a point as you can conceive, it was really hot, but then is expanded out, and the energy that made it hot was spread around as the size of the universe became larger, so the INTENSITY of the heat at a given spot became less, ie it cooled. It's expanded so much that the temperature of the energy that was in the big bang is now a uniform 2.7K. At least that's what the cosmologists say, anyway. It's some pretty mind streching stuff. --GangofOne 07:51, 17 April 2006 (UTC)
- A true vacuum would only transmit heat, not absorb it. That is, while radiation could pass thru it, there would be nothing in it to absorb the heat. So, eventually that flashlight beam will heat some distant matter, depending on whether you believe in a collapsing universe, etc. Also note that space has a very low density of matter, so has a low, but not zero, capacity to store heat. So, you could heat up a large volume of space with very little energy. StuRat 21:20, 17 April 2006 (UTC)
- Yes, though I'm not sure which side StuRat is supporting. I still don't really get you GangofOne. If you shine a light through me, or any other type of matter, I experience a slight increase in temperature, I may even be able to feel it, and much of the light is reflected but almost none of it goes through to the other side of me. There is a noticeable loss of energy as the light beam hits me. If you shine that same flashlight into vacuumed space, the beam travels through and there is no loss of energy so I don't see how you can use the word "absorbed".
Someone said that temperature had to do with "the rate of change of entropy when you increase or decrease the internal energy", but the article he linked to states simply that temperature is the relative molecular activity, which would support the fact that temperature doesn't exist in a vacuum.
I do believe what you say about constant levels of b.b. radition, but I would consider those as potential temperature, or unabsorbed energy. There's probably a more scientific way to say that : [. freshgavinΓΛĿЌ 06:47, 18 April 2006 (UTC)- Light is energy. When the light leaves the flashlight it enters the space. That's all I mean by absorb. Just what thing has it.
- The article you refer to thermodynamic temperature Says: "Strictly speaking, the temperature of a system is well-defined only if its particles (atoms, molecules, electrons, photons) are at equilibrium." Note that it is allowing the case where the "particles" are photons and only photons; "electromagnetic radiation" is the older way to express what is also called photons. Conventionally, they aren't considered "matter" (although if you did, the problem in question would define itself away, because the so-called vacuum now has "particles" in it.) Anyway, I feel I have to somehow to convey to you is that "empty space" with no light going through it is different from "empty space" with light going through it. In the later case, the space has energy that's not in the former. And if it's in the special case of "thermodynamic equilibrium" then it has a well defined number which characterises its intensity, know as its 'temperature'. What your intuitions is telling you (when you say "I would consider those as potential temperature, or unabsorbed energy.") is that without matter there, there is no temperature. What I am attempting to convey is that just the presense of photons and nothing else, has a temperature. In the thought experiment above I attempted to make it more intuitive by considering a closed evacuated cavity. It has a well defined temperature that you can measure. You can stick your hand in and sense it. A thermometer isolated from the sides reads the same temperature as a thermometer measuring the side of the cavity. The only connection of the thermometers is via the photons. (This cavity I discribe is the paradigmatic black body that's in all the textbooks.) Try me again if still obscure. GangofOne 07:27, 18 April 2006 (UTC)
- Yes, you're making yourself perfectly clear. Like I said above this seems much like an argument in semantics. Even your vaccuum box thought experiment could be argued by me as containing unabsorbed energy that is absorbed by the walls and the thermometer thus bringing about a non-zero temperature. It could just as easily be argued your way. I guess that's one reason why people want to figure out if photons should be considered matter or not. freshgavinΓΛĿЌ 05:43, 19 April 2006 (UTC)
- Light is energy. When the light leaves the flashlight it enters the space. That's all I mean by absorb. Just what thing has it.
- Yes, though I'm not sure which side StuRat is supporting. I still don't really get you GangofOne. If you shine a light through me, or any other type of matter, I experience a slight increase in temperature, I may even be able to feel it, and much of the light is reflected but almost none of it goes through to the other side of me. There is a noticeable loss of energy as the light beam hits me. If you shine that same flashlight into vacuumed space, the beam travels through and there is no loss of energy so I don't see how you can use the word "absorbed".
Street Lights[edit]
Would anyone know how bright a basic street light is in lumens? Not a traffic light but one of those lights that illuminate streets and highways after dark. I'm sure they're not all the same, so a reasonable range would do quite fine. Thanks! Loomis51 04:43, 17 April 2006 (UTC)
- According to this site.. its says that a good street light is 20 lumens per square meter (or 1.2 candela per square meter) while for a poor street light is 0.1 lumens per square meter (or 0.006 candela per square meter).... Hope that helped... :-D .... Jayant,17 Years, India • contribs 09:12, 17 April 2006 (UTC)
Once again, Jayant, your level of knowledge is beyond mine. I'm not sure what to make of the "per square meter" part. From what I understand, 20 lumens is not very bright at all ... it's about the brightness of a flashlight if my information is correct.
I know you've been very generous with your time, but the information I need is:
1) What type of bulb is in a standard street light. Incandesent? Fluoresent? 2) How much brightness does it produce? 3) How many watts of energy does it require?
Thanks again! Loomis51 17:25, 17 April 2006 (UTC)
- I can understand your confusion in understanding the units... The brightness of a light source is measured in Illuminance which is lumens per meter square... generally when we normally use lumens, we actually mean lumens per meter square...but we unwittingly drop the per meter square part... and i think the 20 lumens (per square meter) is wrong.... and i also think i might have found out the answers for all the other questions as well.... Here it goes...
- 1) According to the article Street light, Today, street lighting is most commonly achieved using high-intensity discharge lamps, often sodium vapor lamps. Such lamps provide the greatest amount of illumination for the least consumption of electricity.
- 2) & 3) I had a bit of trouble finding about the brightness of a street lamp since brightness is a vague term...nevertheless, i found this site which lists the brightness and the wattage required for different lamps in a table...it also lists some extra information as well... i think you would be more interested in the metal halide and the low and high pressure sodium lamps as they are the most commonly used nowadays...
- I hope that you find that information helpful...and i once again apologise for the 20 lumens per meter square information... If you have anymore questions please dont hesitate to ask me..Cheers!! Jayant,17 Years, India • contribs 18:35, 17 April 2006 (UTC)
I believe the confusion here is that the asker asked how bright the light SOURCE is, which would be in lumens, and the reply was in terms of how brightly the street was lit, which would be in lumens per square meter. The height of the light, among other things, would effect the density of light on the street, even when the brightness of the light source is held constant. StuRat 20:39, 17 April 2006 (UTC)
Thanks again, Jayant. (Thanks to StuRat too.) I'll need some time to digest all this information so I probably won't bother you with more questions until I have all this figured out more or less in my head.Loomis51 00:56, 18 April 2006 (UTC)
- It works like this. Briefly, you get a certain amount of light out of the bulb: a certain number of lumens to play with. Now, when you're illuminating something – a street, for example – that light gets spread out over a certain area. If you mount the lamp up higher, you can illuminate a larger area, but each unit of area gets a smaller fraction of your total light. The amount of light each unit of area receives is measured in lumens per square meter; it tells you how brightly illuminated a given patch of ground is.
- The metal halide and sodium vapour lamps used for street lighting range in brightness from a few thousand to upwards of a hundred thousand lumens, depending on the location and application. A 100 watt incandescent light bulb, for reference, delivers about 1700 lumens. You may find these tables useful: [39]. TenOfAllTrades(talk) 01:29, 18 April 2006 (UTC)
If you want a little more information, I can add that, at least here in my home state the illumination guidelines for the level of illumination on the road surface is recommended to be an average of about 0.6 foot-candles to about 1.5 foot-candles. It depends on the function of the road (residential, arterial, highway/freeway).
I can also bring home a table of lamp wattage and lumen output and add it here tomorrow. One that I can remember off the top of my head is that a 200 Watt lamp has an output of 22,000 lumens. In design practice the output is depreciated to 62% of that. The two depreciation factors are first that a lamp will gradually decrease in output over its lifetime, and also that the fixture will become dirty over the life of the lamp. The heating and cooling cycles cause the fixture to breathe, and dirt accumulates on the lamp and the reflector. So a design that requires 0.6 fc on a roadway using 200W lamps will actually be illuminated to a higher level when everything is brand new, but the average illumination over the lifetime of the system will be closer to the design target value.
HPS bulb output values:
Historically Traffic Ops used values which were reduced to 62% of total output, to account for ballast and dirt depreciation. Newer analysis software handles the depreciation automatically, so the full initial light output value must be known.
The total Lamp Lumen Factor (also called Maintenance Factor) is calculated as:
LLF = LLD x LDD x BF
LLD = Lamp Lumen Depreciation, = 73%
LDD = Lamp Dirt Depreciation = 85%
BF = Ballast Fac tor
LLF = 73% x 85% = 62%
Lamp size, Watts
Lumens (full output) Lumens (reduced to 62%)
100 W 8900 5500
150 W 16000 9920
175 W
200 W 22000 13640
250 W 275000 17050
310 W 37000 22950
400 W 49000 30400
Luminaire classifications:
Short – Medium – Long
Cutoff- Semi-cutoff – Non-cutoff
Type II – Type III – Type IV
Short, Medium, and Long refer to how far light is directed up and down the roadway. Guidelines for use: Spacings given as multiples of the mounting height (MH)
Short: 4.5 MH
Medium: 7.5 MH
Long: 12.0 MH
Cutoff describes how much glare is to be expected from the fixture.
Type II, III, and IV designations indicate how far the fixture directs light across the width of the roadway. Type II = narrow, and Type IV is widest.
Lamp mounting height: Wattage Mounting height (ft)
70 15 100 20 200 25 250 30 310 35 400 40
Lighting conversion formulae: Lumens / sq. ft. = Ft-Candles
Ft-Candles x 10.7639 = Lux Lux x 0.0929 = Ft-Candles
Illuminating Engineering Society of North America
www.iesna.org
Roadway Lighting, book no. RP-8-00
Electric Charges[edit]
Why does positive charge attracts negative charge and negative charge repels negative charge? I know it does but why? Does it have something to do with Quantum Electrodynamics?
- It sure does! In quantum field theory, like charges attract if they couple to a spin-0 or spin-2 field, and they repel if they couple to a spin-1 field. The photon of quantum electrodynamics is a spin-1 particle, so like electric charges repel. The graviton is a spin-2 particle, so masses attract. Melchoir 07:30, 17 April 2006 (UTC)
Thanks for replying but what does couple mean? Also I'm a bit confused when you say spin-0 and stuff but thanks anyway!
- how come photons has to do with electric charges?
- Some of the terminology is explained a bit at Coupling (physics) and Scalar field. Photons are the carriers of the electromagnetic force, so electric charges interact by exchanging photons. It takes years to learn all the subtleties behind these concepts, and I'm not quite there yet! Melchoir 18:51, 17 April 2006 (UTC)
The simplest illustration (that I'm aware of) of the relationship between spin and attraction/repulsion is in chapter I of Anthony Zee's Quantum Field Theory in a Nutshell. The book, although likely the simplest text on Quantum Field Theory, is by no means accessible to a general audience—and it looks (from skimming the section) like it relies on the particular forms of the scalar, photon, and graviton propagators—so I fear there's no way to explain this phenomenon convincingly in words. -- SCZenz 23:48, 17 April 2006 (UTC)
- Word. It's also at the end of Peskin and Schroeder ch.4. Melchoir 00:16, 18 April 2006 (UTC)
Remember to think in three dimensions. Use the simplest example, a hydrogen isotope consists of a positive proton and one orbiting negative electron. This isotope is so unstable it would need to ionically bond immediately or it would transform. The attraction that you refer to is actually the result of the vector that these particles follow. A positive charge follows an outward vector and a negative charge follows an inward vector. That is why they naturally bond, they run into each other. I think that attraction is a poor word to describe particle velocities. Attraction is way too slow. I AM THE AUTHOR OF THIS EXPLANATION AND CAN ONLY CITE MYSELF. IN ORDER TO VERIFY IT, YOU MUST NOT DISPROVE IT.
Mozilla Firefox[edit]
I use Firefox and I want to conserve my "Favourites" list. How can I save it somehow? (I have a complicated structure with overlapping bookmarks in different folders.) Thanks! ttse92
- Click on 'Favourites/Bookmarks', then click 'Manage favourites/bookmarks' - a window should open. In it, in the menu bar, click File -> Export.
- I hope this is accurate, I'm re-translating Firefox from Polish into English right now :) --Ouro 08:47, 17 April 2006 (UTC)
Right got that thanks!
Alcohol and medicine[edit]
Is it safe to consume alcohol while taking prescribed medication ? Some people tell me that the alcohol dilutes the medication and flushes it out of my system too soon for it to have any worthwile effect, while others say it agravates the illness and make it worse. Yet others wil say it makes no difference. Can you please clarify this for me ?
- It depends on what kind of medicine you are taking, the type and why you are taking it. In general, it is advisable to avoid alcohol consumption during medical treatment. Alcohol may have a different effect on anything you take, depending on the chemical structure of the medicine. Always read the label. --Ouro 08:50, 17 April 2006 (UTC)
- Generic redundant answer. You really should consult your physician or pharmacist before consuming alcohol with your specific medication. Some medications do not react with alcohol and some do. Also an interaction could possibly be caused by multiple medications with alcohol. Only your doctor or pharmacist knows what medications you are on, and which interactions should give you worry.--Tollwutig 14:23, 17 April 2006 (UTC)
You didn't say what kind of medication you're taking, so I can't help you. Erik the Rude 16:12, 17 April 2006 (UTC)
- If you ask this question to a medical professional they will look up both drugs (alcohol and whatever else it is) in a huge table of known drug interactions and contraindications. For great justice. 18:30, 17 April 2006 (UTC)
Another factor not mentioned above is that some medications have their first pass through the liver - the same route to the bloodstream used by alcohol. If the liver is busy with one task it can be far less effective on the other - or worse, you can overstress your liver, not something you want to contemplate. This is even true with some simple household medications (ISTR paracetamol and alcohol is a very bad combination, for instance). But in any case, without knowing what the drugs are, we're left with generic answers, and even if we knew the type of drug our advice would be nowhere near as good as advice your doctor or pharmacist could give. Pop into your local chemist's/pharmacist's and ask there. Grutness...wha? 01:45, 18 April 2006 (UTC)
Thank you all for your kind contributions and explanations. I was recently prescribed Co-Amoxiclav Tablets BP (500/125mg) for 5 days for a bad phlegm-producing cough and slight elevation in temperature accompanied by aches and pains, runny nose and sore/inflamed throat from the coughing. I have not started to flap my arms yet !! The doctor also gave me cough syrup to get rid of the phlegm and Paracetemol for the aches and pains and to reduce inflamation. When I asked the doc about alcohol I was told it differs with patient's strength, mental wellbeing and tollerance of the medication and the strength and type of alcohol. I avoided the alcohol but was always curious as to why we were given different advise from different people especially those non-doctors who advocate hot whiskey with sugar and lemon while yet others recommend a glass of red wine daily regardless of doctors orders. Once again thank you all for your advise. - Futiwali
Complementary to the answers already provided, alcohol/medication combination issues can be explained in a large part by drug-drug interaction. One of the main components of drug metabolism is the liver. What makes the liver so special is that it contains a wide variety of enzymes in the cytochrome P450 family. (You can find here everything you ever want to know about P450 enzymes.)
More specifically, alcohol (i.e. ethanol) is metabolized in the liver by the CYP2E1 (or cytochrome P450 allele 2E1) enzyme. At any given time, your liver has only a limited reserve of CYP2E1, so when you consume alcohol, a significant quantity of this enzyme is used to metabolize the ethanol. Say that at the same time, you take a drug that also needs the same enzyme for its metabolism, for example acetaminophen (or Tylenol). You can now see the problem: alcohol mixed with Tylenol will deplete your liver of CYP2E1 thus letting through a toxic quantity of ethanol and acetaminophen, which is not healthy. The complete drug metabolism process is much more complex, of course, but I hope that this can illustrate the mechanism involved.--JLdesAlpins 23:24, 19 April 2006 (UTC)
How do people lose mass?[edit]
When someone loses weight, how exactly does it leave the body? It seems unlikely that it's moved from fat cells or whatnot into the digestive tract; are waste chemicals from fat metabolism filtered through the kidneys and urinated out? Is the carbon bonded to oxygen and breathed out as CO2? grendel|khan 09:16, 17 April 2006 (UTC)
- The fat cells contribute a lot of the weight of the human body..so when you are exercising, the fat gets converted into energy...so the weight of the body decreases... Jayant,17 Years, India • contribs 09:26, 17 April 2006 (UTC)
- What, like e=mc^2 converted to energy? Given the figure of 3500 kCal per pound of human mass gained or lost that I've been given (around 15 MJ), compared to the energy in that mass were it actually converted into energy (around 4.1*10^10 MJ), you're about nine orders of magnitude off. I don't think it works like that. grendel|khan 10:48, 17 April 2006 (UTC)
- This is not true. Mass is only converted to energy in a nuclear reaction. In the body, fat is broken down into fatty acids through the process of catabolism. This involves chemical reactions that release chemical potential energy. I believe these fatty acids are then passed out through the body as urine or excrement. I believe this is the correct explaination but I am not sure - I just wanted to correct the perception that fat is converted directly to energy and this reduces a person's mass. Cedars 10:51, 17 April 2006 (UTC)
- (Edit conflit with Cedars) Um, no. e=mc^2 only applies to nuclear reactions (a nuclear reaction is one where the nucleus of an atom changes). Fats (adipose tissue) store energy in the form of chemical bonds. Breaking those bonds (and discharing the left-over waste molecules) is how the body loses weight. Raul654 10:51, 17 April 2006 (UTC)
- Um, no. E=mc^2 applies perfectly well to chemical reactions. It is just not a significant effect given the amounts of energy involved in breaking and forming chemical bonds. Relativistic effects are significant for heavy-element chemistry though, as the innermost electrons of atoms with high nuclear charges move fast enough to have a significant relativistic mass. Which in turn leads to a contraction of the atomic radius. A practical example is the color of gold. An energy level difference which otherwise (and in lighter metals) would be in the UV range is shifted down to blue part of the spectrum. Leading to absorption of blue light and the yellowish color. Now, to answer the original question here: The main products of fat metabolism is CO2 and water - I think you can guess the respective ways those leave the body. --BluePlatypus 12:29, 17 April 2006 (UTC)
- Um but cellular respiration does not include many heavy metals. Thankfully you did answer the question. Fat metabolism creates water and fatty acides. Cellular respiration of those fatty acids creats CO2 and more water. Molecularly water is rather heavy. Generally the human bladder which holds only several fluid ounces can at the same time hold half a pound of weight. (Want to know how much you bladder holds. Measure yourself with a digital scale before and after urinating.) --Tollwutig 14:30, 17 April 2006 (UTC)
- Nobody said cellular respiration included heavy metals. I was making the point that relativistic effects do occur in chemistry. I didn't say that you needed heavy metals for the energy of a chemical reaction to lead to a E=mc^2 loss of mass. All chemical reactions give that loss of mass, it's just not significant, as I said. That particular relativistic effect is not more significant for heavy elements than for light ones, because the electrons involved in chemical reactions are the outermost (valence) electrons, not the innermost ones. In fact, that effect is smaller for heavy elements, because their valence electrons have lower kinetic energy than those of light elements. (Also, water isn't particularily heavy in a physiological perspective. The density of the human body is very close to that of water, since we're mostly made of it) --BluePlatypus 19:05, 17 April 2006 (UTC)
- Um but cellular respiration does not include many heavy metals. Thankfully you did answer the question. Fat metabolism creates water and fatty acides. Cellular respiration of those fatty acids creats CO2 and more water. Molecularly water is rather heavy. Generally the human bladder which holds only several fluid ounces can at the same time hold half a pound of weight. (Want to know how much you bladder holds. Measure yourself with a digital scale before and after urinating.) --Tollwutig 14:30, 17 April 2006 (UTC)
- Um, no. E=mc^2 applies perfectly well to chemical reactions. It is just not a significant effect given the amounts of energy involved in breaking and forming chemical bonds. Relativistic effects are significant for heavy-element chemistry though, as the innermost electrons of atoms with high nuclear charges move fast enough to have a significant relativistic mass. Which in turn leads to a contraction of the atomic radius. A practical example is the color of gold. An energy level difference which otherwise (and in lighter metals) would be in the UV range is shifted down to blue part of the spectrum. Leading to absorption of blue light and the yellowish color. Now, to answer the original question here: The main products of fat metabolism is CO2 and water - I think you can guess the respective ways those leave the body. --BluePlatypus 12:29, 17 April 2006 (UTC)
- So all the relativity stuff is fascinating, but the answer to the original question is, yes, the fat gets oxidized and thereby converted to CO2 and water, which is disposed of in the usual ways, through the lungs, skin, and excretory organs. That overwhelmingly accounts for the accounts-payable side of the mass-balance ledger; the loss of mass corresponding to chemical energy is a negligible effect. --Trovatore 19:11, 17 April 2006 (UTC)
Why are there no EOS-compatible third-party camera bodies?[edit]
There are a variety of third-party EF lens mount camera lenses available from manufacturers like Tamron or Sigma, made to fit the Canon EOS system, specifically Canon EOS cameras. The EF lens mount uses an electrical-only system to adjust the aperture and focus from the body. (There's a nice picture
First, why can't I find any information about the electrical properties or data structures used by those electrical contacts? Are all third-party lens manufacturers under NDA with Canon? Why would they not make these specifications public?
Secondly, why are there third-party lenses manufactured, but not third-party camera bodies? I've seen interest in things like monochrome-only sensors (getting black and white from a color sensor involves dealing with the Bayer filter and interpolation), or in-body image stabilization, or real-time previewing, the last two of which are available in other SLR bodies, just not the ones that Canon makes. So why doesn't Sigma or Tamron make an EF-compatible body? grendel|khan 10:40, 17 April 2006 (UTC)
- Perhaps it's because of licensing and patents? Obviously Canon don't want other manufacturers using Canon's technology to sell their competitors' cameras. Perhaps (I'm guessing) the vital, patentable parts of the EF mount system are all in the body, while making EF-compatible lenses is just a matter of "coincidentally" having the contacts in the right place on your lens. Alternatively, perhaps Canon make their money selling cameras, and don't make so much on their lenses. Then they'd have an interest in allowing third parties to produce lenses, so making their cameras more attractive propositions. HenryFlower 12:23, 17 April 2006 (UTC)
- Could any patents on the original lens design be in effect? The spec was released in 1987 (according to Canon EF lens mount), so that any patents would be at least nineteen years old now. I'm sure they have new patents on image stabilization and diffractive optics, or whatever their shiny new toys are, but the mount itself can't still be under patent, can it? grendel|khan 02:44, 18 April 2006 (UTC)
- Another posibile reason why the lens specs aren't available is that if the third-party manufacturers don't get any specs from Canon then they will have spent time and money reverse engineering the spec and don't want their competitors benefiting from their work and so are keeping their (reverse engineered) specs secret. -- AJR | Talk 23:42, 17 April 2006 (UTC)
First use of taps/faucets[edit]
When did taps/faucets first become used in domestic settings in Europe? ~M Cain
- Hint : the french name is robinet, and the same in Bahasa, Galego and Greek. It's coming from Robin, a person name and the name of the lamb in the chanson de Renard, which (the story) gave names to other animals, as renard (fox), that was goupil (latin vulpes) before.
- So ... the object came after the period when family names were formed. --DLL 19:34, 18 April 2006 (UTC)
- Eh, what? That makes no sense to me. Anyway, the palace of Knossos had plumbing a few thousand years ago, and the Romans also had plumbing (the word refers to that- since Romans used lead pipes, 'plumbum' being lead.). --BluePlatypus 20:16, 19 April 2006 (UTC)
Comment about a Wikipedia Science Entry[edit]
The Wikipedia entry for Paracelsus makes no mention of the fact that Paracelsus invented a medical term. Specifically, Paracelsus invented the term 'synovia'. Credit is given to Paracelsus for inventing the medical term 'synovia' in the Definition given at the following URL: http://www.medterms.com/script/main/art.asp?articlekey=5684.
If you would, please examine the given URL references and update your various Wikipedia Online Resources accordingly. —The preceding unsigned comment was added by 66.24.11.3 (talk • contribs) 12:55, 17 April 2006.
- Interesting! I don't think that's a reliable source, but you can always bring it up on Talk:Paracelsus or Talk:Synovium and see what others think. Melchoir 20:07, 17 April 2006 (UTC)
Why does Cola go flat quicker in a mug than in a glass?[edit]
I, and a few friends, have noticed that Cola and, to a lesser extent, other fizzy drinks seem to lose their "fizz" quicker when you pour them into a ceramic mug than when they are in a glass. We thought initially it was the thickness of the vessel that made the difference as mugs are generally thicker than glass tumblers. But when a very thick glass tankard is used the drink still stays fizzy.
I thought it might be something to do with the amount of imperfections in the glaze on the ceramic mug compared to those in the glass.
I'm sure my friend's nonesensical suggestion that the bubbles can't 'see' out of a ceramic mug and therefore get scared and pop quicker, is not the answer. So can anyone offer a sensible solution? --Ukdan999 16:54, 17 April 2006 (UTC)
- I guess maybe its because the carbon-di-oxide in the coke reacts with the ceramic and the coke becomes flat faster...or it could be because of the ceramic mug is porous and it might allow the gas to escape out of its walls... Jayant,17 Years, India • contribs
- I don't know the answer, but I think you are on the right track to wonder about the "imperfections" in the glaze. The bubbles tend to form around nucleation sites, typically any sort of relatively rougher surface. As an extreme example, my hypothesis is that if you poured coca cola into a cup lined with sandpaper, it would go flat much much quicker. As an aside, there were experiments done with boiling water - the behaviour of boiling is quite different if water is boiled in a nearly perfectly smooth container...but this is a digression. --HappyCamper 17:38, 17 April 2006 (UTC)
- While not a total answer probably it has something to do with this http://www.coolquiz.com/trivia/explain/docs/drinks.asp . The porous nature of ceramic would allow more CO2 to escape through the sides than glass.--Tollwutig 17:45, 17 April 2006 (UTC)
- Would the ceramic still be porous even though it is glazed? --Ukdan999 18:31, 17 April 2006 (UTC)
- I think the much more plausible explanation is that your mug is wider than the glass, which provides more surface area, which lets the gas escape faster. I doubt it's a chemical reaction - both glass and ceramic materials are inert, at least with respect to the contents of a soft-drink. The nucleation-site idea is a thought, but a glazed ceramic surface isn't really much less smooth than a glass one. --BluePlatypus 18:53, 17 April 2006 (UTC)
- I don't think it's down to the surface area as the effect still happens when I use a ceramic mug and an almost identically-sized glass/pyrex mug. Even using a very wide glass tankard, the coke still stays fizzy longer than when it's in a standard coffee/tea mug. --Ukdan999 19:15, 17 April 2006 (UTC)
Let me suggest a psychological reason. Since you see the bubbles on the sides of a glass container, it seems "fizzier" than if in an opaque container where you can't see the sides. This effect would be more pronounced with an opaque liquid. I suggest you try a clear carbonated beverage and see if this reduces the effect. Also, try evaluating the "fizziness" while blindfolded, to try to mask this effect. StuRat 20:20, 17 April 2006 (UTC)
I think the key is in HappyCamper's answer; the idea of a reaction surface or nucleation site is often used in chemistry - in my chemistry class we found that the reactions were faster in scratched glassware as there was somewhere that the reaction could occur.
- While I agree that a rougher surface will provide more nucleation sites, I don't agree that glazed ceramics are rougher than glass. StuRat 22:20, 17 April 2006 (UTC)
- The answer is definitely because of nucleation sites--once bubbles begin to form at a rough point in the surface, they draw more C0² molecules to the site. The bubbles grow larger and soon attain enough buoyancy to break free and rise to the top. This process continues until the soda reaches equilibrium; with many more nucleation sites, the soda goes flat much quicker. Your typical ceramic mug is much rougher than glass. As an example of this in action, if you were to drop a Mentos in a soda, for instance, the soda will boil over releasing its CO2 in a flash because Mentos provide an incredibly rough surface area, and as it quickly melts away, it's constantly exposing more nucleation sites for the gas to come out of solution.--Fuhghettaboutit 22:33, 17 April 2006 (UTC)
- I think it might be because the ceramic is rougher, than the glass and thus has more nucleation sites, there is more surface area, and the roughness is prime. Oh, Fugettaboutit already said that. --
 Mac Davis] ⌇☢ ญƛ. 05:19, 18 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 05:19, 18 April 2006 (UTC)
The fact that you believe the effect is more pronounced with coke than with clear fizzy drinks prompts me to side with StuRat on this. Sounds like you're a victim of the placebo effect. freshgavinΓΛĿЌ 06:27, 18 April 2006 (UTC)
- Admittedly I haven't tried this with other fizzy drinks - not recently at least - but I really don't think placebo is involved as the difference between the mug and the glass is very clear. --Ukdan999 21:19, 19 April 2006 (UTC)
- I know I'm quite late here, but I might be some good. I believe that the glazed cermaics provide more nucleation sites and allow the Co2 to fizz out faster. But What I believe is not important. It's what I can show. To that end I will now go and measure the molecular roughnesses of a glass surface and a glazed ceramic mug on my Atomic Force Microscope. I will post results when availible. --Chapuisat 20:37, 24 April 2006 (UTC)
- And the results are in. From previous work I have a Rq (roughness) of 0.3 nm for silica glass (taken over a 1 micron by 1 micron area). My measurements on a glazed ceramic mug show a Rq roughly 3-5 times that. So it looks like glazed ceramics (or at least one type of glazed ceramic) is roughly then glass. --Chapuisat 20:46, 24 April 2006 (UTC)
A mystery chemical...[edit]
I've heard that there is a chemical you can taste or not taste based on genetics.
In fact, I've been in an expiriment where this had happened. The Science class went around to the other classes at the school with pieces of paper with the chemical on it. They gave a piece of paper to each of us, and asked us if we could taste it. Unfortunately, I could. I remember the chemical having an intense bitter taste.
Does anybody here know what chemical this is? Thanks in advance. --P. B. Mann 17:27, 17 April 2006 (UTC)
- Ah yes, that chemical is PTC, or phenylthiocarbamide. --HappyCamper 17:33, 17 April 2006 (UTC)
- I've heard/read (possibly even on this very website) that some people are unable to taste or smell cyanide due to a certain gene. --Ukdan999 18:35, 17 April 2006 (UTC)
- In regard to the cyanide answer, for people who can smell it, it smells like walnuts or other assorted nuts. Very helpful for a person who conducts autopsys on a regular basis.
- That's the one. Thanks HappyCamper! --P. B. Mann 18:45, 17 April 2006 (UTC)
- Geez, I sure hope they weren't giving you cyanide in class, that's poisonous ! I've also heard that Asians have receptors to taste monosodium glutamate which the rest of us lack. StuRat 20:10, 17 April 2006 (UTC)
- heh --Froth 21:44, 17 April 2006 (UTC)
- There's also the so-called Asparagus Gene. Asparagus makes urine smell different. Sometimes. But scientists don't know if the reason why only some asparagus-y urine smells different is because only some people possess the genetic ability to process the asparagus into the odd smell, or if it is because only some people possess the genetic ability to smell asparagus-y urine.--Anchoress 02:21, 18 April 2006 (UTC)
- I would say there is a smelly chemical in asparagus which is not broken down, but comes out in urine smelling the same. You could just have people smell other people's "asparagus urine" to determine if some people lack the smelly urine or if some lack the ability to smell it. StuRat 03:31, 19 April 2006 (UTC)
- I believe this experiment was in fact done. The results, if I recall, were that some people produce the Asparagus Urine Scent, and others don't. Meanwhile, some people - not necessarily the same people - can smell the Asparagus Urine Scent, and others can't. The experiment was mentioned in a book, maybe one by Ira Flatow. -- Filliam H Muffman 20:17, 21 April 2006 (UTC)
Smartness[edit]
Is smartness innate, or is something that can change? The word, smartness, seems so subjective and imaginary. Is there any scientific definition of smartness? I am working on a topic about the smartness of nowadays people. I can only think of one agreement, income is direct proportional to intelligence. Does anyone have any suggestions of agreement, that can find data to support it instead of theoretical support?
- Are you speaking of IQ or general knowledge? IQ of an individual while it varies is usually relatively the same. (Someone with a Low IQ isn't likely to become a genius, and unless there is physical damage someone with a high IQ usually doesn't dramatically lower their IQ.) Children do tend to have a higher ability to learn than adults. As for overall knowledge this can vary greatly, depending on IQ, age, education level, and socio-economical background.--Tollwutig 17:54, 17 April 2006 (UTC)
Actually, my exact topic is "are people getting smarter?" and this is a statistical project, so I do not specifically talk about IQ or general knowledge. What I asked to do is finding agreements and related data to support my answer to the question. At first, I thought about finding data of IQ score, but most of the data of IQ score I can find is estimated. Now, I am working on income, if people earn more money, they are possibly smarter. So is there any suggestions of things like income that have a relationship with smartness?
- Again define your concept of smartness. This is the problem with your question. Undoubtedly dealing with financial issues, Richer people are smarter, but can those same rich people say fix a computer, or their automobile? Both of which require a good bit of smartness, both in knowledge and logical thought processes, but are not going to be as rich as a CEO per se. Smartness comes in different forms. And I do not think wealth is a good indicator of smartness.-Tollwutig 18:26, 17 April 2006 (UTC)
Other than wealth and education level, any suggestion of being a good indicator of smartness? Thanks.
IQ is your best bet, but there are still problems. There just isn't a really good mathematical statistic for how smart someone is as different people learn differently, and have different affinities for how and what they learn. --Tollwutig 19:36, 17 April 2006 (UTC)
- IQ is okay for some purposes, but it's always being normalised, so it won't be of much help if you want to determine a population's average trend in intelligence over time.
TollwutigO questioner, were you given "are people getting smarter?" as a project, or did you choose it? If it's the latter, I'd actually advise you to change your project to something a little more concrete. Melchoir 20:49, 17 April 2006 (UTC)
Start here: The Bell Curve. WAS 4.250 21:04, 17 April 2006 (UTC)
- I can't believe nobody's mentioned the Flynn effect. Oh, and the Bell Curve is an enormously controversial book. --Robert Merkel 21:33, 17 April 2006 (UTC)
- Smartness can also be interpreted as how you use what you have to achieve a satisfying life. Last year The Economist had an article about their own measurement of what they called a Quality of Life Index. One could argue that A is smarter than B because she achieves a better QOL with the same resources, (or the same QOL with fewer resources). Maybe the figures required for such calculations would be easier to lay your hands on. --Seejyb 22:18, 17 April 2006 (UTC)
- I saw that and immediately thought that The Economist ripped off Douglas Adam's observation (paraphrasing from distant memory): Humans always thought themselves smarter than dolphins because humans had cities, computers, war, and all dolphins ever did was muck about in the water playing games all day. Conversely, dolphins thought themselves smarter for exactly the same reason. --Kainaw (talk) 01:14, 18 April 2006 (UTC)
- That's a remarkably accurate quote. I doubt he's the only person who's thought along those lines, though. Regarding other "good indicator[s] of smartness," consider perhaps IQ of parents, IQ of siblings, IQ of children, IQ of friends, education level, SAT scores... Black Carrot 03:07, 21 April 2006 (UTC)
Platinum as a catalyst for Hydrogen Combustion[edit]
I am having trouble finding a source that verifies that platinum or palladium causes hydrogen to combust in air without a flame. Could someone point me in the right direction? --Joe 20:03, 17 April 2006 (UTC)
- Perhaps you mean hydrogenation? Isopropyl 20:38, 17 April 2006 (UTC)
- In the presence of oxygen, the platinum and palladium catalysts used for hydrogenation may "spark". If hydrogen is present too, it will combust. Hydrogen will normally burn with a flame that is not visible. Here's an example [40] (item 17 on list) of a warning of this danger, but you can probably find others on Google. --Ed (Edgar181) 21:36, 17 April 2006 (UTC)
- You might wish to read the article on Döbereiner's lamp - a lighter built on that principle. Dr Zak 19:38, 19 April 2006 (UTC)
Basically, I am looking to coat a surface with Pt to heat it up when hydrogen comes in contact with it. Is this feasible and how would i calculate the heat flux based on the surface area of the Pt deposit? Thanks, --Joe 21:56, 17 April 2006 (UTC)
- So what's it you are after? The idea is to measure hydrogen in a gas stream? At what concentration and temperature? What is the source of the gas? Dr Zak 19:38, 19 April 2006 (UTC)
I want to be able to calculate the heat created on the substrate if I have a given Pt surface area, H2 concentration of M and H2 pressure of P. The H2 is coming from a Tank and is at a steady state. --Joe 05:39, 20 April 2006 (UTC)
Fixing a broken monitor[edit]
I've managed to break my monitor - it now renders red to black, and green to yellow. After an investigation, I found the culprit: a severely bent pin in the plug (I knew using the hammer was a bad idea...), and after some more googling I've found that many other people have had similar problems, and I've also heard tell of replacing either the cable itself, or the broken plug, but no instructions on how to do either. The monitor is a Dell; an M991 according to the sticker on the back, and I can get my hands on a replacement cable/plug easily enough (ripping it out of another Dell monitor upstairs - and no, I want to continue using this one because it's bigger), but I don't know how to remove nor install either. Any relief from this blue/yellow world is appreciated, Sam Pointon United FC 20:30, 17 April 2006 (UTC).
- The first thing I would try is bending the pin back to the proper position using needle nosed pliers. You will likely need to bend it past the correct position to account for spring back. The pin might break off, in which case you really will need to replace it, but it's still worth a try. StuRat 22:12, 17 April 2006 (UTC)
- First, realize that messing around with crt monitors is potentially dangerous. Which plug are you talking about? Can you post some photos? For great justice. 22:20, 17 April 2006 (UTC)
- Unfortunately, the pin is bent awkwardly (a double-bend, and right up against the side and bottom), so my efforts to reshape it have shown this avenue to be impractical if not impossible. --Sam Pointon United FC 22:22, 17 April 2006 (UTC)
- I don't have a digital camera, so here is an approximation of the monitor's back in ASCII art:
-----------------------
/ (1) \
| |-----------| |
/ | | \
| | (2) | |
/ ------------- \
###############################
| [o](4) |
| <W>(3) |
--------------------------------
Key: 1 = raised section.
2 = label w/ model info, serial number, etc.
3 = power cable
4 = signal cable - the offender.
- I think that the signal cable is removeable, because it is not actually fixed in, but rather is plugged into a slot (albeit one I don't know how to take it out from), and there's just enough leeway to move it slightly in said slot. --Sam Pointon United FC 22:33, 17 April 2006 (UTC)
- Oh, also, to answer what you actually asked, the plug I was talking about was the one that goes into the graphics card. --Sam Pointon United FC 22:38, 17 April 2006 (UTC)
- I think that the signal cable is removeable, because it is not actually fixed in, but rather is plugged into a slot (albeit one I don't know how to take it out from), and there's just enough leeway to move it slightly in said slot. --Sam Pointon United FC 22:33, 17 April 2006 (UTC)
- OK, so the easiest way to do this is going to be to get a replacement cable, and then cut them both in half, and join each wire in the cable to the corresponding one, so you end up with a new plug on the end of the old cable. For great justice. 22:40, 17 April 2006 (UTC)
- ... But don't fiddle with the cable where it enters the monitor casing. The Dell M911 cannot be unplugged on the monitor side. The wires are connected inside the monitor casing - no plug, and dangerous and difficult to connect. For me, fitting a new computer-side plug would be the reasonable DIY way to go, but splicing the cable may be technically easier. Good luck with your efforts. --Seejyb 22:55, 17 April 2006 (UTC)
- I agree - you may be able to lop the plug off, and fit another one, but most of the modern ones are molded and not as friendly to DIYers as the old days, so I assumed cable splicing would be easier... For great justice. 00:04, 18 April 2006 (UTC)
- The problem with cable splicing is that you'll probably be left in the dark about the pin assignments of the wires. So I'd really recommend splitting open the computer-end plug and replacing it that way, even though it'd probably require a bit of cutting and prying. --BluePlatypus 09:12, 18 April 2006 (UTC)
- I agree - you may be able to lop the plug off, and fit another one, but most of the modern ones are molded and not as friendly to DIYers as the old days, so I assumed cable splicing would be easier... For great justice. 00:04, 18 April 2006 (UTC)
Seagull[edit]
What is teh exact species (just one? or more than one?) of "seagull" that is most commonly found in Toronto? What about New Brunswick?--Sonjaaa 21:09, 17 April 2006 (UTC)
- Turns out this is a little more complicated than it first seems; apparently gulls are a wide variety of birds with subtle variations and overlapping ranges, and there's not even a firm conclusion on exactly how many gull species there are or where the lines are drawn. Some species also interbreed - believe it or not, we have an article (okay, stub) on the hybridisation in gulls. Looks like your best bets may be the Herring Gull, Great Black-backed Gull and/or the Ring-billed Gull. Looks like there's a gull out there for everyone. --ByeByeBaby 23:02, 17 April 2006 (UTC)
Psychotherapy and talk therapy[edit]
Are psychotherapy and talk therapy synonymous? I'm working on treatment of mental illness. --Neutralitytalk 21:44, 17 April 2006 (UTC)
- I don't think they are quite the same. Most forms of psychotherapy are directed in some way, such as the classic "tell me about your relationship with your mother", while I think of talk therapy as undirected. That is, just talking about a problem, with no particular guidance, also helps people out, to some extent. Note that no specific training is needed for this method. StuRat 21:47, 17 April 2006 (UTC)
- The Mayo Clinic seems to consider talk therapy to be another name for psychotherapy. See [41] --Seejyb 22:35, 17 April 2006 (UTC)
- You can imagine the benefits of advertizing "talk therapy" over "psychotherapy". freshgavinΓΛĿЌ 06:20, 18 April 2006 (UTC)
- No. Psychotherapy can be any form of psychiatric therapy, (which may or may not actually be therapeutic). Some of it involves talking, some of it doesn't. Cognitive therapy for treating phobias can be quite hands-on. (actually confronting whatever you're afraid of) Note that "psychotherapy" and "psychotherapist" are quite loose terms, and you often don't need any formal qualifications to call yourself a psychotherapist. Which basically means there's a lot of nutty ideas out there in this field. (So that article is wrong- methods described as psychotherapy may not be based on any credible psychological theory) Contrast this to a psychiatrist who is usually a medical doctor trained in the mental health field. --BluePlatypus 18:12, 19 April 2006 (UTC)
pushing on your eye[edit]
What is the name of the "kaleidoscope" effect you see when you push gently on your eye for several seconds?
-Froth 21:43, 17 April 2006 (UTC)
April 18[edit]
Do blind people dream?[edit]
I've had a look at the Dream article but can't seem to find any information: Some friends and myself were having an interesting conversation of what people who are blinds - of which have no knowledge of any kind of visual anything, e.g blind since they were born - dreams are like, if these happen at all. I understand dreams tend to show previous actions or feelings, and i see the main way of showing this is via sight, so what would a person who has no visual perception of shapes, colours etc dream like. Would/Do they involve colours, emotions, tastes, smells, etc to a greater extent to that of a non-blind person? I really would like to hear peoples feelings on this. And I am gravely sorry if my question has gone to offend anyone. -Benbread 00:04, 18 April 2006 (UTC)
- Blind people, from my experience have particularly heightened hearing senses. They may dream purely in sounds. I doubt that your query will offend people. It seems very genuine to me. Ansell 01:26, 18 April 2006 (UTC)
- Although they might get offended at being called blinds! freshgavinΓΛĿЌ 06:17, 18 April 2006 (UTC)
- If you google "blind dreaming" you'll find there is a lot of research on this, i.e. [42][43] [44]. Seems like a hot question in cog sci research. --Fastfission 02:43, 18 April 2006 (UTC)
- Gravely sorry! Are you saying you are sorry enough to kill yourself?? --
 Mac Davis] ⌇☢ ญƛ. 05:16, 18 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 05:16, 18 April 2006 (UTC)
- Fascinating question. Thanks. Being blind means having a limited or non-existent capacity to use one's eyes. Dreaming has nothing to do with the eyes, because it has nothing to do with seeing external objects. If a blind person can imagine anything at all - and of course they can - they imagine it in symbols as everyone else does. They may not have the rich array of images that a sighted person has, and the images they do have may not accurately represent physical objects, but they can still feel the shape of objects and have a sense of what they look like. Those "feeling images" would constitute their core stock of images that come to them in dreams. And as most sighted people know, dreams can often produce images of things one has never seen in real life, so why shouldn't this happen to blind people as well? JackofOz 09:02, 18 April 2006 (UTC)
- Thanks for the help everyone - Sorry about the use of "blinds", that was likely a typo on my part, I wrote that question rather late at night last night feeling rather tired - See why I needed the comment on trying not to offend anyone? ;) In regards to your comment, JackofOz, I get the feeling that if a blind person cannot comprehend what a colour is (not that colours are anything but our interpretation of light radiating at different wavelengths, and theres plenty of information on some people who are blind are able to see colours), would these dreams have no visual element to them at all, or would it be a simulation of what someone would see, but without colours, or would it just involve other senses? As i cannot imagine someone who has never used their eyes before would know what this feeling would be like, just as people cannot understand what a bat or dophins "sonar" senses are like, except by watching simulated images. Again, thanks a lot, and i'll take a look at those links :) It'd be nice to see the Dreaming article have some information on this, assuming that i haven't missed its appearance since i last fully read that article. -Benbread 10:21, 18 April 2006 (UTC)
- Well, the world had no trouble accepting monochrome TV, so the absence of colour per se does not seem to be a big problem in visualising images. How about this for a counter-question: Do babies in utero dream? And if so, what would they dream about? They've never used their eyes either. JackofOz 02:17, 19 April 2006 (UTC)
- Thanks for the help everyone - Sorry about the use of "blinds", that was likely a typo on my part, I wrote that question rather late at night last night feeling rather tired - See why I needed the comment on trying not to offend anyone? ;) In regards to your comment, JackofOz, I get the feeling that if a blind person cannot comprehend what a colour is (not that colours are anything but our interpretation of light radiating at different wavelengths, and theres plenty of information on some people who are blind are able to see colours), would these dreams have no visual element to them at all, or would it be a simulation of what someone would see, but without colours, or would it just involve other senses? As i cannot imagine someone who has never used their eyes before would know what this feeling would be like, just as people cannot understand what a bat or dophins "sonar" senses are like, except by watching simulated images. Again, thanks a lot, and i'll take a look at those links :) It'd be nice to see the Dreaming article have some information on this, assuming that i haven't missed its appearance since i last fully read that article. -Benbread 10:21, 18 April 2006 (UTC)
music software and poison question[edit]
Is there software (relatively cheap) where you can cut and paste parts of a music track? Say like you only want 3 min. of a 35 min. song. Where can I get more information on this product? Also, a totally unrelated question: is there in existence a potion or chemical or whatever like that used by Juliet in Romeo and Juliet? Thanks. schyler 03:02, 18 April 2006 (UTC)
- You mean like Audacity? Actually I just use SoX but it only has a command-line interface and might be hard to install on your platform (which you forgot to mention). —Keenan Pepper 04:19, 18 April 2006 (UTC)
- And yes, sure, there are chemicals in existence which can knock people out like it did to Juliet. I can't think of any specific names at the moment, though. -- Daverocks (talk) 09:47, 19 April 2006 (UTC)
- Are you sure about that Daverocks? I'm sure there are chemicals that can knock someone out, but can they make them appear to be dead without killing them, allowing them to awaken naturally a long while later without ill effect? It would have to slow their heartrate an enormous amount, slow their breathing (and make it shallow so as to be invisible) and simulate rigor mortis. 57.66.51.165 13:42, 25 April 2006 (UTC)
If the Speed is C.[edit]
If the Velocity of an Object is equal or greater than the Velocity of Light. The Mass is infinitve as per the equation m = Mass at Rest / (1 -( ʃV*V / C*C)). Does infinitive here it keeps on changing ? Please explain and sorry for my BAD Formulae Qutoing I am new to Wiki.
- I think you mean this formula, similar to those at Relativistic mass:
- As v approaches c from below, the relativistic mass increases without bound, assuming that m isn't zero. To accelerate a massive object to the speed of light, then, requires an infinite amount of energy. There's no meaning to going beyond c. Melchoir 04:22, 18 April 2006 (UTC)
- Have you read Introduction to special relativity? —Keenan Pepper04:26, 18 April 2006 (UTC)
Thanks For That. Yes I meant that formulae itself. Till now Science havent managed to defeat the velocity of Light. But I hope there will be a time for that. So if the speed is C. no need to exceed it. Then would give 1 and then 1 - 1 would give 0. Then No amount of Mass at rest would give Infinite. But Every Object Occupies space and has Mass. THen What will be the Mass. One more question why does the Mass change when Velocity changes. Even though it notably changes for 3 X 10^6 m/sec^2 there is always a small change. Plz explicate very easily and understandingly for the age of 15. Thanks in advance.
- Every particle that can be observed at rest has a rest mass, which is a constant property. If you want to think about what the relativistic mass - the one which increases with velocity - means, then think of it in Newtonian terms: mass is an object's resistance to acceleration. So the faster an object goes, the more energy you need to make it go even faster, which (appears as though/means that) its mass is increasing. Yes, if you could accelerate something from rest to c, then it would have an infinite mass, but it would require an infinite amount of energy to do so. The only objects to travel at the speed of light - photons, etc. - cannot be brought to travel at any other speed, because they have effectively zero rest mass. Confusing Manifestation 09:02, 19 April 2006 (UTC)
Thanks for Everything. One More Doubt you said " but it would require an infinite amount of energy to do so" So are you truing to say that that is not possible.--Oasa 10:34, 20 April 2006 (UTC)
- Yes. Keep in mind, though, that velocity is relativistic, so some "weird stuff" happens, but you should take a course in relativity or read Theory of relativity and related articles. Also, photon velocity can vary depending on what it is travelling through, but I suppose it always travels at "the speed of light" by definition. Andrewjuren(talk) 07:57, 25 April 2006 (UTC)
(No heading)[edit]
Sir, i want to know details about GIS and also wnat to know there is any job vaccancy is there.
Please send me complete details
Thanking You,
My e-mail id is (email removed to protect you from spam)
- Depends on what you mean by GIS. We have articles about Gruppo di Intervento Speciale, an Italian special operations unit, and Groupe d'Intervention Spécial, an Algerian one. –Mysid 11:26, 18 April 2006 (UTC)
- And if you want a job then email Jimbo.
- And if you want people to take you seriously, use a spell checker when you apply for jobs. Phileas 02:32, 19 April 2006 (UTC)
- And if you want a job then email Jimbo.
- (IATA: GIS) is clearly Gisborne Airport. For details, you could check a Geographic Information System here, or even do a Google Image Search here. The airport is operated by the Gisborne Regional Council, but sadly they currently have no job vacancies. But why are you asking, anyways? If you have a Guaranteed Income Supplement, as you seem to be claiming, it seems to me that you hardly need a job. --ByeByeBaby 06:09, 20 April 2006 (UTC)
Dieting, metabolism and "starvation mode".[edit]
I've heard lots of people saying that if you skip meals or stop eating as a way of losing weight, it doesn't work. The reason they give for this is that your body goes into "starvation mode" and your metabolism slows down, meaning that your body works more efficiently and you don't use up as many calories. To clarify - say you burn 300 calories from an hour of exercise normally, (according to their reasoning) you would burn fewer calories than this if you hadn't eaten for 24 hours beforehand. I'm slightly sceptical as no one seems to be able to give me a reason for why your body does this. So - is this "starvation mode" a scientific fact, or is it something they tell teenage girls in the hope it will prevent eating disorders? How much difference does this "starvation mode" make? And, if it's more efficient, why doesn't your body run on "starvation mode" all the time? Not trying to diet myself, I was just wondering. Thanks in advance. Bodil 08:44, 18 April 2006 (UTC)
- You're absolutely right, if someone continues to miss meals and starve themselves, their rate of motabolism will go down, and they will use less energy. This is most evident in the amount of energy you're using "ticking over", for example when sleeping, just staying alive. So, by effectively starving yourself you're still losing a lot of weight because you're simply not inputting any calories into your body, but still using less calories overall - Still if your eating less calories than you're using, regardless of how slowed your metabolism is, you'll still lose weight, but it's far from ideal, or safe.
- In regards to healthy dieting, the reason people are overweight is because they're eating too many calories and not using them up enough by not exercising (or by exercising a lot, but eating an incorrect diet), and thus these excess calories are stored as fats. Thus by eating only the calories you require and then exercising to burn calories and increase your metabolic rate your body wont go into starvation mode, but you've got to be eating regular meals, eating small regular meals is a great way to not feel "starving" while still eating less. I'm no dietition, but I hope i have helped you. -Benbread 10:11, 18 April 2006 (UTC)
- Thanks, Benbread - that helped clarify things :-). Bodil 11:18, 18 April 2006 (UTC)
- Also note that when you do go into "stavation mode" you're likely to put fat back on when you do eating regularly again as the body tries to replenish it's reserves. The best formula for dieting is calories burned > calories gained = weight loss. Thats overly simplistic but true. Better than cutting out meals it's best to eat less. Stop eating when you're about 80% full it takes a while for your brain to catch up to your stomach. The best way to lose weight is to consult your Doctor or a nutritionist.--Tollwutig 13:56, 18 April 2006 (UTC)
- Thank you for your answer Tollwutig, I appreciate it :). Bodil 21:19, 19 April 2006 (UTC)
Regarding your question about why people don't just operate in starvation mode all the time for max efficiency, the reason is there is a substantial cost, your body shuts down or reduces all nonessential functions to survive the period of starvation. Your body produces less heat, so you will need to wear more clothes or turn the heat up, it's harder to concentrate, so you won't do as well at complex mental tasks, growth may stop altogether in children, sleep duration will increase, and menstruation will stop in women and sperm production in men. StuRat 01:40, 19 April 2006 (UTC)
- Thanks, StuRat - a great, clear answer with interesting points. This reference desk thing is brilliant, I feel a lot more informed now! Bodil 21:19, 19 April 2006 (UTC)
- You're quite welcome. :-) StuRat 09:31, 20 April 2006 (UTC)
Electrical Engineering[edit]
In a potential or current transformer,is the induced current/potential out of phase or in phase? Because i have come across two books conveying different meaning....
- It depends on which way it's connected. - 128.32.48.131 00:49, 19 April 2006 (UTC)
- As said above it all depends on how you label your diagram of the transformer core. Plugwash 22:07, 22 April 2006 (UTC)
Ballistics[edit]
Does the effect of ballistics apply only due to air resistance present on earth and absent in space?is it because of the fact that a projectile would be able to pierce the air better if rotating than if not rotating about its axis parallel to direction of motion...
- Once again, please remember that this place is an encyclopedia (really, really!). I just checked out the entry on ballistics, perhaps you will be much happier doing it yourself? --Zeizmic 12:09, 18 April 2006 (UTC)
Take your coat off or you won't feel the benefit[edit]
This is something people often say to me if I keep my coat on when I sit down in a coldish bar or cafe, presumably meaning that I should save my coat for when it's really needed, ie when I go outside again. Personally, I can't see the logic in it, but when I question its wisdom people look at me like I must be crazy. Could someone spell out the logic/science please, so that I can refute the myth (if indeed it is a myth) next time it happens? Thanks, John Walker
- There's a little thing in here about the myth. [45] I'm a skinny guy who gets cold a lot, so I know it is important to maintain your core temperature, and build up a heat reserve. --Zeizmic 12:05, 18 April 2006 (UTC)
Many thanks for this, shows I didn't make it up! It also states categorically that I was right! JW
- I agree that the idea of taking your coat off to feel warmer seems sort of silly, but what I'd like to know is where you find coldish bars or cafes in wintertime. In my experience, in places with cold winters, they tend to keep it far too hot inside when it's cold outside. The reverse phenomenon can be noted in the summer. It irritates me because I have to keep stripping and re-bundling. --Trovatore 17:24, 18 April 2006 (UTC)
Yeh, I know what you mean - but believe me there are plenty of cold pubs in Britain. JW
The only way I can see that they would be correct is if you overheat with your coat on, then sweat, then go outside soaked with sweat, which would make you colder. So, put your coat on when you are cold, and take it off when you get hot, and you should be fine. StuRat 01:29, 19 April 2006 (UTC) True! Thanks - JW
- You could tell them to mind their own business. Seriously, why would anyone even say that to you? Black Carrot 15:51, 19 April 2006 (UTC). It's quite common where I come from - maybe it's more parents saying it to kids, but I've definitely heard it as an adult. JW
- When I visit the house of somebody who keeps it cold inside, I am reluctant to wear my coat, as that seems to be a criticism of them keeping their house so cold. However, if it's really cold, I don't care if I insult them. StuRat 20:37, 19 April 2006 (UTC). Yeh, you think they'd take the hint! JW
- "I must be coming down with something; I'm feeling cold all the time. Think I'll keep my coat on." - Couldn't care less if they feel insulted by that - it's my health. Gardar Rurak 06:20, 22 April 2006 (UTC). Yeh, and recent evidence has shown that keeping warm really does help fight the cold viruses. JW
The World[edit]
How many people are in the world as of 2006?
- The US Census Bureau has webpage estimating US and world populations. [46] --Ed (Edgar181) 12:10, 18 April 2006 (UTC)
- List_of_countries_by_population also tells you - Notice the search bar on the left. -Benbread 12:16, 18 April 2006 (UTC)
6,506,930,627 WAS 4.250 18:20, 18 April 2006 (UTC)
Necessary truths[edit]
Most scientists (I think) would agree that there are certain necessary truths. For instance, the Pythagorean theorem seems axiomatic: we couldn't imagine a universe (I assume) where it wouldn't be true. Are the more complex physical laws necessary truths? For instance, a body's kinetic energy increases with the square of the body's velocity: is that a necessary truth, or could the universe have been constructed so that it weren't true? — Asbestos | Talk (RFC) 14:07, 18 April 2006 (UTC)
- Well, the pythagorean theorem is derived from axioms. The axioms have to be taken for granted, but the derivation does not. There are no axioms in the physical science, but useful things can be done, predicted, and made using the theories we have. Indeed, the "truth" you cite demonstrates that useful things can be done with inaccurate theories: the kinetic energy formula is surely pre-relativity? Notinasnaid 14:29, 18 April 2006 (UTC)
- One can imagine a universe existing with any number of different values/properties/laws/etc. The question is whether or not it would develop into what we would consider a universe or not. Two articles which might be of interest in these regards are anthropic principle ("If the properties of the universe were different, would we be here to contemplate it?") and Gödel's incompleteness theorems ("Can an all-encompassing axiomatic system be constructed which can prove all mathematical truths?"). --Fastfission 15:09, 18 April 2006 (UTC)
- Start here: Philosophical skepticism WAS 4.250 18:16, 18 April 2006 (UTC)
I should point out that the Pythagorean theorem does not hold for non-Euclidean geometry and so almost certainly is not strictly "true" in our universe; but on small scales and away from strong gravitational fields, space is nearly flat and so it almost completel accurate. — Knowledge Seeker দ 01:39, 19 April 2006 (UTC)
how can someone kill themselves in...[edit]
Ok if i was in a padded room, naked, fed and watered, (can't drown myself or resist to eat) and temperature is room temperature how could one kill themselves? I can't hold my breath because i would simply be unconcious and start to breath.86.129.71.11 15:35, 18 April 2006 (UTC)
- Old age. Seriously, self-inflicted neck injury or slashing wrists with fingernails, but I don't think there is a method that is reliable. But if you're suicidal, that might explain the padded room in the first place. Peter Grey 16:05, 18 April 2006 (UTC)
- Regarding the wrists, it might be easier to use your teeth. Fredrik Johansson 16:55, 18 April 2006 (UTC)
- Usually you do this in a bath : gore might fill in the wounds, so remember to gnaw more before fainting. --DLL 17:19, 18 April 2006 (UTC)
- Regarding the wrists, it might be easier to use your teeth. Fredrik Johansson 16:55, 18 April 2006 (UTC)
- Bite your tongue and swallow the blood and keep the wound open and flowing with your teeth. Nobody will see you are injured until you've bled enough that there is little likelyhood that they can help. --Kainaw (talk) 18:24, 18 April 2006 (UTC)
- Eat your own head. A Clown in the Dark 21:53, 18 April 2006 (UTC)
- how bout, death by peanutbutter, what? no article? (;205.188.116.74 23:43, 18 April 2006 (UTC)
- Eat your own head. A Clown in the Dark 21:53, 18 April 2006 (UTC)
- Bite your tongue and swallow the blood and keep the wound open and flowing with your teeth. Nobody will see you are injured until you've bled enough that there is little likelyhood that they can help. --Kainaw (talk) 18:24, 18 April 2006 (UTC)
- Oh, come on guys, you can do better than that! Jesus tap-dancing Christ! Tearing at your wrists, indeed! Why not just tear your own throat out? It's a hell of a lot more delicate, and nearly impossible to fix. Of course, to do it properly would require a bit more hand strength than you probably have, but it's not like there's any shortage of excercise time in a padded room. I would suggest tearing off something even more delicate, but it'd be a lot more painful. I like the 'biting the tongue' idea too. If you'd like something more challenging, you could also tear open some of the pads, tie the cloth into a rope, and strangle yourself. Or, for an even bigger challenge, you could try drowning yourself. If you can master your coughing reflex, you could inhale all your water instead of drinking it, which should quickly fill your lungs. Even once you passed out, you would still be getting inadequate oxygen, and should slowly asphyxiate. BTW, you never mentioned them removing your waste, and death by disease is a time-honored tradition among prisoners. Black Carrot 01:53, 19 April 2006 (UTC)
- I'm not sure if it'd be possible in the situation you've given, but another approach is to try and break whatever light source they give you and use the glass as a razor. --Fastfission 01:58, 19 April 2006 (UTC)
- If you lean yourself up against the wall in such a way that your head is bent forward with your chin into your chest, I'd say it would be pretty easy to damage your spine by letting your body drop down off the wall, thus putting a lot of strain on the back of your neck.
I'm not sure if this is possible but I've always wondered; if you sit kneeling on your heels for long enough, you lose circulation. If you maintain the position for long enough, couldn't you get gangrene or something? freshgavinΓΛĿЌ 05:22, 19 April 2006 (UTC)- If you think of the situation the world is in long enough you'll die of a broken heart.
- In response to the tounge biting/blood response, Japanese ninjas, secret agents, and other assorted spies have been instructed to bite off their own tounges. They would use the severed tounge to choke themselves, should they ever encounter such a situation. This has been proven to be pretty darned effective; seeing that it's hard to prevent against and fairly easy to execute when you've been told how to do it. 68.52.56.111 03:23, 20 April 2006 (UTC)
- How about intentionally choking on your food? Just toss in a chicken wing while taking a deep breath. freshgavinΓΛĿЌ 03:36, 20 April 2006 (UTC)
- Pretty possible to do, if you had something big enough (i.e. a chicken wing). Seeing as how I have a varying degree of knowledge on the subject, it's time to end this question once and for all:
1. If you are in a psychiatric ward with padded walls, naked, fed, and watered, it isn't uncommon for them to force-feed you liquid based food, sometimes even introveinously if you are a really obnoxiously suicidal patient. So, you can't choke yourself with your own food, nor can you drown yourself with water, as they will NOT allow you to eat/drink your own sustinance.
2. The light-breaking method is impossible, as the light fixtures (that is, if you HAVE one) are made of polycarbinate glass that WILL not shatter. So, you can't cut youself.
3. Also, please note that many times if you are in what the docs call the 'unreactive' section of a psyche ward, you are in a straightjacket 24 hours a day, 7 days a week. This elimates the ability to slash your own wrists, pull out your neck, or gore/self-mutilate yourself to death in any way. Even the idea of damaging your spine as mentioned above is practically impossible in a straight jacket, as is the idea of losing circulation, or consuming your own feces. It is possible to escape from a straightjacket by A) dislocating your shoulder [easiest overall], B) Using cutting tools , or C) wearing the fabric down [this takes the longest]. However, I don't think the average person would be able to get out of one. If you did happen to get out of the straightjacket, you could practically beat yourself to death if you wanted to, among other things. The choices of death are pretty much endless once your get access to your limbs.
4. The only real choice you have left is what has already been mentioned: bite your own toungue, casuing you to choke on your own blood, or to bite your tounge off completely and use it to choke yourself. Even this has been contemplated for in some cases, when a white plastic attatchment is put on the tounge in some psyhe offices, which prevents you from biting your own tounge. But, that is pretty rare.
5. Please also keep in mind that most of the time when you are in a psyhe ward you are heavily sedateded and heavily medicated with drugs that will affect your mental state towards thoughts of not killing youself. It'll be pretty hard to kill yourself (or even THINK, really) when you are full of what is comically close to horse tranqilizers and 100x doses of prozac/zoloft.
So, in the end, it's quite possible, but pretty damned hard to kill yourself in a situation like that. It would probably be easier to escape from a psyche ward than it would to kill yourself in one. If there are any other questions on anything related escapeology, feel free to bounce questions off of me anytime.
The Linguist 04:33, 20 April 2006 (UTC)
How about biting into the padding and eating so much of it that your insides clog up?
- How do you bury yourself in a chained and locked coffin under cement and dirt for four days and come out alive? freshgavinΓΛĿЌ 06:18, 20 April 2006 (UTC)
- That one is actually simpler than you might think. There are two parts to any good con: deception and stealth.
1. Was the coffin inspected (I mean THROUGHLY inspected) before the guy got into it? There could be tools inside of it like a pick, flashlight, trowel, lock-picking tools, oxygen tanks, a knife, or etc. Many times they hide these under the frilly/padded sections on the bottom of the coffin. They just tug on the pads really hard to expose hidden compartments underneath.
2. Was the magician inspected? The most common place for them to hide tools is in their shoes (boots), although in the underarm and the hair is fairly common as well. Sometimes even the mouth is used.
3. The guy really isn't IN the coffin for four days. Many times, the spot where the trick is going to be preformed has been picked weeks in advance (despite what you may have been led to believe). The common way to do it is just dig a tunnel underground below where the coffin will be buried, then when the guy is buried he digs DOWN instead of up (before the cement can really harden) to get to the secret chamber below. I remember that one guy who did it (it might have been Chris Angel) even had a television down there for him (and the two guys helping him) to watch while they were waiting.
4. So, the guy basicly stays in the coffin for 2-3 hours MAX while digging himself out, then sits around for 3 and 3/4 days underneath the ground.
There might be more complicated ways to do it, but that is the most expedient way to get it done. 68.52.56.111 17:50, 20 April 2006 (UTC)
- I was only half-serious about the question, but although there is most definitely a rather simple answer, I don't think (in general) that's it.
In general the challenges I've seen were much less transparent than that. Hundreds of people watching, magician wearing little clothing, all the "inspection" of the coffin mumbo jumbo. Of course, I remain a sceptic about some of that stuff ... it is POSSIBLE to stage a trick with 20 people watching, although that would take a lot of payoffs, and a trick like that is really all too un-romantic for me to want to believe.
Like you said, though, it's definitely not hard to assume that the guy simply doesn't stay in the coffin for 4 days, and it's assumptions like that that I usually make when attacking "majik trix".
David Blaine (among other less notables) does a long-term ice box isolation trick in which he spends days confined in a box of ice without clothing or nutrition; the box is almost transparent and is usually placed in a busy downtown area. Guiness has a "submerge yourself in ice" challenge and a few guys can handle up to a couple of hours, and I have no doubt that David Blaine doesn't rank anywhere near any of them in terms of superior condition for ice-cold submergence. freshgavinΓΛĿЌ 05:43, 24 April 2006 (UTC)
Animal Dream[edit]
Do animals dream ?
- How could we possibly know if they did? Rapid eye movement occurs in all mammals and birds, but that doesn't mean much. —Keenan Pepper 17:13, 18 April 2006 (UTC)
- Why would that not mean much? It occurs in all mammals and birds, but isn't known to occur in any other animals. That does mean something: Given that we (mammals) all act the same (eye movements, twiches, EEG patterns of brain and muscle activity, irregular heartbeat, etc) during what we call REM sleep, given that we know humans dream during REM sleep and given that we know REM sleep is more similar to the awake state than NREM sleep, it seems more reasonable to assume that all REM-sleeping animals dream than the opposite. There's also the nature of the motor activity, we know humans move in their dreams (although normally with restricted motor control). Anyone who's seen their sleeping cat paw at thin air instinctively draws the conclusion they're dreaming. Baby platypuses tend to make swimming motions with their forepaws while sleeping. The platypus is also the animal that has the most REM sleep. :) ("Monotremes and the evolution of REM sleep", Siegel et al, Phil. Trans. R. Soc. Lond. B, 353, 1147-1157) --BluePlatypus 18:23, 18 April 2006 (UTC)
Yes, animals dream. WAS 4.250 18:08, 18 April 2006 (UTC)
- Yes, they do dream according to this and this... Jayant,17 Years, India • contribs 18:13, 18 April 2006 (UTC)
- Yes. Indeed, research with mice show that the same signals are sent out by the motor cortex while the mouse is sleeping as when the mouse is awake, except that this isn't transformeded into movement due to sleep paralysis (or the mousey equivalent). If you're training a mouse to go through a maze, you can not only tell that he is dreaming about walking through the maze, but even where in the maze the mouse is in his dreams! If you need a citation for the paper I may be able to find it, but it's been a while. [Edit: I guess a similar study is referenced in Jayant's second link]
- That said, we don't know anything about the mouse's experience of dreaming. We don't know if the mouse is "seeing" himself go through the maze, or if the brain is just rehearsing the steps it needs to go through the maze, without any attached conscious experience. This distinction is currently outside the realm of modern science to evaluate. — Asbestos | Talk (RFC) 20:00, 18 April 2006 (UTC)
Yes, animals dream, though their dream sleep seems to be different to human dream sleep and more attuned to rehearsing survival skills, IIRC. Anyone with a cat will know that they dream - occasionally you can see them mimicking their awake movements while sleeping (I've even seen one bat an invisible mouse backwards and forwards before "dream chewing"!) Grutness...wha? 06:24, 19 April 2006 (UTC)
Who builds the best cars?[edit]
Can someone tell me which car company builds the best cars in terms of build quality and reliability? I am guessing it is the Toyota/Lexus brand but I am not sure.
- It depends on what you mean by the best in quality...its a personal choice... and from the cars i have seen or handled (which is not many, considering that i am not allowed to drive yet) i've found the Toyota and the Nissan brands to be quite suiting to my taste..Jayant,17 Years, India • contribs 17:28, 18 April 2006 (UTC)
- Consumer Reports does a number of surveys on things like automobile quality. You could check the latest Buyers' Guide or the most recent car buyers' issue of the magazine, and see which brands and specific models have the lowest rate of reported problems. --Serie 21:17, 18 April 2006 (UTC)
- It varies greatly in time and by model. Short answer: there is no good way to tell, every brand / company has its strengths and weaknesses. Find the car you like, with the features you want, at a price you're willing to pay. — QuantumEleven | (talk) 11:32, 20 April 2006 (UTC)
- Well, it usually goes something like this.
Foreign Car = takes way longer to break down (esp. asian-made) = charge you out the ass for replacement parts American car = breaks down way sooner = replacement parts are cheap
So, the question is, do you want to pay incrementally over time (american made cars) or just a large sum whenever something goes wrong (foreign)? 68.52.56.111 18:06, 20 April 2006 (UTC)
(Limiting the scope of the question to rugged terrain) A series of field tests performed by the Canadian Forces equipment testing facilities (somewhere around Ottawa, Ontario) in the mid-'90s came up with an interesting findings: The two toughest commercial cars (SUV type) were the Range Roover and the Hummer. What was surprising is that even though these two cars have dramatically different design (the Roover is a mobile living room while the HUMVEE is a nut-cracker), they perform equally in all tests (60-degree slope braking, 45-degree sideway inclines, 3-foot mud pits etc.) only but for one test. The Roover failed the "deep-V" gutter test, because the front bumper prevented the front wheel to make contact with the wall on the other side of the V-shape gutter. (The HUMVEE, given that its front wheels are far forward could gain traction on the other side of the gutter and climb back out.) Therefore, if you expect to navigate through a lot of deep-V gutters, the Hummer is your best car. If you prefer utter comfort, go for the Roover.--JLdesAlpins 18:08, 21 April 2006 (UTC)
current affect of technology[edit]
Use of technology has resulted in abuse in nature and man himself: if yes then how; if no then how?
- Hello! Glad to help. See Prometheus first. Goodbye and thank you for your question. --DLL 17:06, 18 April 2006 (UTC)
- The question presupposes that man is unnatural or somehow outside of nature; that technology is unnatural or somehow outside of nature. Realizing that man and technology are as natural as beavers building damns and birds building nests and ants farming aphids results in the understanding that "abuse of nature" is merely a trickery with words designed to support a position not supported by the facts. Then again, the facts do support the idea that man is altering both himself and the world around him in unprecedented ways achieving effects both wonderful and frightening. WAS 4.250 21:11, 19 April 2006 (UTC)
- I don't know if you're talking about facts or words. Yes, beavers use technology. This is not what people mean though when they speak of the ails of technology, by which they usually mean human-made-technology post-industrial revolution, which I think we can all agree on as being in many respects quite different than beaver dams. I think the man/nature divide in this sense is not meant to be a statement about whether or not man is a part of nature, but to differentiate for the purposes of accountability/responsibility. In any case... Heidegger's The Question Concerning Technology would make for the most interesting answer to this assignment. It's not the easiest read for someone not used to Heidegger though, but there's a pretty nifty guide available on the web which goes through the argument step by step... --Fastfission 01:54, 20 April 2006 (UTC)
brain cramp[edit]
If muscle cells are required to function anaerobically, they will convert pyruvate to lactic acid to regenerate the NAD+ needed to continue glycolysis. This build up of lactic acid can cause muscles to “burn” or even cramps to develop. If you think really hard, harder than you have ever thought before and don’t breathe, can you get a “brain cramp”?
The brain doesn't really work like a muscle, so no. A Clown in the Dark 21:50, 18 April 2006 (UTC)
- Brain cells can't function by anaerobic metabolism. If you think hard and don't breathe, you get what people refer to as "brain dead". --Serie 21:51, 18 April 2006 (UTC)
I have experienced something like this, but it's more of a dull ache than a sharp muscle pain. I attribute it to having used up the available glucose in a portion of the brain. StuRat 01:17, 19 April 2006 (UTC)
Human Cloning.[edit]
Hi there, I wonder if anyone would be kind enough to answer my question, which is:
Let us assume that human cloning is now possible. Let us assume that a human has been made infertile (unable to reproduce) due to being irradiated by, lets say, a massive amount of gamma rays. Would the clone of that human being be fertile or infertile?
Thank you, Norman Crompton. Lancashire, England.
- Fertile. Infertility by mechanical damage (castration, gamma radiation, or any other means) isn't inheritable. --Serie 21:54, 18 April 2006 (UTC)
- Infertility isn't really inheritable, if you think about it. Just because a person is infertile does not necessarily mean that every cell is infertile. If the gamma rays that the person was exposed to damaged the particular cell that was used to harvest the cloned DNA, and if the genetic damage was sufficient to affect the reproductive capabilities of the person, it is conceivable that the clone could also be infertile. Isopropyl 23:53, 18 April 2006 (UTC)
- There are types of infertility that are medically correctable, so in that case the infertility could still be inheritable.
- the clone could also be infertile -- true, but in this case the infertility would be entirely unrelated, unless the cell used for the cloning was also responsible for gamete production in the original person. The damage done to each cell is different, so the gamete cells could, say, be unable to produce energy, but some other cell might have become triploid or so. Then the clone might be all triploid and infertile for that unrelated reason. Of course, this means that irradiating part of a person, then cloning them from the damaged tissue could create the opposite condition: an infertile clone of a still-fertile person, even if the clone was made from reproductive tissue. Damage from the irradiation could cause the cell to no longer code for a fertile human without preventing it from being fertile where it was. --Tardis 21:23, 19 April 2006 (UTC)
Note, however, that if the individual was exposed to a level of radiation sufficient to cause sterility it would likely also cause genetic damage. Thus, any clone would be more likely to have mutations. Not the ability to see through walls, as in a sci-fi story, but perhaps an increased risk of cancer might be observed. StuRat 01:11, 19 April 2006 (UTC)
stove hood electric motors[edit]
I have bought a stove hood from Germany but I live in Canada. I have ran the proper 240 V cable and 15 A breakers, but the fan runs on 50 Hz compared to 60 Hz in Canada. Can the fan operate properly with no ill effect to either the motor or the electronic side of it? Thank you in advance. Nanika
- The 20% faster AC frequency will only drive the fan a little faster in practice. It probably is a DC fan with a rectifier because you don't want to use an AC motor in a variable load situation anyway. Just run it for five minutes, then turn it off and feel it. As long as it's not too hot to touch, you should be fine. --James S. 00:51, 19 April 2006 (UTC)
- Either that or its a universal motor. Indeed i strongly suspect that it is. In any case the advice above is sound, run it for a bit and if nothing gets worryingly hot it should be fine. Plugwash 22:15, 22 April 2006 (UTC)
Air embolism?[edit]
Hello! I am concerned with one thing that hapened to me when I was doing one endovenous injection , I injected 2ml of air in the blood circulatory sistem of a pacient. It was error, because someone who prepared the seryng didn't purged the extensor device.
It could make any damages? How long it take the damages to ocour? How much air in the blood can provoque an emboli by gas?
I contacted the paciente 5 days latter and he was working and fine, should I concerned more with this incident?
Thank you.
- Air embolism says: Small amounts of air often get into the blood circulation accidentally during surgery and other medical procedures, but an air embolism which shows symptoms is relatively rare.... Air can be injected directly into the veins either accidentally or as a deliberate act. Examples include misuse of a syringe, and industrial injury resulting from use of compressed air. However, usually this will neither suddenly stop the heart nor cause instant death, unlike in fiction stories where this is used as a method of murder.... Symptoms of an air embolism depend on where the bubbles lodge. They range from skin rashes, joint pain, visual disturbances, balance disturbances, breathing difficulties, extreme fatigue/lack of strength, numbness, paralysis, unconsciousness and death. If the embolism occurs in the coronary arteries of the heart, a heart attack will occur. If it lodges in the lungs, a pulmonary embolism will occur, resulting in shortness of breath and chest pain.
- This source says: Frequency of clinically recognized VAE (venous air embolism) following CV (central venous) cannulation is less than 2%.
- So, it seems you're probably fine, especially if the patient has none of the symptoms. --James S. 00:09, 19 April 2006 (UTC)
April 19[edit]
Potato diet[edit]
Is it possible to live on a diet of just potatoes and water? What would the potential adverse effects of such a diet be?
Thankyou
- Death probablly though that would likely take a while. Off the top of my head you'd end up with at least
scurvyand lack of Essential amino acids to deal with. Almost certainly other issues too. Plugwash - Apparently there is vitiman C in there though it may be lost depending on how the potatos are stored and treated. but in the long term living on any one food is likely to leave you difficiant in something. Plugwash 00:46, 19 April 2006 (UTC)
- You might not get scurvy but you would get beriberi, kwashiorkor, osteomalacia, pellagra and most of the other maladies in Category:Malnutrition. —Keenan Pepper 02:41, 19 April 2006 (UTC)
- Taking just one of these: beriberi seems to be caused by a thiamine deficiency, but is most common in those that subsist on rice. Indeed, potato suggests there is thiamine in potatoes: 0.11 mg per 100g. This is around 10% of the RDA, so 1 kg of potatoes would ward off beriberi. Of course, that amount of green potato could kill you. Notinasnaid 08:45, 19 April 2006 (UTC)
- You might not get scurvy but you would get beriberi, kwashiorkor, osteomalacia, pellagra and most of the other maladies in Category:Malnutrition. —Keenan Pepper 02:41, 19 April 2006 (UTC)
- Not to mention you'd be sick of eating potatoes. freshgavinΓΛĿЌ 05:10, 19 April 2006 (UTC)
- Let's not forget that such a High starch diet would quickly steer the individual towards lots of fat build up. Which leads to it's own set of problems. Obese but mal-nurished.--Tollwutig 15:39, 19 April 2006 (UTC)
- I don't agree on the obesity part. The Irish poor existed almost exclusively on potatoes for quite some time with very little obesity incidence. StuRat 20:32, 19 April 2006 (UTC)
- What nutritional disorders were common among the Irish? —Keenan Pepper 21:39, 19 April 2006 (UTC)
- If what is written across the 'pedia is to be trusted, there must have been little adverse effects to the Irish potato centered diet in the 17-1800s. Seems that there wasn't much of a problem (relatively speaking) until they started running out of potatoes. freshgavinΓΛĿЌ 06:13, 20 April 2006 (UTC)
- I suspect that, even if the Irish diet was 90% potatoes, the remaining 10% (beer ?), must have been enough to prevent nutritional disorders. Although this diet probably did have long term consequences, contributing to the rather low life expectancy at Ireland at the time. StuRat 00:45, 22 April 2006 (UTC)
Not if the potato and water are pure. But dirty water , dirty and maggoty potatos, yes. (Same with pure white bread vs. bread made from vermin infested grain. Prisoners really could live on bread and water when the bread was not pure.) WAS 4.250 21:22, 19 April 2006 (UTC)
- Good point. Maggots especially are full of valuable protein. —Keenan Pepper 21:39, 19 April 2006 (UTC)
I'm anosmic and as such I can tell you fairly accurately what happens when you don't vary your food intake. With my 'handicap' food is a nuisance rather than a pleasure so I have more or less done this although never with potatoes - too much of a hassle. My worst experience was spaghetti and nothing else over the course of 4-5 months. The high amount of starch is comparable although potatoes also contain various minerals and vitamins which might prolong the effects.
- First you loose muscle mass, most of it over the first 3 months at an impressive rate - you don't gain weight (fat) unless you're eating a lot of it. I only ate once a day so it wasn’t an issue for me although it may be if you eat 4-5 times daily.
- Then your immune system gradually deteriorates - you will become sick much more easily. I had a cold almost constantly for the last month and I think wounds and bruises took much, much longer to heal although that may just be paranoia.
- Fatigue and laziness ensues after 2-3 months and increases steadily - you sleep more, can focus for shorter periods of time and start having headaches when you concentrate. You reach a point where reading a book becomes difficult because you can't remember what happened ½ paragraph up. And no, I'm not a bad reader... I read a fair bit at a civilized rate.
- Then you reach the point where if you get up quickly you will feel dizzy and woozy; your vision “blackens” and you loose balance and coherence. First only for a few seconds but later it will increase to as much as a few minutes. You learn to not hurry…
- Then you start to pass out whenever anything more demanding than processing spaghetti occurs to your body – i.e.. drink a beer, smoke a cigarette. It’s not a gradual thing either – maybe you light up a cigarette one day – and it’s like switching off a machine. Bang, you’re out.
- You wake up in the hospital after you passed out at the bus stop.
- You expand your food budget.
That’s pretty much it. I’m sure you can prolong any of these effects by eating vitamin pills, supplementing with small amounts of meat or just eating a fruit once in a while – but I’d advice you to eat varied – there’s probably a ton of side effects which isn’t immediately obvious or may only become harmful after longer periods of time. There's a reason why some people live til they are 60 and others till they are 110 - and genes aren't all of it. Regards, Gardar Rurak 03:38, 22 April 2006 (UTC)
- A very informative answer. Which brings up the question: When are we going to be able to forget about cooking food altogether and just secrete necessary nutrients through a tube or super-drink? freshgavinΓΛĿЌ 05:31, 24 April 2006 (UTC)
- Oh, we've had the technology and knowledge for that for ages. It's probably still safer to get vitamins from normal food sources, as they tend to be packaged in an easily digestable way (we've evolved to process them like this), but food pills/paste/drink is quite feasible. Thing is, there isn't much of a market for it. People like to eat. 57.66.51.165 14:32, 25 April 2006 (UTC)
- We have the technology, but we don't seem to be able to forget about cooking. freshgavinΓΛĿЌ 06:01, 26 April 2006 (UTC)
- Oh, we've had the technology and knowledge for that for ages. It's probably still safer to get vitamins from normal food sources, as they tend to be packaged in an easily digestable way (we've evolved to process them like this), but food pills/paste/drink is quite feasible. Thing is, there isn't much of a market for it. People like to eat. 57.66.51.165 14:32, 25 April 2006 (UTC)
- Its certainly feasible to make food (a super drink would probablly not work too well unless it was very thick) thats prepared in a factory, lasts a long time in its prepared state, can be eaten with no further preperation and satisfies a persons nutritional requirements. Its called a MRE but its expensive and most people would rather have freshly cooked food given the choice. Plugwash 21:32, 25 April 2006 (UTC)
Embryo in an Egg[edit]
In which part of a bird's egg, would you find the embryo? In the White or in the Yolk? Thanks Adrian - Malta 212.56.130.105 04:29, 19 April 2006 (UTC)
- You don't, if you're talking about chicken eggs, they're not only sterile, but unfertilized, so no zygote to be found--64.12.116.74 04:35, 19 April 2006 (UTC)
- Perhaps you might be interested in reading Egg white ad Egg yolk. -- Rick Block (talk) 04:46, 19 April 2006 (UTC)
- Free range chicken eggs are sometimes fertilised (presumably only if there's a rooster around). It's no problem, just a little speck of colour in the yolk.-gadfium 06:47, 19 April 2006 (UTC)
- In the yolk, if the embryo is present. The white is mainly for protection and nutrition for the embryo Wikicheng 06:42, 19 April 2006 (UTC)
- I always thought the embryo rests on the yolk, so you would find it at the interface between the white and yolk --Dunstan 11:26, 20 April 2006 (UTC)
- As odd as it sounds, the egg yolk is actually a single large egg cell. The genetic material is located on the surface of the yolk. The embryo develops on the side of the yolk. It starts out disc-shaped but later develops into a well-formed embryo. If you want to get technical, the morula and blastula stages are somewhat different than they are in mammals (the Wikipidia articles I just linked to focus on mammalian development). The egg white, on the other hand, consists of protein and water and does not contain genetic material. Ginkgo100 04:21, 25 April 2006 (UTC)
3D modelling techniques[edit]
It is easy to comprehend a printer printing a 2D image, but how does the 3D polymer model maker work?
- One way of modeling is building up layers of material. Basically, the printer deposits a uniformly thick 2-D layer on a substrate. This substrate is moved down a little bit and the printer deposits another layer on top. The layers build until there is a 3-D model. Refer to Fused deposition modeling or Stereolithography for more detail. --Joe 16:49, 19 April 2006 (UTC)
- A CNC machine can also carve a 3D model out of block of material, such as steel, while an LOM machine cuts layers of paper and glues them together to form an approximate "wooden" model. StuRat 20:22, 19 April 2006 (UTC)
Diagrammatic[edit]
What is this and who is it named after?
http://img106.imageshack.us/img106/7508/tb44ri.gif
Thanks
- that looks like a diagram describing how an electron (the left arrows) is transformed into a positron (the right arrows), first by emitting a gamma ray (the lower purple line) and then by interacting with a 'q'. That's all I can work out sorry, hopefully someone else can shed some light on it --Dyn.iinet.net.au 16:24, 19 April 2006 (UTC)
- That's a Feynman diagram, obviously named after
Tina Fey and Iman. No, wait: Richard Feynman. --ByeByeBaby 19:45, 19 April 2006 (UTC)
- That's a Feynman diagram, obviously named after
Thank you very much
Psychology: processes in mind when man meets an unexpected thing[edit]
While a talk at science fiction-related forum, we encountered a question without clear answer. Whatever an abstract question, it interests us. Imagine you have suddenly met a real extraterrestrial. What will happen in a man's mind who sees something that "defies all logic"?
We have various POVs:
- a) "Would any normal mind be forever snapped if something so incongruent suddenly popped in front of my face?" + "Perhaps they are so incongruent from what we can handle that our minds simply block them out."
- b) Man will experience a "shock", which will wear off after some time(may be, a matter of hours, days or weeks).
- c) "Most people would completely freak out if they were confronted with anything outside of the expected." + "Some people stay in shock for the rest of their lives."
It would be especially interesting to hear a comment of a psychologist. ellol 16:28, 19 April 2006 (UTC)
- I don't think any of those things would happen. A life form relatively like ours would take some getting used to, that's all. A totally different life form may not be recognized at all, however. For example, if the electromagnetic storms on the Sun are some form of life, we may never recognize them as such, especially if they only have one "thought" every million years. StuRat 20:10, 19 April 2006 (UTC)
- I don't think people would have any problems at all. They'd have a little disbelief for a while, that's all. We've encountered plenty of new species, (although none extraterrestrial). The first time Europeans encountered the platypus the immedate reaction was "Oh that's a fake! Who put that duck-beak on a beaver?". Which also shows how we immedately try to fit new things into the things we already know. When Marco Polo first saw a Rhinoceros in Sumatra, he immediately went through the "list of known animals" in his head, found the closest match and drew the completely wrong conclusion: "That's a very ugly unicorn." of course, with more experience we all know that platypi are perfectly normal platypi and not mutilated beavers and rhinos are just rhinos and not obese unicorns. Similarily we'd pretty soon accept an alien being for what it was. It's worth pointing out that unicorns don't actually exist. That didn't stop Marco Polo from accepting it once he saw something he thought was one. So presumably, most of us today have read enough about extraterrestrials to be prepared to accept the fact if we did actually run into one. --BluePlatypus 20:35, 19 April 2006 (UTC)
- Now that I think about it, it's noteworthy that there's are a lot of people who are so prepared to accept extraterrestrials that they'll choose them to explain things even when there are other, far more likely, explanations. :) --BluePlatypus 00:49, 20 April 2006 (UTC)
- Thank you for the interesting answer :-) Sometimes people have a too pessimistic outlook on future progress. E.g., I've heard that before the first human flight into space, there were claims that a man couldn't live in weightlessness. ellol 05:51, 20 April 2006 (UTC)
- The idea of "snapping" or "shocking" the mind with such an event seems to be related to the motif of harmful sensation. There's very little in the way of substance to it. --Tardis 21:31, 19 April 2006 (UTC)
Thank you. And I've posted your answers in forum at distantworlds.net . ellol 06:04, 20 April 2006 (UTC)
- This is Wikipedia. Things that "defy all logic" happen all the time here. -- Filliam H Muffman 20:27, 21 April 2006 (UTC)
Evolution of sex[edit]
How were male and female sexes developed from single cell organism thru evolutionary process?
- Check out our article on sex, which links to our article on evolution of sex. TenOfAllTrades(talk) 18:18, 19 April 2006 (UTC)
Point in ocean most distant from any land mass?[edit]
I was wondering whether anyone has calculated the GPS coordinates of the point on the world's oceans furthest removed from any land mass. I am guessing that Bouvetoya qualifies as the most removed island from any other landmass, but as for the maritime point I'm guessing it's somewhere in the North Pacific or the Arctic. If you've figured this out, please let me know. Thanks!
- Check out extreme points of the world#Remoteness; it sounds like you want "Point Nemo". — Lomn Talk 21:15, 19 April 2006 (UTC)
Thanks! I should have found that... sexy
Mistake In The Way Wikipedia Search Handles Escape Code %27?[edit]
I put "MacConkey's agar" into the Wikipedia search box built into my web browser (Opera) and I was taken the search page as though I'd search for "MacConkey%27s agar". I clicked on the 'MacConkey%27s agar' link to make a page called 'MacConkey%27s agar', and it instead took me straight to MacConkey's agar. I think this is a mistake, no? --Username132 (talk) 20:11, 19 April 2006 (UTC)
Congratulations, it looks like you've discovered a bug in MediaWiki's search not escaping the "%" character. Someone should probably report this on the MediaWiki bug tracker, but I don't particularly like the premise of having to do the song and dance of registering just to report a bug - surely a valid email address should be all that's needed?--Sam Pointon United FC 21:39, 19 April 2006 (UTC)
- Actually - scratch that, it looks like a deliberate thing done by the string-to-link converter, automatically expanding/collapsing out %xx escape codes. Don't blame me, it looked like a bug at first. --Sam Pointon United FC 21:42, 19 April 2006 (UTC)
- My guess is that your Opera Wikipedia box expands all the advanced characters and then directly tries to access the page, which bypasses Mediawiki's expanding/collapsing automaticisms. Rather silly thing for a search box to do, but it doesn't appear to me that Mediawiki is doing anything strange. freshgavinΓΛĿЌ 03:48, 20 April 2006 (UTC)
How To Get Free Clean Water?[edit]
I was wondering how travellers get their clean water? -Username132 (talk) 20:18, 19 April 2006 (UTC)
Usually using money. WAS 4.250 21:27, 19 April 2006 (UTC)
Usual methods include boiling, filtration, and chemical disinfection (iodine or chlorine pills). Visit a place that sells camping gear (like REI, or see rei.com) and they'll have tons of stuff like this. Most developed countries have drinkable tap water though. Phr 21:29, 19 April 2006 (UTC)
- Sorry, I didn't mean like that, I meant the travellers that move around the UK and other devloped countries in caravans. Some have let themselves in to a disused industrial site. I'm wondering how they tap in to the mains supply? --Username132 (talk) 22:51, 19 April 2006 (UTC)
- At a guess, there may well be standpipes or fire hydrants accessible, or just random mains plumbing that can be used if they find the right tap to turn it on. -- AJR | Talk 00:11, 20 April 2006 (UTC)
- And that's assuming that they're health concious. I'm sure a good number of them don't get clean water, and just deal with the side effects. freshgavinΓΛĿЌ 03:43, 20 April 2006 (UTC)
Does too much water make you poo?[edit]
I've heard a theory that drinking "too much" water can make you need to defecate. Is this possible? --Ukdan999 21:23, 19 April 2006 (UTC)
- Try it and see; then give us your report. GangofOne 21:29, 19 April 2006 (UTC)
- Not in my experience. If you drink too much water, it upsets your electrolyte balance and your kidneys excrete it as clear urine. See Water intoxication. —Keenan Pepper 21:46, 19 April 2006 (UTC)
Not true, but abstaining from liquid can constipate you. Brian G. Crawford 23:34, 19 April 2006 (UTC)
Why are no land animals bigger than elephants?[edit]
Long ago there were dinosaurs maybe 5x the size of today's elephants. They all got wiped out by a meteor, but so did everything else that was large, and it all had to evolve again. Is there some reason that elephants are the biggest that we have now? Could dinosaurs survive anyplace in today's ecosystem? Or is there just not enough oxygen in the atmosphere or something like that? We do have aquatic mammals (whales) that are even bigger than dinosaurs were. Phr 21:33, 19 April 2006 (UTC)
- I'm not an evolutionary biologist, but today's dominant creatures appear to all be warm-blooded. It takes an absurd amount of energy to keep an elephant warm, and it only gets worse as the animal gets larger, so that won't happen without a good reason. Presumably we could re-develop gigantic exotherms, but the speed and adaptability advantages of the endothermic mode would likely prevent the megalizards or so from competing well. --Tardis 21:49, 19 April 2006 (UTC)
- see Paul Colinvaux, Why Big Fierce Animals are Rare, mentioned in overpopulation and see also http://courses.washington.edu/anth457/energy.htm ; it's about thermodynamic efficiency, like Tardis says. Also, bigger land mammals would have to be slow moving, and thus at a disadvantage. --GangofOne 22:32, 19 April 2006 (UTC)
- Two other notes are that the Cenozoic era has only been going for 65 million years, where the dinosaurs had the Mesozoic, which lasted for 180 million years, so they had a lot longer to evolve bigger. Elephants are the biggest land mammals ever, so it's not like mammals are regressing in size. (Of course, early humans probably killed most of the megafauna, so there did use to be a greater variety of big animals.)
- Yes, but the largest dinosaurs lived during the Jurassic. It took only about 80 million years for the earliest dinosaurs to evolve into Brachiosaurus, for instance. Also, it's not at all clear that in today's habitats, no animal could get larger than an elephant. It could be that circumstances simply haven't come together to favor the evolution of a larger animal. There is an element of chance in evolution. Ginkgo100 04:10, 25 April 2006 (UTC)
- The second note as far as a possible limiting factor is that there's also a structural element involved; bones, muscles, tendons, etc. have a certain strength. The strength of a bone is more-or-less proportional to the cross sectional area, but the weight of an animal is proportional to the volume. So if you made an elephant twice the size (i.e. doubled every dimension), the bones would have roughly four times the strength of the original ones, but they would have to support a creature that was eight times as heavy. This is one of the reasons why the largest organisms tend to be marine; the salt water counters the effect of gravity. So we have whales versus elephants; large insects versus lobsters and so on. --ByeByeBaby 23:46, 19 April 2006 (UTC)
- Thanks, yes, I understand about how the square-cube law affects elephants, and GangofOne's remarks about energy efficiency and overpopulation make sense. But those lead to back to the original question (recast): if elephants are about as big as land animals can get, how could there ever have been dinosaurs? What was different? Phr 00:05, 20 April 2006 (UTC)
- Note that he said elephants (or mammoths, according to StuRat) were the largest land mammals. Dinosaurs were not mammals. — QuantumEleven | (talk) 11:18, 20 April 2006 (UTC)
- Well, some constraints on size are cooling systems, bones that can supports one's mass, getting sufficient oxygen, getting sufficient food, and a nervous systems fast enough to react in time to prevent disasters. As for nervous systems, even humans need a somewhat distributed nervous system, where reflexes act quicker than the brain can react. Without this we would step on a nail and have it go all the way through our foot before we reacted. In a much larger animal, you would need local nerve centers to control things like walking, independently from the brain. While these abilities probably existed in dinos, they were lost when they were wiped out. They could evolve again, and likely would have in another hundred million years. But, since people get nervous around animals much larger than them, we aren't likely to permit evolution of larger animals to continue. StuRat 01:02, 20 April 2006 (UTC)
- It's all a question of ecology, not physiology. If it was the latter, we wouldn't have had dinos in the first place. From what we know, the climate and atmosphere aren't really terribly different from back when the dinosaurs lived, so that's not the problem. The likely (IMHO) reason is that when the dinos got knocked out, it was followed by (or consisted of) a period of cold climates. During that critical period, only the small lizards survived, but the mammals apparently did better. Today, while the climate can once again support larger lizards, the ecological pressure from the mammals presumably doesn't permit them to evolve that way. --BluePlatypus 03:22, 20 April 2006 (UTC)
No one knows and there is a lot of debate among the experts. See Giantism.html. WAS 4.250 20:36, 20 April 2006 (UTC)
- ":I'm not an evolutionary biologist, but today's dominant creatures appear to all be warm-blooded. It takes an absurd amount of energy to keep an elephant warm, and it only gets worse as the animal gets larger, so that won't happen without a good reason." in fact this is incorrect. the total heat needed increases but heat is lost only at the surface. So heat is easier retained in a larger than a smaller animal. Many large warm-blooded animals such as elephants have a problem with excess heat removal not heat generation. FT2 (Talk) 19:37, 26 April 2006 (UTC)
Cylinder Gap[edit]
What are the advantages of having a wide/short cylinder gap when tuning up a revolver? The booklet I read gave very leinant settings, from .002-.009. What is the difference between the varying settings? What setting makes a revolver function correctly for the longest period of time? Does it vary from revolver to revolver? Thanks, 161.45.196.221 21:44, 19 April 2006 (UTC)
- From Speer Reloading Manual: Number Thirteen:
Industry specs call for a MAXIMUM gap of 0.012 inch. Unfortunately, there are many revolvers in use, both originals and replicas, that exceed this value. Realistically, 0.012" is quite generous. For light loads, this gap should be much smaller–close to the minimum dimension that still allows a cylinder to freely rotate when it is dirty...An excessive gap allows too much gas to escape. There isn't enough pressure left to push the bullet down the bore. The lighter the load, the more likely that a large gap will cause a bullet to stop in the bore.
- Hope that helps. EricR 23:05, 19 April 2006 (UTC)
- Ah, I see. Thank you for responding so throughly. So that would mean that the gap for a standard FMJ round would be smaller than when using a hot-loaded round of the same type due to the fact that a hot-loaded round would produce more pressure and therefore need a bigger gap; however the gap for an unjacketed round would be smaller than the gap for a standard FMJ round due to the decreased size of the bullet and therefore less pressure required to push it down the bore/barrell.
- I do have one more question, though. How does the cylinder gap come into play when firing an unjacketed round? I have encountered 'leadening' before, where firing too many unjacketed rounds causes excess lead and grit to foul the bore of the revolver and cause malfunctions (namely, the hammer refuses to operate). If one set the cylinder gap to a wider setting as opposed to a shorter one, would this problem be avoided more easily? I am aware that 'leadening' is a unavoidable eventuality when firing unjacketed rounds, but would a longer cylinder gap postpone the onset of it when firing the rounds?
Thanks, 68.52.56.111 02:42, 20 April 2006 (UTC)
.wav to .ogg[edit]
I have the .ogg software installed on my computer, and I have no difficulty listening to .ogg files. However, I have a .wav file on my desktop; I open the file and try to save it as .ogg, but the .ogg option is not available.
How do I change the sound file to .ogg and save it on my desktop again? I have interested in uploading the file to Wikimedia which does not accept .wav files.Patchouli
- Thank you.Patchouli 23:42, 19 April 2006 (UTC)
- Or Foobar 2000. - 131.211.210.15 07:58, 20 April 2006 (UTC)
Waxwing Birds[edit]
is the cedar waxwing bird extinct? healthy? is it's species healthy? thank you
- The Cedar Waxwing is doing fine, and sends it's regards. The Japanese Waxwing] is listed as a nearly threatened species, however. --ByeByeBaby 23:21, 19 April 2006 (UTC)
April 20[edit]
chainmail armour?[edit]
how did they make this even before they had metal working tools?Cyoda mackbuy 21:27, 7 April 2006 (UTC) (copied from the archives page, where it was wrongly asked zafiroblue05 | Talk 00:48, 20 April 2006 (UTC))
- Very slowly and at great expense. Although in a broad sense, they would have had some metal working tools as soon as they had metal. Even today, making chainmail is expensive and labour-intensive compared to ordinary fabrics. Peter Grey 06:25, 20 April 2006 (UTC)
Fatal Blood Loss[edit]
How much blood can a person lose and still survive? At what point (i.e. how many pints or liters, considering normal is ~9 pints or ~5 liters) does blood loss become fatal? KT24.124.51.110 01:16, 20 April 2006 (UTC)
- Well, off the top of my head, a hemorrage is any blood loss of more of 500 ml, and our article on bleeding says that after 1 liter lost, it can be fatal. Titoxd(?!? - help us) 03:53, 20 April 2006 (UTC)
- "Hemorrhage" refers to any amount of bleeding. How much blood can someone lose and still survive depends on a number of factors: their general health, presence of underlying medical conditions (for example, coronary artery disease, how much blood they started with, and if they receive timely resuscitation or transfusion. - Cybergoth 01:30, 21 April 2006 (UTC)
- That'd have to be an exceptional case. Blood donation routinely takes 1 litre, so clearly for most people 1 litre is a safe amount. It sounds like the "drowning in 1 inch of water" ..... possible but unlikely. I would suspect the death point comes when the body cannot compensate for the traumatic loss of blood pressure and either suffers cardiac arrest or loss of brain function leading to unconsciousness and (if unhalted) death. The body also reacts to certain conditions by convulsive constriction of major blood vessels too which may be a factor. FT2 (Talk) 19:31, 26 April 2006 (UTC)
Script for Wikipedia[edit]
Is there a script that can change my homepage (using Opera) to the wikipedia page relative to that day? (IE, it's April 20th, therefore my homepage is set to http://en.wikipedia.org/wiki/April_20). I was wondering if one existed, or if this already exists serverside (like a "Special:Date" page that does this). If not, what would be the easiest way to write a script/program to do this? I was thinking of using either Javascript or Java. - BlazeWizard (Not registered) - 3:58 PM.
- I believe you can do it if you create a template in your user space that redirects to the page of the date, and there is a variable in mediawiki that gives you the date. Let me check it out. freshgavinΓΛĿЌ 05:51, 20 April 2006 (UTC)
- Like this: User:freshgavin/Sandbox/Redirect to date page. It was much easier than I expected. freshgavinΓΛĿЌ 05:55, 20 April 2006 (UTC)
- That doesn't redirect, though — at least not for me. It seems that mediawiki isn't recognizing the redirect construct and is instead just treating it as a list. — Asbestos | Talk (RFC) 15:45, 20 April 2006 (UTC)
- Yeah I noticed that and I was looking through MediaWiki to see why it doesn't recognize it. Apparently in a case like this (it is treated as a special page) redirects are ignored by MediaWiki, even with the ?redirects=yes tag. I'm pretty sure there's no way without JS or bruter, as described below. freshgavinΓΛĿЌ 07:02, 21 April 2006 (UTC)
- That doesn't redirect, though — at least not for me. It seems that mediawiki isn't recognizing the redirect construct and is instead just treating it as a list. — Asbestos | Talk (RFC) 15:45, 20 April 2006 (UTC)
- Like this: User:freshgavin/Sandbox/Redirect to date page. It was much easier than I expected. freshgavinΓΛĿЌ 05:55, 20 April 2006 (UTC)
Thanks for the replies guys, I did however come to a very simple solution to do this. Simply create my own html page with a javascript that uses my computer's date to generate a url, then redirect to that url. Here:http://en.wikipedia.org/wiki/User:BlazeWizard - BlazeWizard 7:447 20 April 2006 (UTC)
Speed of Light[edit]
I actually have two questions:
1) Has any headway been made in the field of quantum physics suggesting that Einstein's assertion that the speed of light is the definitive speed limit that any object can move is no longer accurate? (sorry for the run-on sentence!)
2) This may be a silly question, but bear with me. When at a concert or some other event where "flood lights" are used, it appears to my eye that the lights actually "flood" from there source to their destination. In other words, it appears that I'm witnessing light travel from its source (the flood lights) to its destination (the stage). I'm sure I can't possibly be witnessing the actual speed of light in action. That can't be right. Yet it still appears that the light beam, in a split second, is travelling from its source to its destination. Assuming I'm not actually witnessing the light travel, what optical illusion is at work here?Loomis51 04:18, 20 April 2006 (UTC)
- 1) It's not an assertion, it's a consequence of certain postulates. (which could be called assertions) It can only be false if A) Nature suddenly starts ignoring the rules of logic we've used to describe it so far (In which case Science is useless and we shoud all just go home) or B) One of the postulates of relativity is false. AFAIK, nothing in QM has invalidated those postulates.
- 2) Presumably you're just seeing the light intensity increase to its maximum as the bulb gets warmed up. --BluePlatypus 05:17, 20 April 2006 (UTC)
- To expand on BluePlatypus' answer to 2), your eye can probably see the bulb (begin to) light up before it is bright enough to (noticeably) spread light to the surrounding surfaces, and your brain fills in the sequence by assuming that the light flows from the bulb (which appeared brightly first) to the surrounding surfaces. freshgavinΓΛĿЌ 06:33, 20 April 2006 (UTC)
- Also... in general, when a light illuminates a distant object you should be able to see only the light itself and the object. You shouldn't see the light passing between. If you do, it's because the light is bouncing off (and illuminating) something between. Outdoors, there is often water vapour in the air. For indoor concerts there may be deliberate smoke or mist for atmosphere. So when a floodlight it turned on, it looks as if you are seeing the light "pass through the air". In some cases the heat of the light will change the character of the things in the air close to them, as well. Notinasnaid 10:57, 20 April 2006 (UTC)
Thanks to all of you for your input. It was very thought provoking. In fact it provoked this follow up question: Suppose there exists one bright star adjacent to a large nebula one light year in width and several hundred light years away from earth. (I know a nebula is obviously a 3D phenomenon so "one light year in width" may appear odd. What I'm suggesting is merely that the light that passes through the nebula from the star would take one year to pass from one side to the other.) Suppose there are no other stars in the vicinity to provide anywhere near as much illumination as the one star mentioned. Now suppose the star goes super-nova. Would we then be able to see the speed of light "in action"? Would the nebula slowly darken, over the course of a year, begining at one side and ending one year later at the other?
One other question: Do there exist any "stray" stars in intergallactic space not belonging to any galaxy?Loomis51 23:23, 23 April 2006 (UTC)
- Yes. They're also called rogue stars or hypervelocity stars. There are also rogue globular clusters. [Note to self: Create rogue star article.] -- Filliam H Muffman 01:35, 25 April 2006 (UTC)
- There are also stars observed in galaxy clusters that aren't clearly associated with any particular galaxy within it, particularly around the central galaxy but not bound to it. --Bth 11:16, 26 April 2006 (UTC)
Camparison of 1st magnitude star and light bulb[edit]
I'm trying to give a someone comparison of brightness. Approximately how far away would a 60-watt light bulb have to be to have the same aparant brightness as a first-magnitude star? Bubba73 (talk), 04:21, 20 April 2006 (UTC)
- First of all, as I've recently learned (thanks to Jayant!) wattage is not a measure of brightness, it's a measure of electricity required. Nonetheless, it would appear to me that you could virtually stick the 60-watt bulb in your eye and it still wouldn't compare to the brightness of a first-magnitude star. Loomis51 04:41, 20 April 2006 (UTC)
- A first-magnitude star isn't really all that bright, if I'm reading apparent magnitude and absolute magnitude correctly. However, intensity decreases with the square of the radius...that probably factors in somehow. I'm not a physicist, but that's my shot at it. Isopropyl 04:48, 20 April 2006 (UTC)
- Since you're giving someone a comparison, why not turn on a 60-watt light bulb, look at it, go outside, and look at a first-magnitude star? I'd recommend Spica from Virgo, or Pollux from Gemini. --Bowlhover 15:49, 20 April 2006 (UTC)
- In order to represent the brightness of a star in "human" units like lumens instead of "objective" units like watts, you need to know the spectrum of the objects involved. The reason that a 100-watt light bulb doesn't produce "100 watts of light" is that its emissions follow a blackbody spectrum (like most everything) and most of it isn't visible; moreover, it doesn't even quite radiate 100 watts of photons because it conducts some of its energy away through the air. But we can try a couple of very very rough approximations:
- Just compare the total radiance of the two sources, neglecting conduction for the bulb and the differences in the spectra (which are not necessarily all that great; tungsten light bulbs can be run at up to 3000 K, and the Sun's photosphere is "only" 5800 K — but remember that we could be talking about a blue supergiant star at great distance from us, too).
- Consider the maximum theoretical luminous efficiency as noted in the light bulb article, and compare that with the known luminous output of the light bulb (850 lumens, from the article).
- For either of these we need the luminosity of your first-magnitude star at some distance. Let's suppose (very unphysically; this part is just bookkeeping, not physics) that the star is at the distance of the Sun but still appears first magnitude, so its luminosity (output at all wavelengths) is .
- This is obviously times the output of the bulb. The inverse square law says that to get equivalent brightnesses, we need a distance ratio of , so the bulb should be at distance.
- Alternatively, rate the star (with ) at , which is times the output of the bulb, giving as the appropriate distance.
- So "across town" (for some size of town) is probably the best we're going to be able to say without considering the luminosity function. Hope this helps. --Tardis 17:59, 20 April 2006 (UTC)
- My calculation: I found on a website that the Sun is 10^24 times as bright as a 400 watt bulb. I took that to be approx 6.7 x 10^24 times as bright as a 60-watt bulb. That is a 62.06 difference in magnitude between the sun and bilb, putting the bulb's absolute magnitude at 66.86. Distance for that to have an aparant magnitude of 1 puts it 6.73 x 10(-13) parsecs away, 12.9 miles. Bubba73 (talk), 18:13, 20 April 2006 (UTC)
Google Calendar-Palm synchronization[edit]
The new Google Calendar is absolutely amazing, but of course a glaringly lacking feature is the ability to communicate with or syncrhonize with PDAs. I use my Palm Treo extensively, and despite the advantages of an online calendar like Google's there is no way I can switch to using it if it can't talk with my Palm. I Do you think that Google is working on a way to enable synchronization? Does anyone have any ad hoc solutions? I was thinking about synchronizing my Palm with Outlook instead, then exporting the data to GCal, but I'm not sure how the reverse would work. I think I could use Outlook to pick up the data from Google's Ical web page, but it would be a pain to manually re-synchronize every day and besides, I worry that just copying the entries back and forth would lead to errors, such as multiple copies of an event appearing each time the data are imported. Any ideas or suggestions? — Knowledge Seeker দ 06:57, 20 April 2006 (UTC)
- Right now, I don't know of any solutions, but given the way additional utilities like Gmail Drive have been created by third-party users (not to mention the plethora of sites using Google Maps), my tendency would be to wait for a couple of months; I'm sure that Google knows that PDA sync is a big missing feature, and I'm equally sure there's a thousand good hackers who really want to sync Palms with Google calendar. I've had a multiple-copies of events problem with a Palm in the past (in fact, there were a couple of bad syncs, so I wound up with four copies of everything). Personally, I'd be tempted to see if a good solution isn't right around the corner, either from clever open-source hackers or Google itself. I know it's not the answer you're looking for, but I'd rather keep with the old system until June rather than risk screwing up all my schedule data. --ByeByeBaby 23:33, 20 April 2006 (UTC)
Optimal Resolution For Graphics Card[edit]
My old graphics card allows 1024 x 800 at 70 Hz, but down at 800 x 600 only 60 Hz. Now I thought that a lower resolution would be easier on the graphics card and allow a higher frequency - do graphics cards find higher resolutions easier to produce? --Username132 (talk) 07:15, 20 April 2006 (UTC)
- Generally, a higher res will have a lower refresh rate, but there are exceptions. Maybe someone else can explain the exceptions. StuRat 08:02, 20 April 2006 (UTC)
- The refresh rate is the rate the screen is refreshed. in LCD/TFT screens where only changes alter the image it is not very important in Cathode ray tubescreens it does matter since a low refresh rate can be visable to the eye in the form of a "flickering" image. Generally speaking a higher refresh rate is easier for your eyes. Monitors/video cards however have trouble displaying higher resolutions at high refresh rates. It is recommended to use a refresh rate of at least 75Hz on a CRT monitor. The actual digital signal of a Video card is converted to a analog one via the videocards RAMDAC video cards with a old/low quality ramdac might have trouble displaying high resolutions with a high refresh rate (higher datastream)But to sum op your question. No they should not find it easier.. try setting the refresh rate manually ;) --Magicmasta 12:44, 20 April 2006 (UTC)
- Basically, 800x600 and lower resolutions are for some reason different than higher ones, and have been like that for a while, though it didn't always used to be that way. 640x480 and 800x600 are often limited to low refresh rates with new video cards/on new monitors and I don't really know why but I've always assumed it was because 800x600 and lower resolutions were no longer "priority resolution ranges" for monitor companies and so they don't bother programming them to perform with optimal settings. Notice that the color range is also limited with low-res settings, thought that may be because of a completely different reason.
There may be a technical reason that high-res monitors/video cards can't handle high-freq/high-color settings with low-resolutions, possibly to do with pixel size, but unfortunately I know little about electronics so I can't give you a straight answer : (. freshgavinΓΛĿЌ 05:25, 21 April 2006 (UTC)
Quantum Electrodynamics[edit]
An electron (1) repels an electron (2) because one of them (1) emits a virtual photon which alters the other's path (2) and thus recoils (1). Why does it "know" when to emit the photon when encounters another electron? Also, is it that the electron created the virtual photon from nowhere? Can anyone also explain why an electron attracts a proton? Is it similar to the previous phenomenon? Thanks! - Just Love Science
- It doesn't have to know "when" to trade a virtual photon. The way it actually works is that the probability for the repulsion of the two electrons is given by a sum of all possible ways for the virtual particle to get exchanged. This is an example of quantum weirdness, it's a general phenomenon in quantum theory: you get from the initial state to the final state by summing the amplitudes for all possible paths between. The electron scattering has to sum not only over all the ways to trade a photon, but also the ways to trade two photons, three photons, a photon that splits and recombines in the middle, etc, etc, etc. As for the difference with attractive forces (like between a proton and electron), there really isn't a difference. The minus sign from the charge ends up determining the direction of the momentum transfer, so it goes in the other direction, but it's still a virtual photon exchange. -lethe talk + 07:44, 20 April 2006 (UTC)
Apollo Missions Purifying Agent[edit]
Given that the sodium salt of the purifying agent is cheaper and more commonplace why do you think it wasn't used on the Apollo missions?
I assume you mean the carbon-dioxide absorbing filters for the air, which IIRC used lithium hydroxide. I always thought that was to save every gram of weight possible -- 1 mole of sodium hydroxide weighs 40g, 1 mole of lithium hydroxide weighs 23 grams. i.e substituting lithium for sodium gives you the same CO2 absorbtion capacity for 0.575 the mass. Malcolm Farmer 09:19, 20 April 2006 (UTC)
In addition to the weight issue, sodium hydroxide is extremely hygroscopic—it very readily absorbs water from the air. If you leave a pellet of sodium hydroxide (NaOH) on the lab bench, it will sit there absorbing water until it dissolves into a little puddle of concentrated NaOH solution. Obviously this would present handling problems, particularly in space. While LiOH is also hygroscopic, it is to a much lesser extent. So safety and ease of handling are a couple more reasons to use lithium hydroxide. TenOfAllTrades(talk) 13:26, 20 April 2006 (UTC)
Virtual LBW accurate ?[edit]
Well if you watch Cricket matches. You would possibly see a LBW appeal. Then the T.V. Broadcaster or the necessary concerned would show a virtual Field with no Batsman. And the Ball would be in motion to show whether it was really Leg Before Wicket (LBW).But is it 100% accurate ? Will it be able to take completely the wsing, the pitch , the wind etc. --Oasa 10:48, 20 April 2006 (UTC)
- I don't think anyone would claim it's 100% accurate. I would imagine that issues like wind shouldn't be important, though, because the trajectory is presumably based on the video of the ball -- any effects like that would be already present in the data used for the extrapolation. But I don't know for sure how they come up with the projection. --Bth 11:02, 20 April 2006 (UTC)
- I heard somewhere that, that technology was used to track missiles... so, i guess its pretty accurate...but if a batsman happens to come forward and hit the ball on full toss...the technology might fail in predicting the balls movement after if it had bounced... Jayant,17 Years, India • contribs 15:31, 20 April 2006 (UTC)
- The system is called Hawk-Eye, and its accuracy is apparently "measured in milimetres"[47] (that claim comes from the people who make the software for it, so should perhaps be seasoned to taste.) -- AJR | Talk 23:55, 20 April 2006 (UTC)
Eggs[edit]
I feel very daft asking this, as I'm sure there is a very simple explanation: When chicken hens lay eggs, are they already fertilised (ie if you keep them in a warm place they will eventually hatch), or are they only fertilised later? If the latter is true, how (as the egg is enclosed in its shell and sealed off from the outside, save for very small pores for breathing)? And what is the biological point of hens laying unfertilised eggs with no rooster around (as I presume is the case in most commercial egg production factories), as they will not get any chicks out of them? — QuantumEleven | (talk) 11:27, 20 April 2006 (UTC)
- The eggs are internally fertilized. That's what sex is for. Most aquatic organisms however, do external fertilization, like salmon for instance. English I presume? --
 Mac Davis] ⌇☢ ญƛ. 11:54, 20 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 11:54, 20 April 2006 (UTC)
- More specifically, the fertilisation happens in the oviduct before the egg is surrounded by the yolk,albumen, shell, etc. As for the "biological point" of laying unfertilised eggs, I'm not sure, but given that the tendency exists it's certainly been bred for over millennia of domestication (and of course in general the trait only has to not be selected against once present -- there doesn't necessarily have to be a positive "point"). (Some info here but none on the "point" of laying unfertilised eggs. Semi-interestingly, searching for this brought up a vast amount of abstruse controversy about vegetarians about whether eggs are veggie-kosher and if so which sort.) --Bth 12:15, 20 April 2006 (UTC)
- As a moment of wild speculation, I might guess that the purpose of laying unfertilised eggs is somewhat similar to the purpose of menstruation - to make sure that everything is in prime condition (dare I say it, "fresh") for fertilisation. Confusing Manifestation 12:18, 20 April 2006 (UTC)
- Heh, you have edit conflicted me as I was adding that to my answer. --Bth 12:23, 20 April 2006 (UTC)
- As a moment of wild speculation, I might guess that the purpose of laying unfertilised eggs is somewhat similar to the purpose of menstruation - to make sure that everything is in prime condition (dare I say it, "fresh") for fertilisation. Confusing Manifestation 12:18, 20 April 2006 (UTC)
- More specifically, the fertilisation happens in the oviduct before the egg is surrounded by the yolk,albumen, shell, etc. As for the "biological point" of laying unfertilised eggs, I'm not sure, but given that the tendency exists it's certainly been bred for over millennia of domestication (and of course in general the trait only has to not be selected against once present -- there doesn't necessarily have to be a positive "point"). (Some info here but none on the "point" of laying unfertilised eggs. Semi-interestingly, searching for this brought up a vast amount of abstruse controversy about vegetarians about whether eggs are veggie-kosher and if so which sort.) --Bth 12:15, 20 April 2006 (UTC)
- It is most certainly a trait which was specifically bred for -- there is very little evolutionary advantage that I can see in laying unfertilized eggs (though perhaps if you were expecting predators to steal a few, having "extra" eggs might decrease the likelihood of them getting one of the fertilized ones). In any case, in the case of modern chicken breeds (such as the Leghorn (chicken)), they have been specifically bred to be able to produce high volumes of unfertilized eggs. --Fastfission 15:36, 20 April 2006 (UTC)
- Copulation in birds happens before the egg is laid. Without copulation, the egg is infertile. Birds and other animals, including reptiles and fish, can become "egg bound" if they do not pass eggs after ovulation; this may be a reason for the laying of infertile eggs. --Ginkgo100 04:02, 26 April 2006 (UTC)
- It is for the purpose of menstration. In human females, they pass eggs regardless of whether the egg is fertile or not, only the eggs aren't quite as.. enormous. Chickens undergo the same thing.
White spots in nails[edit]
I sometimes have white spots in my nails. People say it's due to a lack of calcium, but I'm not sure whether that's true or not. What do you think?
- Here's a nice article. [[48]] There are, apparently, zillions of possible causes. --Zeizmic 14:04, 20 April 2006 (UTC)
- It is not a sign that you lack calcium. - Cybergoth 01:45, 21 April 2006 (UTC)
- I heard it was because you banged your nail on something, and it's an imperfection resulting from the collision.
about jobs inbioinformatics in india[edit]
Sir , I want to know that can an computer engineer can enter in bioinformatics field.If so then how . Please guide me .
- Sure. If you look at the bioinformatics article, you'll see all the other field that relate to it. Having some knowledge of statistics, DNA, protein folding and evolution will probably put you at an advantage. I know, though, that the Bioinformatics Master's course at my old university, Edinburgh University, really only expected knowledge of coputer science. I'd recommend looking at the department websites for universities in your area. — Asbestos | Talk (RFC) 15:38, 20 April 2006 (UTC)
Calculating the curve when one wheel is faster than the other[edit]
I'm writing a little program modelling a simple robot. The robot has two wheels, one on either side. It turns by making one wheel faster than the other. I'm just taking into account absolute speed — acceleration is instantaneous. If the left wheel is at speed x, and the right wheel at speed y, how do I calculate the robot's position and orientation at time t + 1?
Any advice would be really helpful! --Mary
- Depends upon how far apart the wheels are.
- Ok, set any distance: one unit. --Mary
- Assume y>x without loss of generalization. Hence the robot translates along a straight line (in the direction of x in the last time step) with speed x. Also the wheel with speed y will try to rotate with an angular velocity (y-x)/d. Where d is the distance between the wheels. You can superpose these motions together to find the position at the next time step. Hope that helps. --coolmallu 16:45, 20 April 2006 (UTC)
Well, I worked it out using the info at http://rossum.sourceforge.net/papers/DiffSteer/. Rather complicated, but I think I got it. Thanks, --Mary
- I didn't understand your comment that "acceleration is instantaneous". That's impossible, an infinite accel would require an infinite force or zero mass using F = ma. StuRat 17:30, 20 April 2006 (UTC)
- Not it is not, all you do start the time when the robot is moving at top speed, then the accelleration is zero.
- I think she means to use the simple approximation that the robot starts at rest at position 0, but then instantly starts off with the wheels going at speeds x and y, so you don't have to worry about the effects of gradual speeding-up. You could just consider starting with the robot already moving. Confusing Manifestation 18:15, 20 April 2006 (UTC)
- She never said she was actually building the robot. It's just a computer model. freshgavinΓΛĿЌ 05:12, 21 April 2006 (UTC)
- I think she means to use the simple approximation that the robot starts at rest at position 0, but then instantly starts off with the wheels going at speeds x and y, so you don't have to worry about the effects of gradual speeding-up. You could just consider starting with the robot already moving. Confusing Manifestation 18:15, 20 April 2006 (UTC)
Here's an answer - I think it's right, assuming there is no side-slipping allowed with these wheels: If x and y are constant, the robot must travel in a circle (a picture would be very useful here, but oh well). The inner wheel will trace out a circle of radius , and the outer wheel will trace out a circle of radius . Thus, in one full rotation of the robot, the inner wheel will have travelled and the inner wheel will have travelled . Dividing by the respective velocities yields the time taken for a complete rotation. From the nature of the motion, the times for the two wheels are equal, so . Simplifying and solving for r, . This is the radius of the robots path for a given d, x, and y. Hope that helped!
--Bmk 23:36, 24 April 2006 (UTC)bmk
bromine[edit]
how the bromine produced industrially?
- Bromine is a chemical element, so it might be more appropriate to ask how it's extracted than how it's produced. Bromine#occurrence does have some details about the extraction methods. StuRat 17:15, 20 April 2006 (UTC)
- The question was probably about the substance Br2, which is produced from brine by electrolysis. —Keenan Pepper 17:26, 20 April 2006 (UTC)
- I was just going to say RTFL Bromine, but gosh-golly it doesn't say. I got this from a USGS report:
Based upon USGS estimates of quantities produced during 2000, Arkansas continued to be the leading bromine-producing State, accounting for most U.S. production. Michigan was the only other State that produced bromine. Mining operations in both States extracted subsurface, bromine-rich natural brines by submersible pump for subsequent processing. --Zeizmic 17:17, 20 April 2006 (UTC)
Keratin[edit]
What is the chemical formual for keratin? Like, water is H2O, and the hydrogens connect to the oxygen at the 100 degree angle. 64.198.112.210 15:57, 20 April 2006 (UTC)
- First off, Keratin isn't a molecule in the same sense that water is, it's a protein. Proteins are chains (sometimes balls of tangeld-up chains) of amino acids linked together. I can give you some clues on which elements it contains, though:
- All amino acids contain Carbon, Hydrogen, Oxygen and Nitrogen, furthermore, keratin is a textbook example of a protein rich in Sulfur, 24% of it being made up from Cysteine, a Sulfur-containing amino acid. -Obli (Talk)? 16:10, 20 April 2006 (UTC)
- I mean chemical structure, sorry. And more like the pictures that you draw to show how molecules built, like the third part of the first picture in molecule. 64.198.112.210 16:01, 20 April 2006 (UTC)
- As stated in my previous answer (which was answering the wrong question), Keratin is made up from numerous amino acids, the sequence of them detemining how they will attract each other and how the chain will bend. I found this picture of how amino acids interact, it's not the structure of keratin, but it shows the general makeup of a protein. -Obli (Talk)? 16:23, 20 April 2006 (UTC)
- Proteins have four levels of structure: primary, secondary, tertiary, and quarternary. For this reason, models of proteins tend to be very complex. Is there a specific reason you need the crystal structure of keratin? Perhaps the information might be found in other ways. Isopropyl 00:31, 21 April 2006 (UTC)
- Three-dimensional structures of proteins (determined mostly by x-ray crystallography, sometimes by NMR methods) are deposited in the Protein Data Bank. That article also tells where one can find viewers. (I like pymol). Unfortunately, when one throws the keyword "keratin" at the database, no hits come up. If you are interested only in the amino acid sequence you will want to look at the Swiss-Prot sequence database. Dr Zak 02:33, 21 April 2006 (UTC)
Intermittent hormone therapy[edit]
I am trying to find information on: intermittent hormone therapy treatment for Prostate Cancer.
Thank you. -------
- Are you talking about testosterone blockers ? StuRat 17:09, 20 April 2006 (UTC)
- I always thought nowadays uses compounds that decrease the level of testosterone in the body. GnRH is the first hormone in the regulatory chain, then come FSH and LH, which in turn act on the level of steroid hormones. GNRH1 analog is another good keyword. And look out for the name of Ralph Hirschmann at the University of Pennsylvania. He had a hand in the research during his tenure at Merck. Dr Zak 22:50, 20 April 2006 (UTC)
- According to Dr. Peter Scardino, who is the chairman of urology at New York's Sloan-Kettering Cancer Center, intermittent hormone therapy is intended to balance the benefits of hormonal control along with a reduction in unpleasant side-effects. As the name implies, it involves periods of hormone therapy alternating with periods where the drugs are interrupted. The hormones are resumed when levels of prostate-specific antigen (PSA) begin to rise.
- Doctor Scardino seems less than impressed with this method, since the side effects of hormone therapy only begin to abate by the time the drugs must be resumed. Since the drug-free periods tend to shorten over time, the implication is also that interruption allows the cancer to progress more rapidly or even develop resistance to hormones more rapidly. Here's a link to his book: Dr. Peter Scardino's Prostate Book--Mark Bornfeld DDS 16:01, 21 April 2006 (UTC)
Really dumb java question[edit]
I can't seem to import correctly in my Main method. My main method is in package a. I have a number of classes in package a.b, including MyObject. When I say
package a;
import a.b.*;
public class Main {
public static void main(String[] args) {
MyObject o = new MyObject();
}
}
it says "Cannot find symbol: constructor MyObject() in location a.MyObject.
If I change the import line to
import a.b.MyObject;
it works fine. Note also that importing, say, Java.util.* works fine. I'm using the NeBeans IDE.
I know this is dumb, but I can't work out what's wrong. Any help? --Mary
- Sounds like you have a name conflict between packages
aanda.b. Perhaps there is aMyObjectin each? Or else you moved classes around and have stale.classfiles. It also sounds like you changed the names for the question; I'd try making a new, clean test with just two classes in new packages, and then gradually make them look more like what you want the real code to be until the problem occurs (at which point the cause should be clearer). (Also, note that computer science questions are now supposed to go on the math desk.) --Tardis 18:12, 20 April 2006 (UTC)
- I guess that was it. There were no other .java or .class files in the folder that I could see, but when I performed a 'Clean and Build' in NetBeans it worked. I guess I don't really understand where NetBeans is keeping its class files, but that seemed to solve it. Thanks! --Mary
Backing up DVDs[edit]
This may or may not be appropriate for this reference desk, but can anyone recommend a piece of widely available, layman-accessible software that I could use to make a few copies of my DVDs (I'm leaving for college and don't want to risk losing my entire collection). I haven't got any nefarious designs, but I expect it's probably illegal anyway. Any suggestions? Bhumiya (said/done) 17:34, 20 April 2006 (UTC)
- Not a lawyer, of course, but as I understand US law (US from your user page), there's nothing illegal about simply copying a DVD for personal use; see fair use. If you decrypt the DVD's contents, you can run afoul of the DMCA, but that's not inherent to copying. The DMCA might also have something to say if you have to "circumvent" (whatever that means) other copy-protection on the disc. I believe that standard DVD burners like, say, Toast for OS X are capable of doing what you want, but I don't have a list of those handy... maybe optical disc authoring software and its links would help? --Tardis 18:30, 20 April 2006 (UTC)
Impurities[edit]
What are the effects of impurities (like Arsenic,Antimoney,Bismuth) Copper. Please explain in detail?
- What are the circumstances? Are you asking how these impurities are found in copper or how you would remove those impurities or what are the properties of impure copper metal, etc? --Chris 19:24, 20 April 2006 (UTC)
A/D converters[edit]
What are the characteristics of A/D converters in data acuisition?
THANK You
- If you type "A/D converter" into the search box up there , you will be taken to our article about Analog-to-digital converters, which tells you a lot about them. -- AJR | Talk 00:28, 21 April 2006 (UTC)
Allergy[edit]
I bought a Hot Tub last year for use in our Scottish Garden. The Tub was made in California by a reputable manufacturer and distributed in Scotland by a highly responsible specialist dealer. My wife and family, as well as several friends, use it regularly and enjoy it immensely. But me? Every time I use it I break out within hours with an incredibly itchy skin, mainly on my back but also on my arms and legs. I have consulted both the manufacturer and distributor and have had great support from them in trying alternatives to the stabilised chlorine granules they normally recommend. These alternatives have included non-chlorine monopersulfate granules. But alas, to no beneficial effect. I religiously clean the filter and monitor the PH, Alkalinity, and Water Hardness and dose them accordingly, and I always shower thoroughly after each Hot Tub. But of all my family and friends, I seem the only one affected. My question is this; Is there a way of treating the water so that I can enjoy the use of the Tub without the adverse side effects described above, or am I consigned for all time coming to be on the outside looking in? The others are quite happy with that latter situation as I make a very good Gin and Tonic for them to enjoy whilst bathing. My Hot Tub suppliers claim not to know of any other similar sufferers in Scotland. Sorry for the length of this whine but thanks in anticipation for any advice.195.93.21.42 18:46, 20 April 2006 (UTC)
- Would you like some cheese with that whine? Hehehe. Could you be more specific as to what chemicals you are using to treat the water? There is obviously more than just chlorine that you could be reacting to. It may even be something as crazy as what material the hot tub is made out of. You may also want to consider seeking the advice of a physician, perhaps a dermatologist or allergy specialist. --Chris 19:20, 20 April 2006 (UTC)
- I would think that bromide and ozonation would be two alternatives --WhiteDragon 19:41, 20 April 2006 (UTC)
You could pay big bucks and convert to a saline spa. These are very mild with almost no irritation. We don't have an article on it, but basically the water is salted to 1/6 of seawater, and an electrolyser produces pure sodium hypochlorite. I've seen them, and they don't produce the halide by-products that are so nasty. --Zeizmic 20:19, 20 April 2006 (UTC)
- My opinion is that if the manufacturers do not have any helpful suggestions you are probably out of luck. Talk to your Physician. Maybe you are hypersentitive to something else?
- It does seem like an allergic reaction, and it could be quite beneficial to find out what exactly it is. Contacting your physician for an allergy test would be a wise course. If whatever is causing it is in one product, chances are good it's out there in other forms as well. (Basicly they test you for a large number of allergens, i believe by exposing your skin to tiny amounts, see if you have any uncommon response) SanderJK 22:41, 20 April 2006 (UTC)
- Are you sensitive to the water temperature (ie. is the water too hot for you)? - Cybergoth 01:52, 21 April 2006 (UTC)
I suggest you dump the old water, carefully rinse the hot tub out, then refill it, but don't add chemicals. Then try it and see if you get the same reaction. This will tell you if it was something in the water causing the problem or perhaps just a reaction to hot water. I've often thought that a distillation process would allow the water to be cleaned without any nasty chemicals. This method would require an exterior tank to keep the distilled water separate from the dirty water, and would require lots of heat, but that wouldn't be so bad if you needed to heat the water anyway. Perhaps a timer could be set to run the distillation process right before you plan to use it. Time to file my patent ! StuRat 07:03, 21 April 2006 (UTC)
I think your skin is reacting to whatever the tub is made of or coated with. As a test, sit in a regular bathtub with the same temperature and add the same chemicals, but don't transfer water from the california hottub. Maybe some sort of coating on your hottub would help. Or a liner? WAS 4.250 10:12, 21 April 2006 (UTC)
Planet Orbits[edit]
On diagrams and pictures, all the planets seem to orbit on one level plane (with the exception of Pluto). Is this correct? Why do all the planets roughly orbit the sun on the same plane? I thought the effects of gravity worked on all planes.
- Mainly because the accretion disk theory says that the planets form from a flattish blob of matter, rather than a spherical one. Pluto tends to be the odd one out in these things, which is why its status as a proper planet tends to be in dispute. Confusing Manifestation 20:02, 20 April 2006 (UTC)
- As an aside, Pluto has lots of other problems too. It's not a gas giant despite the fact that it's on the outer part of the solar system, and it's actually part of the Kuiper Belt, which has many large objects, at least one of which is probably larger than Pluto. There's plenty of more information on those pages and several more I'm sure. EWS23 | (Leave me a message!) 20:13, 20 April 2006 (UTC)
- Also take into account the theory of gravity - the planets will pull each other closer. They may have started on a tilted plane, but when they near each other in orbit, they will pull the plane of their orbits closer. Also, there is the spin of the sun. It is most likely not a coincidence that the planets orbit in the same plane that the sun spins. --Kainaw (talk) 20:52, 20 April 2006 (UTC)
- And the planets all orbit in the same direction the Sun spins (except Pluto) right?
- Yes, all planets in our solar system (including Pluto, actually) orbit in the same direction. Most of the planets also rotate in the same direction, but Venus, Uranus, and Pluto all exhibit retrograde rotation. Prograde and retrograde motion is a good read. EWS23 | (Leave me a message!) 00:39, 21 April 2006 (UTC)
- If memory serves, Uranus is a weird one in that the direction of rotation is a bit of a guess. The best description of it is that it rolls around the orbit. The axis of rotation point directly toward the sun (or directly away from it - depending on your point of view). Since neither axis points "north", it is not easy to say which way it is rotating. --Kainaw (talk) 01:10, 21 April 2006 (UTC)
- Well remembered. The page that I linked above says:
- Uranus rotates nearly on its side relative to its orbit. It has been described as having an axial tilt of 82° and a negative rotation of −17 hours, or, equivalently, of having an axis tilted at 98° and a positive rotation. Since current speculation is that Uranus started off with a typical prograde orientation and was knocked on its side by a large impact early in its history, it is most commonly described as having the higher axial tilt and positive rotation.
- EWS23 | (Leave me a message!) 03:30, 21 April 2006 (UTC)
- Well remembered. The page that I linked above says:
- A couple of other pages of relevance: ecliptic plane and invariable plane. --Bth 23:12, 20 April 2006 (UTC)
Ocean Trenches[edit]
I can't find how they are formed. How are they formed? I am in grade school so i don't know how all of this wikipedia works.
- Read Oceanic trench and subduction. They might be a little heavy going for you, but try them and then ask again back here if there are things you don't understand.-gadfium 22:53, 20 April 2006 (UTC)
- They form when one piece of the Earth ends up going under another through subduction, then there's a crack. --
 Mac Davis] ⌇☢ ญƛ. 04:20, 21 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 04:20, 21 April 2006 (UTC)
The hardest part is geting the scale of things to make sense in your mind. The continents (land) and oceans are just a thin film on a very hot slow rolling "boil" that over billions of years causes the thin surface light-in-weight rock to move like some thin film on the surface of water being boiled in a pot. The relative thinness of the surface of the Earth and the vast stretches of time involved in shaping it are hard to come to grips with for many people. WAS 4.250 09:55, 21 April 2006 (UTC)
- We need a Wikly-pedia for late grade school to early highschool. I find that many of the science articles are somewhat ridiculously post-graduate. --Zeizmic 13:06, 21 April 2006 (UTC)
- Hardly, most are barely under-graduate--152.163.100.74 20:50, 22 April 2006 (UTC)
Yeah...for anything above that, click the links at the bottom of the "heavier" articles :)
April 21[edit]
where do baby internet trolls come from?[edit]
surely the adult form doesn't find any time to reproduce, so where do the little ones come from?5r75r 00:10, 21 April 2006 (UTC)
- Trolls are like prions; they twist innocents into their own shape.-gadfium 00:57, 21 April 2006 (UTC)
- Funny thing - I was accused of being a troll just a couple days ago because I said Americans can be just as stupid as African "savages". Then, later, I said the United States can be (and has been) just as imperialistic as China. Since I disagree with the accepted notions that Africans are inferior to Americans (especially the white ones) and China's government is pure evil, I suddenly became a newborn troll. Now, I don't know what to do. Should I continue thinking for myself and becoming more and more of a troll - or should I dump my brain in the garbage and start making more palatable posts? --Kainaw (talk) 01:18, 21 April 2006 (UTC)
You're a racist then. "Especially the white ones", why do you specifically believe that race of americans are not inferior with a greater degree of certainly than other americians? 12.183.203.184 03:57, 28 April 2006 (UTC)
- I was the one refuting your absurd accusations that Hawaii is under a military occupation equivalent to that of Tibet by China. However, I never called you a troll, although I do question whether you should be permitted out at night by yourself, LOL. StuRat 06:49, 21 April 2006 (UTC)
- I did not intend to imply that you called me a troll. You are a well-respected Reference Desk user. It was an anonymous-coward who called me a troll. Because I feel that nobody else is interested, I will attempt to explain my theory of the Hawaii-Tibet relation on your talk page. Cool? --Kainaw (talk) 12:15, 21 April 2006 (UTC)
- Everyone knows that internet trolls come from under internet bridges. M@$+@ Ju ~ ♠ 22:30, 21 April 2006 (UTC)
- Are you sure the Chinese government is pure evil? It doesn't leave many categories to describe the many goverments featured in List of war crimes does it? Have you tried gas chromatography or mass spectrometry to confirm the degree of purity of the evilness? FT2 (Talk) 19:07, 26 April 2006 (UTC)
help me get into a med school![edit]
I know a lot of the scholarly types visit this place so i was wondering, i want to get into medical school so i can grow up to be rich and famous. the question is, what do you think is the best way top get into a med school? im not very smart. easily confused. and not very motivated. although im sporting a 2.9 gpa in my 5th year as an undergrad so not too shabby. im one of those not very motiveated ppl who neevr fit the cookie cutter nerd mold. and i dont like scinece or biology or math very much. but im a real social out going guy who loves to party and lives off his parents considerable finances so i should have no trouble overcoming my shortcomings. plus i have a convertable bmw and im only 26 years old. not to mention i love to network and have lots of people skillz. could do mad well in the business world but i want to help people. question is. how would my parents money best be spent in helping me get into medical school on my own merit? i dont like tutors mind you their some boring no life book nerd who never get good results anyway. and i do poor on placemnts. question, help me get into med school. also i hate lecturs and lavs bunch of bullshit book learning never got anyone street smarts in medical school--Iwantasportscar 00:18, 21 April 2006 (UTC)
- Consider not going to med school unless you can make yourself study for the MCAT. Isopropyl 00:24, 21 April 2006 (UTC)
you think i could hire someone else to take the test for me. i mean the mcat ppl dont really know what i look like anyway how are they going to know who really took the test or not--Iwantasportscar 00:31, 21 April 2006 (UTC)
- I agree. No offence, but if you just want to be "rich and famous", try to find a profession in which people don't die if you screw up. If you "dont like scinece or biology or math very much", you probably won't enjoy a medical job anyway. —Keenan Pepper 00:30, 21 April 2006 (UTC)
- Also, if you fail your MCAT, you likely will be sued for malpractice multiple times. So, you will never get rich, but you may get famous for your ineptitude. You should consider advice a Norwegian woman told me when I was in Tromsø: Being successful in America is easy. Just go to medical school and be a doctor. If you are too stupid to be a doctor, go to law school and be a lawyer. If you are too stupid to be a lawyer, just be a politician. --Kainaw (talk) 01:24, 21 April 2006 (UTC)
- I think Iwantasportscar really wants to be a writer, and this question is a little exercise. (See also the previous question.) Clearly you have enough talent to get published on USENET --GangofOne 01:42, 21 April 2006 (UTC)
- You could always sell your BMW and buy a hot-dog mobile. You'd be relatively rich (fifty grand in your pocket) and somebody would probably take a picture of you and put you in the paper for being a moron and driving in a hot-dog mobile, thus making you sub-famous for a few days. freshgavinΓΛĿЌ 05:02, 21 April 2006 (UTC)
I apologize but are you serious about this question? If you are as you describe, going to Med school will NOT make you rich and famous. It doesn’t sound like you really have the passion, desire or discipline required for Med School. You have to love what you do and do it well.
- Maybe you could ask a real doctor? "Wow you are the dumbest fucking idiot ever. Instead of your stupid ws.arin.net why don't you try ip2location.com. As for the location in Virginia--that is WHERE VERIZON is headquartered! Put the IP that I used at work into ip2location.com and it comes up LOS ANGELES. You dumb fuck. Speaking of idiots...you are using AOL. And what is your obsession with me? Are you so jealous? Did you aspire to be a doctor but failed? I look forward to you agreeing with me that you are the dumbest fucking idiot ever. I had to say that twice because maybe it didn't get through your thick skull. More about me: I make about 270K per year, and live in a million dollar home. All at the age of 31! :) User:ER MD 10:46, 31 March 2006" (UTC)[49] --GangofOne 19:49, 21 April 2006 (UTC)
- Hey, I think I knew you in college! Ginkgo100 03:56, 25 April 2006 (UTC)
An old adage says that the most reliable two ways to become a millionaire are to marry a millionaire – or divorce one. Since female millionaires are less common than male millionaires, and you clearly want the laziest way, may I suggest emigrating to Canada, Holland or South Africa, all of which countries have a Western style culture and permit gay marriage, and therefore will give you the best percentage odds of success in your quest. FT2 (Talk) 19:02, 26 April 2006 (UTC)
Schizoaffective disorder[edit]
Do shizoaffectives have cycles of mania and depression like bipolar people do?
- There are apparently two types, "bipolar schizoaffective disorder" and "depressive". Why don't you take a look at the schizoaffective disorder article? - Cybergoth 02:48, 21 April 2006 (UTC)
Old people[edit]
Why do old (70+) women develop facial hair? I know they've always had facial hair, but some old ladies are about as hairy as pubscent boys. A Clown in the Dark 01:14, 21 April 2006 (UTC)
- Lack of estrogen after menopause. Men with estrogen treatment stop growing as much facial hair. --Kainaw (talk) 01:20, 21 April 2006 (UTC)
- I believe the ratio of testosterone to estrogen controls facial hair growth, among other things. StuRat 06:40, 21 April 2006 (UTC)
Anatomy[edit]
Acidosis occurs when arterial blood pH drops below what?
- 7.35. I recommend you read Acidosis. A Clown in the Dark 01:49, 21 April 2006 (UTC)
- Actually, you've got causality a bit mixed up there. Acidemia is when plasma pH is less than 7.35. Acidosis is the underlying disorder that caused the acidemia. --David Iberri (talk) 03:04, 21 April 2006 (UTC)
Anatomy[edit]
The predominant extracellular cation is the what?
- I certainly hope Alex would know enough to respond, "No, sorry" to potassium. THe predominant extracellular cation is sodium. The predominant intracellular cation is potassium. That's the whole point of the question! - Nunh-huh 04:32, 21 April 2006 (UTC)
Orca distribution[edit]
According to Wikipedia "The orca is the second-most widely distributed mammal in the world, after the human." but surely rats must be a candidate for this title? Thank you
- This is due to it huge area that the orca has. The ocean is a big place. Some kind of insect would probably come after, or other kinds of whales. --
 Mac Davis] ⌇☢ ญƛ. 04:28, 21 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 04:28, 21 April 2006 (UTC)
Aeration treatment[edit]
I want to know about the "aeration treatment" which is extensively used in water treatment systems. If anyone can answer to the following question regarding the aeration systems,it will be a great help for anyone who searching information about that.
- How does the aeration affect in treating sewage or waste water?
(What will happen to the waste water in the aeration process?)
- Specifications of the aerators used in various occations?
- How does it reduce the BOD & COD level of waste waters?
IF anyone can publish an article about this, It would be great..!!! (Sithara from Sri Lanka)
Basically, it gives the microorganisms in the water all the oxygen that they use up while breaking down (oxidising) the wastes. Biological Oxygen Demand is a measure of how much oxygen is used up by organisms as they try to consume the waste, the end result of aeration should be reduction of the BOD as the wastes have been removed. Sewage treatment should answer your questions. Malcolm Farmer 19:27, 21 April 2006 (UTC)
Basic mechanical reference.[edit]
Doubtless, you are aware of how mindbogling an array there is of guages, types and other descriptions of basic items like screws and threads and wires sizes and so on. Is there any central location (web or print) that has all of it in an easily cross-referenced format? I plan to make excel files eventually, unless someone has them. Thanks! Feel free to respond directly. (email excluded)
Dunno if there's a counterpart on the web, but as far as print goes, you can buy a copy of the current edition of "Machinery's Handbook". Erik Oberg, Franklin D. Jones, Holbrook L. Horton, and Henry H Ryffel are the authors, the publisher is Industrial Press, Inc., New York. It's 2,640 pages, and the ISBN is (at least for the 26th Edition) 0-8311-2625-6.
A much cheaper version is the "Pocket Reference" booklet written by Thomas J. Glover, and published by Sequoia Publishing, Littleton, CO. ISBN 1-885071-00-0 —The preceding unsigned comment was added by 216.163.128.130 (talk • contribs) 13:50, 21 April 2006.
- I have no idea if you care, but there's a biological equivalent: Registry of Standard Biological Parts. Isopropyl 04:53, 21 April 2006 (UTC)
Body decomposition rate[edit]
How long does a body in a fancy tomb take to decompose, versus a body buried simply in a casket in the earth? And does the casket affect the rate? Thank you. PatrickJ83 05:12, 21 April 2006 (UTC)
- I don't know about the rate, but the casket and surrounding concrete vault will most definitely slow decomposition by keeping water and organisms out. Embalming also greatly retards the decomp rate. StuRat 06:34, 21 April 2006 (UTC)
- The decomposition rate is determined by things other than how "fancy" the tomb is: temperature, humidity, acidity, being eaten or not by whatever from bacteria to dogs, method of body preparation. Burial of executed criminals in no casket at all in north european peat bogs was extemely preservative of the bodies. WAS 4.250 09:32, 21 April 2006 (UTC)
- Thank you, but do u think it takes, say, 50 years for the soft tissue to decompose in a 'fancy' tomb above ground and maybe 10 years in a coffin in the Earth? This is under normal circumstances, no preserative beat bogs or anything. Without embalming. Thanks PatrickJ83 17:43, 21 April 2006 (UTC)
- It depends. Louisiana or Montana or Nevada? Humdity and temperature matter. How safe from flys and worms. Buried in clay could be safe from worms, above ground could be fly food. How decomposed is "decomposed"? I recommend you take a piece of meat and put it somewhere and look at every day or so. Maybe even write down your observations. Maybe even have a few pieces of meat; one outside, one in the dark, one in the light... WAS 4.250 18:14, 21 April 2006 (UTC)
- Would a piece of meat really be a good experiment to contrast with a human body? I would have to bury the piece in a coffin in the ground and then bury another piece of meat in a marble tomb, HA! Anyway I digress. The reason I asked this question in the first place is because I was reading in a magazine about a newish trend of people buying above-ground tombs for themselves instead of being buried in the ground as they think it's more noble. But does this slow down decomposition rate? Would a body buried in a marble tomb in a church take, say, 100 years to decompose to skeletal remains? That's really what my question boils down to. Thanks you guys! PatrickJ83 21:34, 21 April 2006 (UTC)
- A better subject for your experiment would be a whole animal carcass, rather than just a piece of meat. Ginkgo100 04:00, 25 April 2006 (UTC)
I've seen news reports on coffins for sale in Japan that have dehumidifiers, interior lighting, and even air conditioning, so be careful when referring to "fancy" coffins. freshgavinΓΛĿЌ 05:58, 26 April 2006 (UTC)
- A text book on forensic autopsy might help, the decomposition of the human cadaver under a wide range of circumstances is a very well studied subject..... sadly most of all in the context of establishing the circumstances of death of a body. FT2 (Talk) 18:57, 26 April 2006 (UTC)
- Try reading Stiff by Mary Roach, or Corpse by Jessica Snyder Sachs, or Death's Acre by Bill Bass. Or go here 64.198.112.210 19:41, 28 April 2006 (UTC)
- Also, you could guess. It take from 50-365 days for an exposed cadaver to reach the stage of dry decay, where the skeleton, along with hair, nails, and dried skin are the only things left. It always surprises me how little people know about decomposition. While I'm not an expert, you can ask me if you need more help. DuctapeDaredevil 19:44, 28 April 2006 (UTC)
Why does some chewing gum disintegrate in your mouth?[edit]
Example: Altoids Sour Apple Chewing Gun disintegrates in your mouth after chewing it for 5 - 15 mins.
Description of phenomenon: The Gum looses all flavor and it begins to get really soft as you chew it...the texture and the elasticity is gone. It them becomes like a glob of paste and disintegrate in your mouth.
Possible answer found on on HighBeam Research but did not have full access to read the article (Chemistry and Industry, May 2, 2005): "Chewing gum can be a disappointing experience of short-lived flavour or grainy texture, largely due to the method of manufacture. There are two basic processes: the first is to mechanically mix gum base compounds/polymers. This tends to produce a nice texture, but can destroy encapsulated flavours, The second method is to compress discrete gum base particles, a process that is gentler on the additives but can result in gum that disintegrates too easily on chewing."
Question: Can some elaborate or confirm if the explanation is true?
- I like that stuff! I think its because there are almost no purposeful elasticity agents in the gum. Its not really gum, its just sugary soury stuff that is like a chewy sweet tart. Most gums have stuff like chicle or plastic-like substances to make them elastic, for the pleasing feeling for the chewer. Sort of how people love chocolate for one reason that it melts just under your mouth's temperature. Mmmmm. --
 Mac Davis] ⌇☢ ญƛ. 11:33, 21 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 11:33, 21 April 2006 (UTC)
- Not exactly your question, but certainly related: http://www.straightdope.com/classics/a2_201.html —Keenan Pepper 14:53, 21 April 2006 (UTC)
- Fantastic. Now we can work on that other important question, "Does your chewing gum lose its flavor (on the bed-post overnight)?" Confusing Manifestation 12:34, 22 April 2006 (UTC)
- Actually, one time when I was younger, I had been chewing some gum for the entire day—some bubble tape, if I recall correctly—and it eventually disintigrated. It first lost its flavour and then, a few hours later, began to taste awfully bitter. A few seconds after that, it dissolved. 163.153.27.11 15:39, 27 April 2006 (UTC)
Speed of Heat through Space[edit]
My question is .." Does heat travel through space at the same speed as Light (close to 300,000 Kilometers per second)?" if it does can I have a reason Thanks Tony Stevenson
- Someone else will no doubt have better information on this than me, but ISTR that both heat and light are radiant energy by-products of electromagnetic radiation and therefore travel through vacuum at the same rate. Grutness...wha? 09:17, 21 April 2006 (UTC)
- Heat in the form of thermal radiation and all light is EM radiation, not a byproduct. But note that heat can take many different forms, not all of which are radiative. Light on the other hand is, well, light, and is always radiative. However, in a vacuum the only transfer of heat is via photons, so yes, through space heat and light are identical in almost every respect (aside from visibility to humans and a reasonable difference in energy per photon). --Tardis 18:23, 21 April 2006 (UTC)
Thanks for the answer, I still don't understand why the heat from the sun in the morning takes a while to heat the earth.. if an atomic bomb was to be let off in space would a spaceman say 600,000 kilometers away be burnt to a cinder in 2 seconds? surely he would see the light before being burnt?
- The time taken to heat up in the morning is nothing to do with the speed of the heat. After all, the heat already left the sun, and has arrived by dawn. The time taken is because there is only so much heat available, and because there is an awful lot to heat up. Notinasnaid 10:15, 21 April 2006 (UTC)
- Assuming the spaceman is not hindered by anatomical difficulties that regulates the speed of which he observes, but instead acts like a neutral, single-photon sensitive sensor, an answer can be given that hopefully isn't very bad. The explosion takes place, and photons will be emitted spherically. Now, after two seconds, ONE photon is destined to arrive at the spaceman before the others, or along with a couple of others. He will detect this photon. Within very little time, other photons will also arrive. Remember that photons will one by one clash into the spaceman, and heat him up until being burnt dead (assuming that is the effect of a nuclear bomb at 600,000 km). The answer to the question is then that yes, he will detect light before being burnt. However, another answer, equally true, says that AS HE DETECTS the first photon, a part of him, even if only an electron or something, will be affected ("burnt"). If you like, he is burnt, but on a very, very small level, because as soon as a photon hits him, energy is transferred, and heat is created on a very, very tiny level. The difference between this and actually having the hole man burn up, is just a matter of how many photons are needed to clash into all of him. Likewise, your earth is heated up as soon as a photon hits, but the more photons arrive over a longer duration, the more heat it gets. 213.161.190.228 10:34, 21 April 2006 (UTC) Henning
- Just to give a sense of scale, any normal nuke at that range will have little to no effect; that distance is nearly one hundred Earth radii. A human (taken to have a ridiculously large presented surface area of 2 square meters) will intercept approximately 4 mJ of radiation of various kinds from a multi-megaton explosion at a range of . This energy, if evenly distributed, will raise the person's temperature by some 15 nK -- entirely insignificant. There might still be some concerns from, say, gamma irradiation damage, especially since there's very little material present to convert the initial reaction radiation into lower-energy forms (like molecular motion or thermal photons), but I suspect that a man on the Moon would be quite safe from all effects of nuclear explosion taking place very nearly on the opposite side of the Moon's orbit (but in line-of-sight; not talking about Earth as a shield here). --Tardis 18:23, 21 April 2006 (UTC)
It is a good answer but not the one I wanted to read... so I lose the bet.. Thanks again Tony
- Heat can be transmitted in two ways: One is convection/conduction, where the heat is transmitted in the form of the motion of atoms and molecules. In that case the theoretically fastest speed it could move at would be the speed of sound in that medium, although it's usually much slower. The second way (and the way the heat of the sun gets here) is through infrared radiation (which, when absorbed by molecules turns into kinetic energy), in that case the heat moves at the speed of light. As for why it takes a while to heat up the earth, that's the same answer as for why it takes a while to heat up a pot on a stove. Note that in the morning/evening, the light is coming in at an angle, and there's less incident light, and more of it is getting absorbed by the atmosphere and not making it down to ground-level. Which is also why we have seasons and why it's colder near the poles. --BluePlatypus 12:14, 21 April 2006 (UTC)
- We're dealing with separate phenomena here. The reason that it takes a while for the Earth to warm up is because of its specific heat. The way that heat is transferred to the Earth by the Sun is not by direct conduction (as there is very little matter in between) but by electromagnetic radiation. The amount of energy delivered is inversely proportional to the wavelength of the light. Tying this to the atomic bomb thing, the reason you don't die when you're so far away from the bomb is because intensity decreases with the square of the radius; doubling the distance cuts the intensity to a quarter. Also, bombs propogate shock waves as fast as possible through their surrounding media, approximately the speed of sound. Isopropyl 13:58, 21 April 2006 (UTC)
In a pure vacuum, heat would radiate at the speed of light, and there would be no convection or conduction of heat. Space, however, isn't quite a pure vacuum, so, while most heat would radiate at the speed of light (as infrared radiation), some would be absorbed by matter in space, and later radiated back. So, there would be some lagging heat which could arrive considerable later. I'm not sure if the amount of lagging heat would be significant or not. StuRat 22:08, 21 April 2006 (UTC)
palaeanthropus palestiensis[edit]
My Harpers Bible Dictionary refers to eight giant human skeletons from around 100,000 years ago being found in a cave on Mt Carmel and calls them Palaeanthropus Palestinensis. I am unable to find any further info on them, could someone direct me onwards. Thamks Hatch
- Never heard of it before, but some research suggests that "Palaeanthropus" is considered a simple synonym of "homo sapiens". http://www.jewishvirtuallibrary.org/jsource/Archaeology/carmel.html talks about the caves of Carmel. The cave may well be "Skhul". http://www.archaeologyinfo.com/homosapiens.htm talks a lot about it, but doesn't talk of giants: "the site is an extremely important one" "The site has been dated from 120 kyr up to 40 kyr by various methods" "This led to the false impression of large cranial capacity". Notinasnaid 10:28, 21 April 2006 (UTC)
- It was made up, there never were any race or species of "giant" humans. --
 Mac Davis] ⌇☢ ญƛ. 12:23, 21 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 12:23, 21 April 2006 (UTC)
Start here: Mount Carmel, Israel and here WAS 4.250 12:39, 21 April 2006 (UTC)
How do I obtain a list of the richest person in Microsoft Access?[edit]
Let's say I have a database of people on earth that has following data fields:
- Continent: Asia, N America, ...
- Country: Australia, ..., Zaire, ...
- Province or State: ...
- City: ...
- Name: ...
- Net worth: ...
I want to know who is the richest person in each country's each province. And I also want to know where can I find him/her (which city). I think I can create a query like this:
| Continent | Country | Province | City | Name | Net worth |
| Group by | Group by | Group by | do nothing | do nothing | Max |
However, in Microsoft Access, you can only do:
| Continent | Country | Province | City | Name | Net worth |
| Group by | Group by | Group by | Group by | Group by | Max |
It results in a very long list also grouped by cities and names. I end up to have a list of the richest Johns, Marys, Toms, ... in each seaside little town. Can I fix it with some SQL commands? -- Toytoy 13:04, 21 April 2006 (UTC)
yea you can and i could help you with the commands. im just a little confused about exactly what you want, could you elaborate a little bit- like what tables you already have and what the different fields are. --modesty 17:04, 21 April 2006 (UTC)
- I would switch to SQL mode in the query window and cut those extra 'group by' statements, that's what's throwing it off. Access usually accepts hand made SQL as well as anything.
- One way you could do this is: (you seem to have some Access knowledge, so I'm skipping all the little details of building queries, linking and so on - let me know, and I'll add them in)
- First run a query, with Continent, Country and Province set to Group by, and Net Worth to Max. Don't include the City or Name fields at all. Save the query, I'll call it Max_Worth, which gives you the maximum net worth in every province -- with no name or city information.
- Second, start a new query. Add the base table, plus the Max_Worth query. Link the Continent, Country and Province fields from the base table and the Max_Worth query. Next, link the Net Worth field from the base table with the last field in the query; it'll be called something like MaxOfNetWorth. Add all the fields in the base table to the query; have all of them set to Group By.
- That should give you a list of every field that matches the maximum net worth for their particular province, with all of the information you need. --ByeByeBaby 03:29, 22 April 2006 (UTC)
- One way you could do this is: (you seem to have some Access knowledge, so I'm skipping all the little details of building queries, linking and so on - let me know, and I'll add them in)
Could a planet succesfully orbit a black hole?[edit]
My understanding of black holes is that they are essentially stars that have mass so great that light/matter can't escape it's gravity. So essentially they work like vacuum cleaners sucking up anything that crosses within their gravitational threshold. Presumably this also would mean that their mass would continue to grow as would their gravitational attraction as they "hoover up stuff". All that said, could a planet (or planets) orbit a black hole safely as our planets orbit the Sun? Or would they gradually be pulled towards the black hole and consumed because of it's ever increasing mass?
I would imagine that the process of a star becoming a black hole would no doubt destroy any orbitting planets of the star, but even so I'm still curious in the hypothetical case. Thanks in advance. Gallaghp 15:21, 21 April 2006 (GMT)
- By the divergence theorem, a spherically symmetric object of a given mass has the same external gravitational field regardless of its radius. If the Sun were replaced by a black hole with the same mass, all the planets would continue to orbit the same way. —Keenan Pepper 14:50, 21 April 2006 (UTC)
- Yeah, that's the common but somewhat incorrect perception. Black holes aren't vacuum cleaners, their gravity works just like that of any other stellar body up until the Schwarzschild radius. That radius is much smaller than the average planetary orbit, so any object could orbit a black hole just as easily as any other stellar object. The thing about a black hole (but not limited to them) is that most of them probably have an accretion disc with matter that's been drawn into orbit. In that disc they'll bump into eachother and the energy loss to friction will lead to them falling in eventually. But the rules for orbiting a black hole are the same for any other object. (Except for within the Schwartzchild radius). --BluePlatypus 14:53, 21 April 2006 (UTC)
- Actually, Gallaghp's question seems perfectly rational to me. I'm not sure that the two answers above me noticed that the question was asking whether a black hole's mass (and thus the Schwarzschild radius) continues to increase, which would thus endanger any orbiting planet. In the general sense, I would assume that the answer is 'yes, the mass will continue to increase and so...'. However, if, say, the Earth were to turn into a black hole, its mass would increase no faster than if it just stayed being the earth (with random meteorites adding to its mass). In fact, it would probably increase in mass slower, as the Schwarzschild radius would be inside the original radius of the earth (so some debris which would have hit the Earth would miss the black hole). — Asbestos | Talk (RFC) 20:15, 21 April 2006 (UTC)
In order to form a traditional astronomical black hole, it would have to be super-massive, which means it would have a much larger gravity well than a typical star and pull in passing objects from much farther away. However, I can imagine a period after which all nearby matter had been pulled in, and the black hole could then capture a passing planet (not sure why a planet would be just flying around the universe, but let's ignore that for now). This would be quite difficult to detect, since not only would there be no light given off, but the gamma ray jets associated with the accretion disk would also be absent. A gravitational lens effect could still be detected, however, on objects behind the black hole from our POV. StuRat 21:39, 21 April 2006 (UTC)
- Actually I'm pretty sure that the capture of a planet by a black hole would release an enormous amount of energy. Some of it would be released as light when the planet was destroyed, outside the event horizon, by the tidal forces; that should heat up the matter to the point that it would give off quite a lot of light, unless the black hole were truly huge. Given a black hole so large that a planet could slip past the event horizon without being vaporized first (anyone care to figure out how big that would be?) I would still expect a massive energy release, but it might be in the form of gravititational waves, which are pretty hard to detect. --Trovatore 22:18, 21 April 2006 (UTC)
- I don't think you are taking "capture" to mean the same thing I meant. I meant the planet would orbit in a stable manner, perhaps at a rather extreme distance, like billions, or even trillions, of miles. StuRat 01:01, 22 April 2006 (UTC)
- Oh, I see. Sure, I don't see any reason that couldn't happen. Of course some of the planet's energy has to be dissipated the first time it approaches, or it'll just fly off again, but I suppose there are ways that could happen. --Trovatore 01:15, 22 April 2006 (UTC)
- As I understand it, a light object on a nonintercepting course with another far more massive object, in space, will do one of the following things:
- If the speed of the light object is much less than the orbital speed at that distance, the light object will spiral inward and eventually collide with the massive object.
- If the speed of the light object is somewhat less than the orbital speed at that distance, the light object will spiral inward toward the massive object until the speed is sufficient for a stable orbit, and the light object will then enter that stable orbit.
- If the speed of the light object is exactly the orbital speed at that distance, the light object will immediately fall into a stable orbit. This, however, is extremely unlikely.
- If the speed of the light object is slightly more than the orbital speed at that distance, the light object will spiral outward until the speed is sufficient for a stable orbit, and the light object will then enter that stable orbit.
- If the speed of the light object is much more than the orbital speed at that distance, the light object will slingshot around the massive object in a parabolic path and then continue on it's way. In an extreme example, the deflection is so slight that the path of the light object appears to be a straight line.
- So, 3 or the 5 cases would result in a stable orbit, eventually, again assuming there is no accretion disk in the way of all this happening. StuRat 01:39, 22 April 2006 (UTC)
- No, in the simple case (no energy transfer), there is no "spiraling". The light object coming "from infinity", or more to the point having a speed at least equal to the escape velocity, will trace out a hyperbolic (or parabolic) orbit past the heavy object, and escape to infinity, unless the perigee of that orbit is smaller than the radius of the heavy object, in which case it will crash.
- To get any other pattern, energy somehow has to be transferred between the two objects, or between the light object and other bodies in the system (moons, accretion disk, etc). I think this might be a plausible mechanism for energy transfer between the planet and the black hole: Tidal forces could act on the planet, changing its spin and therefore its angular momentum, and the angular momentum of the hole would have to change to balance the books, with a concomitant change in its rotational kinetic energy. But the precise calculations would get into general relativity and are quite beyond me. --Trovatore 01:53, 22 April 2006 (UTC)
- I disagree. This isn't the way satellites behave and I don't believe it's the way planets behave either. If orbits were that unstable, nothing would remain in orbit for long. StuRat 19:39, 23 April 2006 (UTC)
- No, it is the way satellites behave, essentially. The difference is that satellites are gravitationally bound to their primaries; that is, they do not have enough kinetic energy to escape. But a body coming "from infinity" always has enough energy to escape, because it had it to start with. Unless, as I said, that energy can be transferred to some other body.
- Satellites don't "spiral" either. Their orbits, rather than the hyperbolas or parabolas traced out by bodies coming from infinity, are ellipses. They fall in to the point of nearest approach (perigee), and fall out to the farthest point (apogee), and repeat; unless there's an energy transfer, the perigee and apogee does not change from orbit to orbit. --Trovatore 19:48, 23 April 2006 (UTC)
http://en.wikipedia.org/wiki/Methuselah_(planet) - If neutron stars, why not Black Holes? - G3, 07:00, 25 April 2006 (UTC)
Another postscript, for the record: What I write above is correct in the Newtonian case and will work as long as you don't get too close to the black hole. It appears that matters are somewhat different if you get within a few Schwarzchild radii of the hole, owing to relativistic effects. See #Black hole horizon problems below. (In the archives, might be in a different week, not sure.) --Trovatore 02:52, 26 April 2006 (UTC)
Vacuum Extraction[edit]
Hello, I've heard of a procedure I'd like to find out more about, apparently Vacuum Extraction is "a new method of non intrusive exploratory digging without the risks associated with normal digging. In the USA legislation has been passed preventing mechanical digging due to costs associated with litigation following damage caused to underground pipes and cables. The method of exploration starts with high pressure water making a hole of up to 8 cms and a vacum system that draws out the debris The cable avoidance ground radar identifies hazards before damage occurs. The waste is held in a tank to be used as back fill or disposed of at water treatment plants if necessary." All I've been able to find on google under Vacuum Extraction relates to a medical procedure used in childbirth so I wonder if this is known by another name. Can anyone help me find out more about this method of digging? Thanks. AllanHainey 14:33, 21 April 2006 (UTC)
- Vacuum excavation? It seems to give a lot of hits. --BluePlatypus 14:45, 21 April 2006 (UTC)
This method sounds like it would have some limitations, especially when dealing with underground water. Only 32 feet/10 meters of water can be supported by a pure vacuum, so a hole with water at more than that depth (or any depth of water continuously leaching in from the sides faster than the vacuum can remove it) would be an impassable obstacle. As a practical matter, the pump impeller would cavitate and fail at far shallower depths. A traditional drill would have no trouble with the water, and could actually benefit from the cooling. I would also think sharp chunks of quartz and such would damage the vacuum, or at least wear it out quickly. StuRat 21:06, 21 April 2006 (UTC)
- We call this 'daylighting' over in the boonies of Ontario. It's only for shallow excavations around existing pipes and such. There is no reason to go deep. --Zeizmic 23:56, 21 April 2006 (UTC)
- You don't necessarily have to suck from the surface. You could always pump compressed air or liquid down to push the dirt out. Alternately, you could build the pump into the drill head, and again push from the bottom rather than pulling from the top. Of course, as Zeizmic says, this technique is really only used or likely to be useful for shallow work. If you're going to go deeper than pipes or cables are buried, then you can switch to conventional drilling equipment once you've got the first few meters of hole opened up. TenOfAllTrades(talk) 03:24, 22 April 2006 (UTC)
Martial Arts feats[edit]
I have seen buddist monks, martial artists, and other assorted people stand on very sharp things (i.e. a sharp edge of a katana) and not be cut by them. Similarly, I have seen people stand on a single sheet of rice paper without breaking it.
Is it some kind of trick, or are they really able to do it? Is they are, how? I'm not into 'Ki' or 'Qui' or whatever the popular word is for it now; I would prefer a scientific explanation above all else.
Thanks! 68.52.56.111 15:30, 21 April 2006 (UTC)
- Hmm, I know it's possible to support your weight on a lot of sharp things together, like a bed of nails, but not a single sharp thing by itself. Were they standing on a whole bunch of katana, or just one? Also, if a sheet of rice paper is curved just the right way, it might spread the forces out and support the weight of a person. By the way, it's spelled qi in pinyin. —Keenan Pepper 15:40, 21 April 2006 (UTC)
- Similar to this monk. Unfortunately, I can't answer your question. Thank me for being useless. --Chris 16:48, 21 April 2006 (UTC)
- I was actually present when a guy (martial artist, cannot attest to the style though) stood with his feet on two katanas. How do I explain this...eh...he stood where the two blades of the katanas were both hitting his feet, as if he were standing on two telephone wires that were next to (parallel to) each other. So I guess the force could have been spread out along both of the katanas. I cannot verify the sharpness of the katanas to you, but he had what I assumed was a Aikido, Iaido, or Kenjutsu (or the like) practioner do a tameshigiri on some rolled up bamboo poles with both of them before he stood on them. Maybe that's the secret to it - he had something in the mats to secretly blunt the swords. However, if the guy was on the up and up, I would like to know how he did it. Thanks. 68.52.56.111 18:45, 21 April 2006 (UTC)
Some thoughts:
- The bamboo was probably struck at high speed with the swords, while his feet were only slowly placed on them. Thus, a much greater force was applied to the bamboo. Perhaps, even impact force was applied to the bamboo by the high speed.
- The bamboo might have also been considerably softer than it appeared, say if it had been soaked in something to make it pliable and/or cut most of the way through.
- He may have had thick callouses on his feet, making them much tougher than they appeared.
StuRat 20:50, 21 April 2006 (UTC)
Think of how you would eat a steak. Would you try pressing or pushing your steak knife through the meat? No. You would move it forth and back in a slicing motion. This is the action which performs the cutting. Tameshigiri doesn't work if you strike like a baseball bat. The swing technique has the hands leading the blade. As contact is made there is a natural tendancy for the blade to draw across the mat in a slicing motion, resulting in a rather crisp cut when done correctly.
Standing or laying on sharp objects does not involve the slicing motion. Notice how carefully they do this... This goes back to pressing the steak knife through the piece of meat. Sure it can be done, with enough force, but the gimmick is that the guys weight on the blades doesn't result in this much force.
Seperating Chemicals[edit]
I noticed that in my first aid kit there are electolyte tablets that have 220 mg of NaCl, 15mg of KCl, and 18mg of CaCO2. Just out of curiosity, how would I got about seperating these 3, especially the NaCl from the KCl? --Chris 16:48, 21 April 2006 (UTC)
- The CaCO2 is easy. Just dissolve the tablet in water and it will precipitate out, especially if the pH is raised. Separating Na+ from K+ is much harder. Chromatography and ion exchange come to mind... —Keenan Pepper 17:10, 21 April 2006 (UTC)
- [This http://www.corrosion-doctors.org/Biographies/DavyBio.htm] is interesting. It indicates that Humphry Davy developed a method back around the beginning of the 19th century to separate potassium from sodium. It used the fact that sodium perchlorate is soluble in 97% ethanol whereas potassium perchlorate is not. (You're going to need perchloric acid, ethanol, and patience....)
- A technique used commmercially is called recrystallization. Again, it relies on manipulating the relative solubilities of the potassium and sodium salts. Starting with a room-temperature saturated solution of sodium and potassium chlorides, add your mixture of salts and heat to high temperature; mix until no more powder dissolves. The solubility of NaCl in water is very nearly constant regardless of temperature, whereas the solubility of KCl increases substantially when the water is heated. Consequently, adding your powder to the hot solution will dissolve KCl and leave powder enriched in NaCl.
- Step two is to filter this solution to collect your NaCl-enriched powder from the hot solution. Step three is to cool the solution back to room temperature. The excess dissolved KCl will precipitate; you can collect it by filtration. TenOfAllTrades(talk) 03:18, 22 April 2006 (UTC)
- I assume it's CaCO3 that's meant? --BluePlatypus 17:27, 21 April 2006 (UTC)
- Hmm, right, wonder why I didn't notice that. CO22− would be the impossible ion carbonite. —Keenan Pepper 17:36, 21 April 2006 (UTC)
Yes, CO22−. Carbonate... sorry. --Chris 21:10, 21 April 2006 (UTC)
History of psychiatry[edit]
Hello, I've been wondering about one specific topic : the evolution of the concept of late schizophrenia. European nosology (early 20th) used a specific concept : "psychose hallucinatoire chronique" (PHC) that is still widely used in France for instance. Modern nosology only uses the concept of late schyzophrenia...so here are my questions :
- is late schyzophreia an accurate replacement concept for PHC ?
- how to explain that despite the DSM-4 and CIM-10, that specific concept of PHC is still used ?
So if you guys have any link / reference article / answer / suggestion... it would be greatly appreciated !
Alkaline seafood dishes[edit]
Is lutefisk the only alkaline seafood dish? I remember reading somewhere that most cultures developed acidic seafood dishes (e.g. Western fish with lemon, Japanese sushi with vinegar...) because that converts the amines like cadaverine and putrescine into their ammonium salt forms and thereby reduces the rotten fish smell. How come the Scandinavians didn't catch on to this? —Keenan Pepper 17:18, 21 April 2006 (UTC)
- Perhaps not the only, but probably the most alkaline. :) Lutefisk isn't rotten and doesn't smell much though, that's Surströmming. They do eat acidic fish too, in the form of pickled herring. Between the salting, smoking, drying, drying-with-lye, pickling and fermenting.. I think they pretty much used up every form of preservation available to them. My guess is that if they had had vinegar, they would've pickled the herring instead of fermenting it. --BluePlatypus 17:42, 21 April 2006 (UTC)
Seahorses![edit]
Hi. I am doing a project on seahorses in my marine biology class. I was wondering what the digestive, skeletal, and circulatory systems were for seahorses. Can someone help me out? Thanks for your time!
- I hate to sound so much like the others...but try searching for 'Seahorses' on Wikipedia, perhaps?
-I already did, but it didn't say anything about the systems...thanks anyway.
- Try this Google search --LarryMac 19:35, 21 April 2006 (UTC)
- 18 months back I visited a seahorse farm in Port Lincoln, South Australia, and afterwards did some of my own research on the syngnathidae. The folks at syngnathid.org were really helpful (so I now know sea horses have only one “kidney”, without glomeruli, on the right side – wow!). The site is dedicated to info about these creatures, mainly related to their breeding in captivity, with the aim of conserving wild populations. Collectively they have thorough knowledge of the sea horses' normal and pathological structure and function. Try asking on that site; people who are fanatic about conservation (in a good and necessary sense) are usually good for asking questions of, since their main weapon is education. --Seejyb 20:42, 21 April 2006 (UTC)
While not an answer to your question, you might be interested to know that there are seahorses in lake Titikaka in Peru. Not only have I heard that it is the highest lake on Earth, but also one of the deepest, and the only freshwater habitat on Earth that supports seahorses. The story I recall is that as the Andes lifted, carying a pocket of the sea with them, the lake somehow remained always filled, and ecologically isolated, so that virtually all of the species there are decended directly from marine forms instead of freshwater species. Thought you might be interested.
Lucid Dreaming[edit]
Is it possible to induce lucid dreaming with a tape recording that says "YOU ARE DREAMING" over and over?
- Sounds like an experiment! Melchoir 21:21, 21 April 2006 (UTC)
- It would work for me, I know in light sleep, I can hear what is going on while I am sleeping at times. Good experiemnt. See if the Lucidity Institute has done it before. --
 Mac Davis] ⌇☢ ญƛ. 03:24, 22 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 03:24, 22 April 2006 (UTC)
I would think something like a taped conversation or animal sounds would be more likely to start a dream. Physical sensations also seem able to cause a dream, like a bandage (or "plaster", for the Brits) once made me dream I had a leach on my arm. StuRat 04:55, 22 April 2006 (UTC)
- The point is not to start a dream, but to start a lucid dream (which the OP helpfully linked above). Melchoir, as I recall, this isn;t so good, as your brain will eventually tune out the sounds of the tape if its keeps hearing the same thing. People who use tapes usually try to time the voices to come on when they expect to be in REM sleep, somewhere around 90 minutes after you fall asleep. If you look online, you'll find all the common methods (looking and your hands and stuff) that are pretty tried-and-true. — Asbestos | Talk (RFC) 23:05, 22 April 2006 (UTC)
Government Suppression[edit]
Is there any eveidence besides reviewing endless lists of patents, to understand, or gain information about, how the government exercises an ability to suppress technological innovations, certain inventions, or scientific data to control the public more than it should. I'm not trying to be some conspiracy theorist, but I have already found astonishing evidence about things I can barely believe in relation to the vagueness listed above. Anyone can go and read some writing about or by tom bearden to see a little bit of what i mean. I can understand the implications of monopolies blah blah and all that. but when something has the potential to beifit all of mankind in profound ways then why else does this seeming oppression prevail?
and does anyone know what this means: e=±((square root symbol) since i couldn't find it)α(multiplied by something that looks like an h with the stem(top part)crossed through with a small line)finally multiplied next by c (the speed of light of course) lastly maybe someone can give me a vague explanation of non-linear optic effect
Retrieved from "http://en.wikipedia.org/wiki/Wikipedia:Reference_desk/Miscellaneous"
- This has been taken up over at Misc. --Zeizmic 23:48, 21 April 2006 (UTC)
Absolute pitch[edit]
I _need_ an absolute pitch. It can be developped. Has anyone got any idea about how to/the best way to attain this target? Thanks. —The preceding unsigned comment was added by 85.50.33.189 (talk • contribs) 21:53, 21 April 2006 (UTC)
- You're one up on me if you have information that absolute pitch can be learned; I always thought you pretty much have it or you don't. Certainly, you can learn some tricks for approximating absolute pitch (for example, learning how particular notes feel in your throat), but I don't think that's quite the same thing.
- Absolute pitch is a mixed blessing for a singer anyway; good relative pitch is much more useful IMO. Absolute pitch can be a particular handicap to an ensemble singer, as it can make it difficult for him to match pitch with the rest of the group. --Trovatore 22:33, 21 April 2006 (UTC)
Holy cow batman! We actually have an article on Absolute pitch! --Zeizmic 23:52, 21 April 2006 (UTC)
- It is entirely possible to learn absolute pitch. The problem is that it is generally considered impossible after the age of Five. This is not true for all people, but is a good standard to go by. Unless you are asking on behalf of your child or a younger person who falls into the "under age Five" category, I would assume that you are over such age, and not in that category, so it may be impossible for absolute pitch to be attained. yaninass2 04:55, 22 April 2006 (UTC)
- It's not as difficult as you think. For me, I can always sing a C to myself, without reference. Using that alone, I can simply relatively pitch other notes. It's not like you need to be able to recognise every note. I think I read about studies which showed that if people listened to a particular song many, many times, then whenever they sung the song to themselves, they would sing it in the correct key, without reference. However, that was only after a lot of listens. So I'm pretty sure it can be learnt through intense practice. As far as I can remember, that's how I seemed to develop it. -- Daverocks (talk) 05:27, 22 April 2006 (UTC)
- Yup. From years and years and years of tuning my guitars, I know the pitch of 440Hz by "instinct". -- Branden 20:43, 24 April 2006 (UTC)
- It's not as difficult as you think. For me, I can always sing a C to myself, without reference. Using that alone, I can simply relatively pitch other notes. It's not like you need to be able to recognise every note. I think I read about studies which showed that if people listened to a particular song many, many times, then whenever they sung the song to themselves, they would sing it in the correct key, without reference. However, that was only after a lot of listens. So I'm pretty sure it can be learnt through intense practice. As far as I can remember, that's how I seemed to develop it. -- Daverocks (talk) 05:27, 22 April 2006 (UTC)
April 22[edit]
Cannabis cultivation[edit]
I have recently become interested in botany and I was wondering - in regards of the cannabis plant - how do I distinguish the female from the male? Regards, Gardar Rurak 02:13, 22 April 2006 (UTC)
- Erowid should have all the answers you need on their Cannabis cultivation page, specifically these two illustrated articles linked from it. Also, you may be interested in our Cannabis (drug) cultivation article. --Aramգուտանգ 02:59, 22 April 2006 (UTC)
- And just why do you want to know? A Clown in the Dark 03:05, 22 April 2006 (UTC)
- Perhaps he's investigating traditional methods of making hemp rope, and wants to make sure there's no THC in the final product, so he needs to know which ones are female so he can discard them :) Remember, assume good faith. --Aramգուտանգ 03:23, 22 April 2006 (UTC)
- Yes - I'm starting a hemp shop selling hemp-tshirts and hemp-slippers and I need to sort out the products of the evil one. Thanks or the reply, it's very informative. Gardar Rurak 03:45, 22 April 2006 (UTC)
- Yes, and I'm sure you will burn the evil ones. Of course, to ensure proper fire safety, you will need to burn them in small quantities. These small quantities should be in wrapping papers to properly contain the fire, and you will need to pull air over the marijuana by inhaling at one end of each unit for proper combustion. Your devotion to fire safety is admirable ! StuRat 04:45, 22 April 2006 (UTC)
- If he really wants to make hemp rope, he should read the hemp article. The proper way to make hemp would be to cultivate the right variety and to use the right part of the plants. this would ensure the final product contains no THC far better then using only males plants (not to mention he won't be very successful if he discards all female plants since he will need to grow more plants which he can't do if he destroys one sex). However it is common practice to remove male plants to increase the amount of THC. If he wishes to do so I really couldn't give a damn but he should be honest or at least not lie about his intentions. If he does wish to lie, and you wish to help him, at least come up with a more plausible lie that actually makes sense. Nil Einne 05:22, 22 April 2006 (UTC)
- If you're worrying about male and female plants, maybe you've already been around cannabis too long. Peter Grey 04:52, 22 April 2006 (UTC)
- Take it easy... I got an answer to my question and everything was all dandy. Look, I live in a society where it is legal and acceptable to grow these plants for personal use so I see no reason why I should lie about it which was indeed never my intention. I realize sarcasm is not easily communicated through text and may not be as prevalent in other cultures as a form of humour - that is regrettable and for this I do apologize. I have previously made my own beer, wine and alcohol - now I want to try this - it does not mean that I am an alcoholic or a pothead - in fact I rarely even drink coffee much less alcohol or pot. Still, as a hobby it's a lot more interesting than sitting on your ass watching sitcoms or collecting stamps all day. You can judge me all you want - that's your business. Gardar Rurak 06:45, 22 April 2006 (UTC)
- Since you are out of the Nasty States, you can grow small amounts for personal use (or you have a medical permit). I had fun with that for a while. There are lots of seed shops. Get the best hybrid you can afford. Put the seeds in little peat seed cups to start. Lighting is the toughest thing to get right. You also have to go through 2 generations to get a good crop, by eliminating the males, and taking cuttings from the females. I don't smoke, and followed the recipes for heating in olive oil, but shortly I started reacting with migraines, and gave it up. --Zeizmic 12:51, 22 April 2006 (UTC)
Price of medicines[edit]
I noticed this in the U.S.-Australia Free Trade Agreement#Pharmaceutical Benefits Scheme 2 article:
- The scheme involves subsidy of the price of certain "listed drugs", with the result that consumer prices for many common medications are a great deal cheaper than elsewhere in the world.
It sounds to me like this claim is most definitely wrong. AFAIK, while the price of these common medications are cheaper then in the US and in other countries without subsidy schemes, the prices are comparable (definitely not a great deal cheaper) then the prices in other countries with similar agencies that subsidy and negoiate for cheaper drugs, e.g. NZ, UK etc. Can anyone confirm this? Nil Einne 05:01, 22 April 2006 (UTC)
Stars on the southern cross - why do some have 7 points and one has 5 points?[edit]
I have been researching the stars of the southern cross, and was wondering why,when you draw these stars on things such as the Australian flag, are the four main stars (Alpha crucis, Beta crucis, Gamma crucis, Delta crucis) represented as 7 pointed stars, and the smaller (epsilon crucis) is represented as a 5 pointed star?? I am guessing it has something to do with double stars and red dwarfs, but I was wondering if there are conventions for this and where I can find info about the rules when drawing a star? Thanks for your help!!
- I would guess it's just a way to differentiate between the brighter and dimmer stars. StuRat 08:30, 22 April 2006 (UTC)
- The seven points on the stars on the Australian flag represent the number of states and territories present at Federation (six states, and one point representing all territories). I'm not sure what the 5-point star is meant to represent, and I'm not aware of any specific conventions for drawing stars. Our article on Flag of Australia has a lot of information you might find useful. -- Daverocks (talk) 08:35, 22 April 2006 (UTC)
- The 6 states and the territories are represented by the 7-pointed large Federation Star (or Commonwealth Star) beneath the Union Jack, not by the stars in the Southern Cross itself. To quote the article:
- "The number of points on the stars of the Southern Cross on today's Australian flag differs from the original design in that the stars varied between five and nine, reflecting the relative brightness of each in the night sky. The British Admiralty, to increase ease of manufacture, standardised the Southern Cross by giving the four biggest stars seven points and five for the faintest Epsilon Crucis. The Commonwealth Star originally had only 6 points, representing the six federating colonies. However, this changed in 1908 when a seventh point was added to symbolise the Territory of Papua." JackofOz 09:03, 22 April 2006 (UTC)
- The 6 states and the territories are represented by the 7-pointed large Federation Star (or Commonwealth Star) beneath the Union Jack, not by the stars in the Southern Cross itself. To quote the article:
- All a bit silly really. As everyone knows, the main four stars only hav five points each (and you can ignore the fifth one :). As far as the Aussie flag is concerned though, as Jack (there's an ironic name, considering the topic) points out, the points represent the states and territories. Originally Alpha Crucis was given nine points, Beta eight, and so on down to epsilon with five. When the flag was altered in 1908, the four larger stars had the number standardised to seven. The number of points on the smallest star remained at five, possibly for ease of manufacture rather than any more esoteric reason. You might also like to check out the Flags of the world website, which has considerably more information. BTW, Australia is not the only country which has an unusual number of points on its stars for the purposes of representing subnational regions. Malaysia and the Marshall Islands are two others which spring to mind which also do this. Grutness...wha? 10:27, 22 April 2006 (UTC)
Grutness, I hate to disabuse my trans-Tasman cousins, but you seem to have somewhat confused the New Zealand flag and the Australian flag.
- The NZ flag has four stars in the shape of the Southern Cross. All four stars have 5 points.
- The Aussie flag has six stars. The smallest star Epsilon crucis has 5 points, and all of the remaining stars have 7 points.
- The Aussie Southern Cross comprises 5 stars: four of 7 points and Epsilon crucis.
- There is a separate Commonwealth Star that appears beneath the Union Jack (I could almost call it the Union JackofOz)
- The stars in the SC do not represent anything other than themselves.
- The only star on the flag that represents the states and territories is the Commonwealth Star. JackofOz 03:00, 23 April 2006 (UTC)
Please read what I wrote. I mentioned that when the Australian flag was changed, the four largest stars of crux had the number of points changed to seven. The Commonweatlth Star was also changed from six to seven points at that time. Since the question referred to the stars of Crux without reference to the Commonwealth star (which is symbolic and has no astronomical significance) I did not refer to it in my answer. The reason for the change of all these stars was to represent the seven regions of Australia (the six states and the territories). Perhaps if the Aussies had chosen a recognisably different flag in the early years of the 20th century rather than one attempting to copy a flag that has been used in New Zealand since the 1860s, the confusion wouldn't have arisen ;) Grutness...wha? 09:43, 24 April 2006 (UTC)
- I did read what you wrote, which was what prompted my response. True, the question referred only to the Southern Cross and not to the Commonwealth Star. But it did say "why do some have 7 points and one has 5 points" and it did specifically mention the Australian flag. Since the New Zealand flag has no stars of 7 points, clearly the question had nothing to do with the New Zealand flag and was referring only to the Australian flag. Nevertheless, you wrote: "As everyone knows, the main four stars only hav five points each". This statement would be true only if you were talking about the NZ flag. But you never stated you were talking about the NZ flag, which is why I thought you must have been confusing it with the Australian flag. I still think that.
- It is not true that "The reason for the change of all these stars was to represent the seven regions of Australia (the six states and the territories)". Please re-read my italicised quote above from the Wikipedia article on the Oz flag. The reason for the change to the stars in the Southern Cross was "ease of manufacture". The only star that has ever represented the states or territories is the Commonwealth Star. If you disagree with any of these statements, you're free to change the article, but expect such changes to be quickly reverted unless you can provide references.
- Re the design itself, and its frequent confusion with the NZ flag, I couldn't agree more that a new design is well and truly overdue. Of course, both flags would need to change, because if we change and NZ doesn't, people will still look at the NZ flag and think it's the Oz flag (and vice-versa). And if we change first, don't you Kiwis dare copy us !! :--) JackofOz 10:03, 26 April 2006 (UTC)
- oh dear. The original title, as stated in the heading, did not mention Australia (although it was mentioned later in the repeating of the question). But it would be nice if you could work out what a smiley refers to. And if the Wikipedia article states that the only reason for the change was ease of manufacture, then it is wrong. The number of stars was brought into alignment at the time the Commonweath Star was increased to a seven-point star. As such, they were changed to seven points primarily because the Commonwealth Star was changed. Ease of manufacture was a reason - but it it had been the primary reason then the number of points would have been either six or five - a far easier number of points for a flag maker to manufacture that seven. thereofore, the reason why they are seven point stars rather than some other number is because of the states and territories.
- James, I'm a reasonable man (obviously), and so I again invite you to come up with a citation for this claim. If you can't, I'm going to have to assume you're practising your "patent nonsense" skills. :--) JackofOz 08:57, 27 April 2006 (UTC)
- As a practicing flagmaker for the last eight years, I can tell you that anyone who thinks that the design of a flag was settled on stars being of seven points rather than five for "ease of manufacture" clearly has no knowledge of flag design or construction. There is NO WAY ON EARTH that the decision to use seven-pointed stars rather than five-pointed stars could ever have been for that reason alone. Consider the common sense here for a minute.
- Premise 1:The stars all previously had different numbers of points.
- Premise 2:A decision was made at the time the Commonwealth Star was changed to make the remaining stars have the same number of points as each other for ease of manufacture.
- Given the clear fact that seven-fold symmetry is one of the most difficult forms to create, replicate, or manufacture, why settle on seven points rather than eight, six, or five? The answer is obvious - because the Commonwealth Star now had seven points. So, why did that have seven points? Because of the number of states and territories. If A is the cause of B, and B is the cause of C, then A is the ultimate cause of C. Therefore, the number of points on the seven-pointed stars of Crux on the Australian flag is due - indirectly but definitely - to the number of states and territories. Not patent nonsense - patently obvious. Boil down the original question and your answer to its minimum: Q - "Why are the stars seven pointed?" A - "Because they're easier to make that way!" Now that's patent nonsense! Grutness...wha? 09:29, 27 April 2006 (UTC)
- As a practicing flagmaker for the last eight years, I can tell you that anyone who thinks that the design of a flag was settled on stars being of seven points rather than five for "ease of manufacture" clearly has no knowledge of flag design or construction. There is NO WAY ON EARTH that the decision to use seven-pointed stars rather than five-pointed stars could ever have been for that reason alone. Consider the common sense here for a minute.
- James, I'm a reasonable man (obviously), and so I again invite you to come up with a citation for this claim. If you can't, I'm going to have to assume you're practising your "patent nonsense" skills. :--) JackofOz 08:57, 27 April 2006 (UTC)
- oh dear. The original title, as stated in the heading, did not mention Australia (although it was mentioned later in the repeating of the question). But it would be nice if you could work out what a smiley refers to. And if the Wikipedia article states that the only reason for the change was ease of manufacture, then it is wrong. The number of stars was brought into alignment at the time the Commonweath Star was increased to a seven-point star. As such, they were changed to seven points primarily because the Commonwealth Star was changed. Ease of manufacture was a reason - but it it had been the primary reason then the number of points would have been either six or five - a far easier number of points for a flag maker to manufacture that seven. thereofore, the reason why they are seven point stars rather than some other number is because of the states and territories.
I’d almost be prepared to meet you half way, but for a couple of points:
- You now acknowledge that the link is indirect, but you seem to make more of it than I think is warranted. Let me give you an analogy:
- Wellington NZ was named after the Duke of Wellington. His name comes from the town of Wellington, Somerset UK. Does that mean that Wellington NZ was named after Wellington UK? Of course not. Not even indirectly. There is an indirect connection between the 2 places, but as for one being named after the other, I don't think so. Maybe this is becoming a semantic argument, so I won't take it any further.
- But even if there wasn’t a logic problem, there’s still an indisputable timing problem.
- This says: "The original design has been changed three times since 1901. First, in 1903 the design was changed so that all but the smallest star in the Southern Cross had seven points, ostensibly to improve the ease of manufacture. In 1906 Australia acquired the Territory of Papua, and to indicate this the number of points on the Federation Star was increased to seven in 1908. This second design change was Gazetted on 22 May 1909."
- The Flags of the world website that you yourself provided above, tells much the same story:
- 20 February 1903: Proclamation that King Edward VII had approved design for the Flag of Australia together with the warrant for Australian registered ships to fly the red ensign. (the design approved by the King differed from the original design in the number of points on the stars and the warrant was republished in Commonwealth of Australia Gazette No. 8*)
- 19 December 1908: Commonwealth of Australia Gazette No. 65 (page 1709) announced addition of 7th point to the Commonwealth Star to represent Australian Territories.
I’m quite prepared to defer to your map-making skills, and I accept your argument that the real reason for changing the Crux stars to 7-pointers must have been something other than "ease of manufacture" (and the refs seem to also be in some doubt on this score). But that does not automatically mean that the only other possibility was to recognise the states and territories. If that were the real reason, the 4 main stars would all have had 6 points not 7, because at the time (1903) there were no territories to be counted, only 6 states.
- Which leaves us with a conundrum - what was the real reason for the 1903 change in the number of points on the 4 main Crux stars? It's clearly neither of the above. I don't know if we're any closer to the answer to the original question than we were 5 days ago (but I've learned a lot more about my flag than I knew before, so I'm not complaining). JackofOz 14:08, 27 April 2006 (UTC)
- I suspect the answer lies buried in the bowels of the archives of the British Admiralty. The change was not something that the Australian government or parliament had any say about. The flag was sent to London, and an altered version came back. Back in those days, Britain still made all manner of decisions for the supposedly independent nation of Australia, and this was one of them. The change would not have been queried in 1903's Australia, but simply accepted. That would explain why there's no information about the reason for the change on any Australian website that I've ever seen. It would be a fascinating project to dig out the dusty Admiralty records and find out about this. Cheers. :--) JackofOz 02:37, 28 April 2006 (UTC)
- This is all getting rather tedious, but still... A lot of that digging has been done in the past by my colleagues at FOTW. You seem to have looked at the first page of that Flags of the World link but not didn't delve deeper into the mysteries on the pages linked from it. The Australian flag was found for many years in several forms with differing numbers of stars (and was still often used with 9,8,7,6 and 5 points, though most frequently with the four largest stars of the Southern Cross each with seven points) until the exact specifications of the flag were formally ratified in the Australian Government Gazette on 23rd March 1934 - considerably after 1903. Admiralty documents do indeed record seven and five pointed stars, however, although this was frequently questioned in Australia. It should be noted, though, that the recognition of New Guinea as a specific territory was only a change of status, as it had already become officially Atsuralian in the 1880s, so its recognition as a seventh region of Australia would not have been surprising even in 1903. Grutness...wha? 02:59, 28 April 2006 (UTC)
Thanks for pointing me in the right direction, Grutness. I have now read the full information very throughly, and I'm impressed with the work you and your colleagues have done. At the risk of arousing your tedium yet further:
- I understand that prior to the 1934 Gazette notice there was considerable confusion about the exact specifications of the Australian flag. However we’re dealing here with the origin of changes made in the early years of the century, before they became fully official.
- Australia changed the Commonwealth star to a 7-pointer in 1908, and for the very specific reason of recognising that we now had not just 6 states, but also the external Territory of Papua, which had been acquired on 1 September 1906 (see below). The fact of the addition of the 7th point was gazetted on 19 December 1908, and the formal design change was gazetted on 22 May 1909.
- However the 4 main Crux stars had first been changed to 7-pointers by King Edward VII in 1903. It is not apparent to me from the website just what the King's (or the British Government's or Admiralty's) reason was at that time, but whatever it was, it certainly could not have been the representation of any Australian territories, for the simple reason that we did not have any territories (external or internal) until Papua came on board in 1906. If they had wanted to represent the states, they would have made the Crux stars 6-pointers - but they made them 7-pointers. It seems the real reason for this has yet to surface.
- The British colony of Queensland annexed Papua in 1883, but this was repudiated by the British Government, which on 6 November 1884 made Papua a direct possession of the British crown, under the name British New Guinea. It was handed over to Australian administration on behalf of Britain in 1901/1902 (sources vary), but it was still the British possession of British New Guinea. It did not formally become an Australian possession until 1 September 1906, when the name was changed to the Territory of Papua. So this was most definitely a change of status. Cheers. JackofOz 11:41, 28 April 2006 (UTC)
work, energy and power[edit]
How much is the power required to raise 300 liters of water per minute through a height of 6 m using a pipe of diameter 2.4 cm if acceleration due to gravity is 10m/s*s ?
- Per guidelines that are up at the top of the page, we don't answer homework questions here. Otherwise, it would be a homework help desk. What forumlae and knowledge can you apply? Did your teacher tell you anything in class about hints to solve it? I'll hint you some. You'll need to know the weight of the water through w=mg. Check the water article for its mass, and convert. Apply the formulas at work and power. Your angle is 90 degrees. The pipe diameter doesn't matter, its there to fool you. Good luck ;) --
 Mac Davis] ⌇☢ ญƛ. 07:19, 22 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 07:19, 22 April 2006 (UTC)
- Pipe diameter is important if you're worried about frictional losses. All else being the same, it takes less effort to move water through a large-diameter pipe than a small-diameter one. --Serie 22:37, 25 April 2006 (UTC)
Light[edit]
A spherical soap bubble of radius 2cm attached to the outside of a spherical bubble radius 4cm. What is the radius of curvature of the common suraface?
- Per guidelines up at the top of the page we don't answer homework questions here, otherwise it would turn into a homework help desk. See this for help. --
 Mac Davis] ⌇☢ ญƛ. 07:21, 22 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 07:21, 22 April 2006 (UTC) - If the header is anything to go by, the answer is about 300,000 km/s. Grutness...wha? 10:35, 22 April 2006 (UTC)
Bound charge density[edit]
Can anybody tell me what is the bound charge density for a uniformly polarized sphere.Is it zero?Thanks!
- Need more info. Solid sphere? Hollow? Conductor/insulator? Isopropyl 15:54, 22 April 2006 (UTC)
For a solid sphere.Thanks.
- Well, you should look up bound charge (which redirects to polarization but does discuss it properly). Then you should think about what "uniformly polarized" means, and apply the formulas. But make sure to consider the surface of the sphere separately, if you care about the surface: assuming that there is nothing outside the sphere, the polarization is certainly not uniform when there's a sphere on one side of you and vacuum on the other! --Tardis 19:30, 22 April 2006 (UTC)
Centre of Gravity[edit]
this might sound adsurd to ask but why is a 4 legged stool more stable than a 3 legged stool? I think it has something to do with the centre of gravity.
- That depends on how you define "stable". If you have an identical stool and you mount four legs on it rather than three, it will be less stable. The reason for this is that a tripod (three legs) is always stable on nearly any surface (try it!). A four-legged stool will always have one leg free in the air on any surface except a perfectly flat one (assuming the four legs are equally long). — QuantumEleven 14:07, 22 April 2006 (UTC)
- It does indeed depend on what you mean by stable. Quantum's answer assumes that it means "not wobbly", whereas I think the questioner means "unlikely to fall over", which is different. With the latter definition, the stability of an object can be defined as the angle by which you need to tilt it before it falls over. This is the angle at which the object's centre of gravity ceases to be above the object's footprint. The further the COG has to shift sideways before it falls outside the footprint, the larger this angle and therefore the more stable the object. A three-legged stool has a triangular footprint, while a four-legged stool has a square one. Take two stools of the same seat size, one with three legs and the other with four, and draw their footprints - a triangle and a square. You will find that the distance from the centre of the triangle to the nearest point on any edge is shorter than the corresponding distance for the square. So, the COG of the three-legged stool has less far to travel to the edge of its footprint than the COG of the four-legged stool. In turn, this means that the three-legged stool has to tip through a smaller angle than the four-legged one before it falls over. This makes it
moreless stable. --Heron 17:18, 22 April 2006 (UTC)
- It does indeed depend on what you mean by stable. Quantum's answer assumes that it means "not wobbly", whereas I think the questioner means "unlikely to fall over", which is different. With the latter definition, the stability of an object can be defined as the angle by which you need to tilt it before it falls over. This is the angle at which the object's centre of gravity ceases to be above the object's footprint. The further the COG has to shift sideways before it falls outside the footprint, the larger this angle and therefore the more stable the object. A three-legged stool has a triangular footprint, while a four-legged stool has a square one. Take two stools of the same seat size, one with three legs and the other with four, and draw their footprints - a triangle and a square. You will find that the distance from the centre of the triangle to the nearest point on any edge is shorter than the corresponding distance for the square. So, the COG of the three-legged stool has less far to travel to the edge of its footprint than the COG of the four-legged stool. In turn, this means that the three-legged stool has to tip through a smaller angle than the four-legged one before it falls over. This makes it
- That's an excellent response, although I think you meant to say that the 3 leg stool is "less stable", at the end. Another "stability" concern is the ability to resist tipping over if one or more legs collapses. With a 3 leg stool, losing any one leg will cause it to collapse. With a 4 leg stool, if the CG remains above the triangle formed by the remaining 3 legs, which is basically a 50/50 shot, then it will remain upright. 5 or more legs would make it even more stable (with 5 legs, any two legs could fail without causing the stool to collapse). So why don't stools typically have more legs ? That would add expense and weight and chair legs don't collapse all that often. Also note that the wobble problem with more than 3 legs is easily fixed with rubber feet on the stool, which will, in effect, adjust the height of each leg (within limits), to compensate for a lumpy floor. 3 leg stools also typically spread the legs out farther than 4 leg stools, to compensate for the "tippiness" inherent in a 3 leg design. The inherent instability of 3-wheel ATVs (all terrain vehicles) actually led to them being banned in many places due to fatal rollover crashes. 4-wheel ATVs are much safer. StuRat 18:27, 22 April 2006 (UTC)
- Minor point: a uniform stool with five legs in a regular pentagon arrangement would fall over if two adjacent legs were lost. --Tardis 19:50, 22 April 2006 (UTC)
- Which would be essentially just as stable as a 4-legged stool. freshgavinΓΛĿЌ 05:50, 26 April 2006 (UTC)
- I disagree. If such a stool lost two adjacent outside legs, you would still have a tripod supporting it, especially if the legs broke off and thus their weight is no longer counted toward the CG of the stool. Note, however, that it would be an off-center 90-45-45 triangular base, not a 60-60-60 base. But, if you were careful how you sat on it, you could still make it work. StuRat 18:24, 26 April 2006 (UTC)
Photon mass[edit]
Photons are massless, but according to E=MC^2, if photons' mass is 0, 0 times the speed of light squared is also zero. how about its energy? photons have energy i think? (2nd part of question moved to next section below.)
- Photons have some amount of mass; see photon. As for atoms, see electron configuration for an overview of orbital theory. Isopropyl 13:51, 22 April 2006 (UTC)
- The E=mc2 equation is actually a simplification of Einstein's result. You can start at Energy-momentum relation for more details. Essentially, there is another term that relates to momentum and not just mass; this is where the energy of a photon is represented. (Despite having a zero rest mass, photons do indeed have momentum.) TenOfAllTrades(talk) 13:53, 22 April 2006 (UTC)
- hmm, photons aren't massless for one thing--152.163.100.74 20:32, 22 April 2006 (UTC)
- Photons are quite massless; I don't know where you and Isopropyl are getting that. Melchoir 20:40, 22 April 2006 (UTC)
- Photons are exactly massless. The formula E=mc2 relates the energy with mass of a massive particle in its rest frame where its momentum is vanishing. However in the case of a zero mass particle, this rest frame simply doesn't exist (a photon never has zero momentum)which forbids one from applying this formula to the photon. For a photon propagating with pulsation w then its energy is given by E= hcw2 with h the Planck constant LeYaYa 21:22, 22 April 2006 (UTC)
- What's pulsation? If you mean angular frequency, the formula is
- If you mean ordinary frequency, the formula is
- Nothing is squared. Melchoir 21:58, 22 April 2006 (UTC)
- What's pulsation? If you mean angular frequency, the formula is
- All of the posters above are correct, but are defining "mass of a photon" differently. Photons have zero rest mass, but nonzero relativistic mass. While it can be argued that talking about the "rest mass" or "relativistic mass" of an object (like a photon) that moves at C isn't terribly meaningful, it's useful if you want answers to questions like this one.--Christopher Thomas 03:01, 24 April 2006 (UTC)
Atomic Model[edit]
My chemistry teacher says that electrons orbit around the nucleus, and my physics teacher says taht it would lose energy if it does. How does the atomic model actually work?
- I've split your question in two, hope you don't mind (makes it easier to answer). Have you read our excellent article on atoms? The short answer is that "electrons orbiting around a nucleus" (in the manner of planets around a sun) was a model of the atom used a long time ago (I seem to recall that it was developed by Niels Bohr, but I could be wrong), and while it's not technically correct per se (your physics teacher is correct, electrons orbiting a nucleus would lose energy and spiral in), it's a useful model for much of chemistry, as, below a certain level of complexity, it can explain effects observed in chemistry perfectly well (in school, it usually lasts until the last years of high school). As for what 'really' happens in an atom, things get a bit hairy... electrons behave in very strange ways, a bit like particles and a bit like waves (check out wave-particle duality if you're interested). Because of the Heisenberg uncertainty principle, we can never know exactly where an electron is, only that it's somewhere near the nucleus. Take a look at the picture at the top of atom - the dark regions (called orbitals) around the nucleus (the dot in the middle) are the regions where the electrons of a Helium atom are most likely to be found, but we don't know where in that region they are at any one time.
- You'd be right to think that this is all very odd - it is. Also, I've greatly simplified things, if you're interested in more detail, ask again, and I'm sure someone with better knowledge of the subject will be able to help you. Have you tried asking your two teachers to sort out their apparent contradiction...? :) — QuantumEleven 14:04, 22 April 2006 (UTC)
- As indicated above, the "real" answer (where real is the best current understanding, not an ultimate truth) is very complex, as well as counterintuitive making it hard to understand. Your chemistry teacher is using a model of the atom that is sufficient to explain most of chemistries dealings with atoms and electrons, probably the Bohr model, without burdening the class with the hard math and strange effects of quantum mechanics. Your physics teacher appears to have started on explaining quantum mechanics, and has indeed touched one of the primary reasons the theory was developed in the first place, because before it no satisfying atomic model existed. SanderJK 14:29, 22 April 2006 (UTC)
- I would assume that the physics proffesor in question is probably explaining simple waves, accelerating charges around a point mass, etc... probably EM waves, but no quantum mechanics at all. am I right? and the bit about electrons falling into a nucleus is probably just a throw away example about moving charges generating waves? something about how if the classical model of the atom were correct atoms would just generate bright EM flashes then disappear into nothingness?--152.163.100.74 20:40, 22 April 2006 (UTC)
- Actually, the planetary model is insufficient for chemistry. How can it explain bonding? A decent chemistry class will have much to say about quantum-mechanical orbitals. Melchoir 19:52, 22 April 2006 (UTC)
- As indicated above, the "real" answer (where real is the best current understanding, not an ultimate truth) is very complex, as well as counterintuitive making it hard to understand. Your chemistry teacher is using a model of the atom that is sufficient to explain most of chemistries dealings with atoms and electrons, probably the Bohr model, without burdening the class with the hard math and strange effects of quantum mechanics. Your physics teacher appears to have started on explaining quantum mechanics, and has indeed touched one of the primary reasons the theory was developed in the first place, because before it no satisfying atomic model existed. SanderJK 14:29, 22 April 2006 (UTC)
- Well, it's true that the electrons would lose energy if they orbited the atom - if you described them as moving charged particles in the classical physics context. However, classical physics don't work on the atomic scale. You need quantum physics for that. Now, from the quantum physics viewpoint, the electrons do orbit the nucleus moving in what's called 'orbitals' - unlike in classical physics they can't have any energy, only certain discrete energy levels which each correspond to a pattern of motion. Motion in this sense is a bit strange concept though. - The electrons have momentum and angular momentum and so on, but they don't "move" in the classical sense of following some trajectory. Rather, all you have is a "probability cloud", that is a given probability that the electron will be at some point in space. So in summary, yes they orbit the nucleus, but they don't do it in any way which is similar to what we usually mean by 'orbit' as in planets. --BluePlatypus 16:18, 22 April 2006 (UTC)
- your general chem proffesor is just teaching you the classical atomic model, and your physics proffesor is doing the same thing, short answer, the classical model was wrong, they don't teach you that in general chem, because general chem is basically just a test to see what percentage of the premeds actually have the patience to get through a chemistry course, most don't, just accept that everything they teach you between your first semester of general chem, and your second semester of organic is basically over simplified material designed to catch up students who may not have ever taken high school chemistry, or come from regions of the country with inferior public education systems--152.163.100.74 20:37, 22 April 2006 (UTC)
In general most things in science have more subtle (or detailed or complex) explanations as you learn more. So you start out simple, then learn it wasn't quite right, and get better (but still not 100%) answers. if you continue to a PhD you'll be the one researching the answers.
The question here centers around the term "orbit". Usually we think of this in terms of classical mechanics, an object orbits, this requires a force, energy is lost in the system, the system winds down. Thats classical mechanics, and this was in fact not a trivial problem. It was the main problem with the "orbiting electron" solar-system type models.
In fact a more accurate model is that when you look at energy within an atom, you have to look at it via quantum mechanics not classical mechanics. In quantum mechanics, the electron is no longer seen as a solid particle, nor does it lose energy unless it moves out of its energy state, or shell. It's no longer a solid object circling, instead its a probabalistic wave-particle whose location is described by equations not by simple X-Y-Z co-ordinates. The "orbit" of an electron in quantum mechanics is a shorthand easy way to say the "energy-state" of that electron, and it doesn't lose energy so long as it doesn't change state.
Your chemistry teacher doesn't need you to know these complexities, so he is simplifying it. Your physics teacher is trying to touch on them without confusing people. Hence you get 2 different views. FT2 (Talk) 03:07, 26 April 2006 (UTC)
What experiments can I run of a polluted water sources (e.g collected from a heavily polluter river)?[edit]
At the beginning, i would like to say sorry to all users that i ask the question such directly since i am in hurry, please forgive my rudeness. Question:
Can any Wikipedia users give me some suggestions of experiments or test that i can do unto a pulluted water sources in a simple secondary school laboratry. My teachers do have some suggestion to me:
Listed below: 1.dissolved oxygen 2.quantity of chlorin ion,phosphate,magnesium ion
However, my teacher told us that if we can run extra experiments that beyond his suggestion list, then we can possiblely score a higher marks in this assessment. By this reasons, i am here to please for your helps to suggest some others experiments that i can carry out. If it don't touble you please also give the steps and equipments require for your sugested experiment. Please remenber that my school's laboratry is not quite well equiped.Thanks~
Timmy Ho From Hong Kong
- A simple litmus test can give you the relative pH of the water. It's not too difficult and the supplies are cheap. Isopropyl 15:52, 22 April 2006 (UTC)
- You could boil off the water and mass the remaining material. Really cheap and easy. Leonardo 16:16, 22 April 2006 (UTC)
- Shake it vigorously, and watch for the formation of suds. These are a good indication of certain types of pollutants, such as phosphates. Compare how long it takes the suds to dissipate with an unpolluted control sample. StuRat 18:03, 22 April 2006 (UTC)
You might also try incubating cultures from it.
- Some types of pollution will increase bacteria count in the water and others (like arsenic) will reduce it, however. StuRat 23:49, 28 April 2006 (UTC)
- The Water quality article gives some tests and it points to the Wastewater quality indicators article. Samw 23:21, 29 April 2006 (UTC)
weight on planets[edit]
suppose you weigh 50 kilograms, or about 110 pounds.What would you weigh on Mars.
- The same, if your scales were calibrated correctly. --Heron 17:23, 22 April 2006 (UTC)
- No, you'd have the same mass if your scales were calculated correctly. You'd still have a mass of 50 kilograms, as a kilogram is a unit of mass, but you'd no longer weigh 110 pounds, due to the lower gravity. Our article on Weight has a handy table of interplanetary conversion factors, and a weight of 110 pounds on Earth (I'm assuming this part -- if you weigh 110 pounds on, say, Jupiter, the answer is different) would weigh 0.377 times as much on Mars which is about 41.5 pounds. Our Weight article has a very good description of the difference between weight and mass, if you're confused about that bit. --ByeByeBaby 17:54, 22 April 2006 (UTC)
- (Edit conflict) That's not true. Weight refers to the force exerted by gravity, so that changes on different planets. The confusion here is because 50 kilograms is a unit of mass, which does not change from planet to planet. So if you weigh 110 pounds, according to this page, on Mars you would weigh 41.5. (In proper metric units, you used to weigh about 490 newtons, and now weigh about 180. It's a bit confusing that many places using metric units refer to peoples' "weights" in units of mass.) -- SCZenz 17:56, 22 April 2006 (UTC)
- My answer was deliberately oblique because I didn't want to do the questioner's arithmetic for him. Besides, I was making the semi-serious point that scales on Mars should either read mass in kilograms, or force in newtons. Imagine you're a cook in a Martian colony. You would want a kilogram of potatoes to register "1 kg" on the scale, not "377 g". After all, you want to know how many stomachs they are going to fill, not how heavy they feel. If the scales
correcteddid not correct for the difference in gravity, then recipes would all have to be different depending on the planet you were on. (Or they could all be translated into moles, which would be even weirder.) Our attempt to colonise the galaxy would collapse in confusion, I tell you! --Heron 18:47, 22 April 2006 (UTC)
- If you weigh 110 pounds, you belong either in school or in the kitchen; since neither institution is available on Mars, the question is moot. (runs away) Melchoir 20:06, 22 April 2006 (UTC)
If we're getting into terminological exactitude, bear the following in mind. When a person says "I weigh 100 kgs on Earth but only 37.7 kgs on Mars", they're really saying:
- My mass is 100 kgs (everywhere in the universe).
- My weight here on Earth at sea level is 100 kgs weight.
- My weight on Mars would be 37.7 kgs weight". JackofOz 02:30, 23 April 2006 (UTC)
If we're really getting into terminological exactitude, bear the following in mind. When a person says "My mass is 100 kgs (everywhere in the universe).", they're really saying:
- I have certainty that the laws of physics as presently understood can be safely extrapolated to all places and contexts of all kinds, both known or unknown.
- I can ignore relativity, or I never travel, or I confuse rest mass with mass :)
- I habitually make assertational statements that I can't be sure are right.
FT2 (Talk) 02:56, 26 April 2006 (UTC)
Long bones[edit]
how do you determine right or left humerus, femur and tibia long bones?
- I believe that right and left in the anatomical sense refers to the right and left of the patient; for instance, this image is looking at the heart from the front, and the right and left sides labelled are those of the patient, not of the observer. There is only one humerus in each arm, and the right and left humerus..es? would be mirror images of each other. The same goes for the femur and the tibia - there is one of each in each leg. As can be seen from the images in the articles linked, all these bones have different shapes, and could hence be differentiated from each other. -- Mithent 20:15, 22 April 2006 (UTC)
Car Tires[edit]
Funny story: my friend's dad accidentally ran over her foot with a Ford Explorer this morning. What is really unusual in my opinion, however, is that she says that it didn't hurt that much, except for a little throbbing afterwards. The bones of her foot are all intact, etc. How does this happen? Does the weight of the car redistribute itself somehow to the other three tires? What is the exact mechanism that doesn't cause one-fourth the weight of the car to bear down upon a foot that is being run over? JianLi 19:16, 22 April 2006 (UTC)
- There is no magical mechanism required. A tire can only exert 35 psi (the tire pressure) on anything it runs over. The lower part (toes mainly) can easily take this. If it runs up higher, the ankle smashes and the foot is a total wreck. --Zeizmic 19:34, 22 April 2006 (UTC)
- Er, that 35 psi business isn't right. Think about an elephant sitting on a unicycle. Certainly not just a few psi there, even if the tire was inflated just to that. Partly, the tire is compressed, so the pressure (and/or temperature) will increase. Also, the material strength of the tire is available to provide force beyond the air pressure. As for foot survival: I'm not sure how the suspension on the car might affect it, but obviously if her foot was level with the road (in a foot-shaped hole, for instance), the car has no way of "knowing" that one of its tires is on a foot, so I would expect approximately 1/4 the car's weight to, er, participate. But it's not like throwing 1/4 of a car at someone's foot; very little energy and momentum is involved, just force. And think about standing, or even hopping, on tiptoes of one foot while carrying a heavy backpack — it's quite possible to generate over a kN of force that way, and the car won't exert that much more. Moreover, that's on tiptoes, not with the foot flat and well-supported. And you'll get a little help if you're wearing anything more than sandals. I don't know what the maximum load on a flat foot is, but hopefully this helps you see how it's possible to be this great. --Tardis 20:10, 22 April 2006 (UTC)
- The 35psi is correct. Consider an 8-inch wide tire. Press down until you have approximately an 8x8-inch square in contact with the ground. That is 64 square inches. At 35psi, that one tire supports over 2,000 pounds. So, it will take over 2,000 pounds to get a 35psi tire to form an 8x8 footprint. At less weight, the footprint shrinks to something like 4x8 (slightly over 1,000 pounds). As you put more weight on a tire, more of the tire comes in contact with the ground, so the psi multiplies to support the weight. The less weight, the less tire in contact with the ground. So, regardless of the weight in the vehicle, when it runs over your foot you get 35psi on every square inch of your foot. Side note: my bike uses 60psi, not 35. Mainly, the tire is skinnier so it needs to be higher. As for weight on a flat foot - the toes can handle a lot of weight. As mentioned eariler, the arch of the foot will compress and get damaged. --Kainaw (talk) 00:13, 23 April 2006 (UTC)
- thanks for your responses! has anyone ever had a similar experience?JianLi 01:11, 23 April 2006 (UTC)
- I think when I was ~8 I put my besandled foot in the path of my dad's sports car. Didn't feel hardly a thing, but it may be confabulation caused by dreaming, happens to me a lot. --
 Mac Davis] ⌇☢ ญƛ. 01:40, 23 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 01:40, 23 April 2006 (UTC)
- I think when I was ~8 I put my besandled foot in the path of my dad's sports car. Didn't feel hardly a thing, but it may be confabulation caused by dreaming, happens to me a lot. --
- The 35 psi doesn't sound instinctively right, but the arguments are pretty good: and 35 psi can hold a lot of weight. And if you think of a 35 pound weight, formed into rod one inch across, that's a fairly heavy thing. Pile up rods like this on end to cover your foot, and it would be uncomfortable, though not crush your foot like a bug. (Not that all bugs crush well.) The elephant on the Unicycle? If the tyres were not large enough or the pressure not high enough, the tyre would first compress, increasing its pressure. Then, because there is more tyre above for the air to go, the tyre would flatten until the wheel was resting on its rim; the tyre may or may not be damaged. So if the elephant ran over your foot, what runs you over is the wheel rims directly, which have a small area of contact and very high pressure: worse than the elephant standing on your foot, which spreads the load. According to some back-of-the-envelope studies, elephants are nothing compared to stilleto heels, which can give "hundreds" of times the pressure on your foot. I think these studies tend to oversimplify (e.g. by measuring pressure on the ground over the entire large foot, then assuming that the parts of the foot in contact with your shoe apply the same pressure; and by ignoring the profile of the sole of the foot, assuming it is a flat surface). Notinasnaid 07:26, 23 April 2006 (UTC)
Sodium Usnate[edit]
Can anyone possibly draw, create or show me the strucutural formula for the monosodium salt of Usnic acid. I asked my chemistry teacher but it is beyound the scope of her knowledge. Tahnk you, Christopher
- Google image search shows us these two diagrams for usnic acid. It appears to be a phenol with three hydroxyl groups; presumably it donates a proton from one of them, but I don't know how to guess which one is the weakest. Melchoir 22:25, 22 April 2006 (UTC)
- My best guess would be that the OH group on the right in the first link is more likely to lose it's H then the other two, since it is close to two doublebonded oxygens, connected via sp2 C-C / C=O hybrid bonds. The double bonded oxygen is very electronegative, and the sp2 hybrid structure would be able to spread the extra electron from a hydrogen dissociation over a high number of relatively low energy states of the molecule, lowering the overall dissociation energy. However, i would not be surprised if the dissociation energies were close enough to each other that in reality a mixture of 3 salts would occur (with fraction depending on the relative dissociation energy of each O-H bond). SanderJK 23:47, 22 April 2006 (UTC)
- Hmm... it sounds like you're describing the one of the left here, the one that's alone on its ring? It's the lone OH that loses its proton in this diagram, which I just found. Melchoir 00:18, 23 April 2006 (UTC)
- Yeah, that's the one i expected, thanks, i meant second link not first i now see. I did some further digging on the subject, it seems the monosodium salt of usnic acid has the trivial name of Binan, and is denoted as C18H15NaO7 . 2.5 H2O in at least 2 references. I also found a link to a resolved crystal structure of Binan, but it isn't really my field of expertise, can't say for sure what exactly it is saying about the structure here. SanderJK 00:34, 23 April 2006 (UTC)
- My best guess would be that the OH group on the right in the first link is more likely to lose it's H then the other two, since it is close to two doublebonded oxygens, connected via sp2 C-C / C=O hybrid bonds. The double bonded oxygen is very electronegative, and the sp2 hybrid structure would be able to spread the extra electron from a hydrogen dissociation over a high number of relatively low energy states of the molecule, lowering the overall dissociation energy. However, i would not be surprised if the dissociation energies were close enough to each other that in reality a mixture of 3 salts would occur (with fraction depending on the relative dissociation energy of each O-H bond). SanderJK 23:47, 22 April 2006 (UTC)
Smoking vs. Chewing[edit]
I have a medical question. Now before you all go on about how I'd be better off asking a doctor, let me just say that I've asked many doctors, and none seem to be willing or able to give me a straight answer.
I'm a former smoker. I finally quit smoking by chewing nicotine gum. The transition was actually quite easy. Now I hardly think of having a cigarette as I find chewing the gum to be more pleasant, both for me, and those around me (no smoke to irritate my eyes, no second hand smoke to irritate the people around me.)
The problem is, unfortunately, quitting the gum is proving to be far more difficult than quitting "smoking" the nicotine.
From what I understand (and correct me if I'm wrong...that's the point of my question), the vast majority of all (or most) of the ill effects of smoking, such as cancer, heart disease, etc... etc... etc... are not the result of the nicotine (the addictive substance in tobacco) but the result of other, non-addictive yet deadly substances, such as tar, carbon monoxide, even certain forms of cyanide (I once read somewhere that there are dozens if not hundreds of different deadly substances in cigarettes.)
Nonetheless, I fully understand that nicotine itself is not the healthiest thing in the world and should be avoided as well.
I'll put my question this way: Let's say on a scale of 1 to 10 we place cigarette smoking as a 10 when it comes to being harmful for one's health. Where then should we put chewing nicotine gum? Is it only half as harmful as smoking, in which case we'd give it a 5? Or is it actually 80% as harmful as smoking in which case we'd give it an 8? Or is chewing nicotine so much less harmful than smoking that we'd give it a 1 or less? Thanks to anyone who may have knowledge to share. Loomis51 21:19, 22 April 2006 (UTC)
You won't get cancer from nicotine gum. Brian G. Crawford 21:24, 22 April 2006 (UTC)
- You are correct, the vast majority of bad effects of smoking are from the smoke itself and not the nicotine. I'm not sure the long-term effects of nicotine gum have been studied, but non-smoking tobacco products like the Scandinavian Snus have been studied intensely, with the conclusion that they're not risk-free but certainly much better than smoking. (There's public debate in those countries whether it should be recommended by physicians as an alternative to smoking, basically the old Harm reduction debate). While these substances are probably still carcinogenic and the risk of cancer can't be ruled out, it's worth noting that the smaller the risk, the more difficult it is to establish the correlation. --BluePlatypus 21:34, 22 April 2006 (UTC)
- I'll concur with what BluePlatypus said. While nicotine in large quantities is an acute toxin, long-term exposure to low levels is certainly a lot easier on your system than smoking. Nicotine raises heart rate and blood pressure, though probably not to a dangerous extent in an otherwise healthy individual. Nicotine is not a known carcinogen, but there is some research to suggest that it may be metabolized to carcinogens within the body: [50].
- Nicotine messes with your brain chemistry in ways that are not fully understood. There is debate (see our nicotine article) about its relationship with disorders including schizophrenia, Parkinson's disease, and Alzheimer's disease. (In some cases it is thought to be a potential risk factor, in others it is being investigated as part of a treatment regimen.)
- Regardless, your health will be better if nicotine is the only toxic substance you're putting into your body, rather than nicotine plus other poisons and carcinogens. You'll dramatically cut your risk of lung ailments, particularly emphysema and lung cancer. You'll lower your risk of COPD and other cardiovascular ailments, too. Finally, you're likely to be happier—you won't smell bad to people around you.
- I don't know if there any long-term studies exist on the risks and effects of chronic nicotine usage. I'm willing to go out on a limb and say that you'll significantly lengthen – and improve the quality of – your life by switching from cigarettes to gum. TenOfAllTrades(talk) 21:58, 22 April 2006 (UTC)
Snus is still tobacco and despite the fact that it isn't smoked, it is still carcinogenic just as chewing-tobacco is. The only difference is that "chewing" tobacco results in cancer of the mouth rather than that of the lungs. It's still carcinogenic, and as such it may be different from nicotine gum. What I'd like to know is the ill effects of nicotine alone when compared to tobacco (whether smoked or chewed.) This leads me to wonder why doctors in Scandinavian countries would recommend snus rather than simple nicotine gum.Loomis51 22:10, 22 April 2006 (UTC)
- Twenty years ago, as I lay on the emergency department trolley, watching my clothes being ripped off by nursing and other medical staff, the admitting doctor, who was a friend of my accompanying wife, who was a nursing sister in the same hospital, told her it might not be a bad idea to call all our family and friends as, "I have never seen anyone survive a blood pressure reading as high as this without either a major heart attack or stroke resulting". I was in intensive care for 3 weeks thereafter. But the cardiologist in charge of my case (a saint) told my wife that as he had spent so much time and effort in keeping me alive, the least I could do would be to give up smoking the 60 a day habit I had developed over the preceding 20 years. I see him every 3 months or so and his first question is always the same, "How much money have you saved since you stopped smoking?". At today's prices for a packet of 20 Benson and Hedges King Size being £4.85 - say $7.46, I am always happy to tell him I have saved about £106,205 or $163.392 (to date). Forget the chewing gum - the best advice I got was to choose whether to live or smoke - "you can't do both". Or even better, "Stopping smoking is easy - all you need to do is cut out the first cigarette of the day".195.93.21.7 23:38, 22 April 2006 (UTC)
- Loomis51, Snus has not been conclusively shown to cause cancer, after a number of long-term studies, including oral cancers of the types caused by chewing tobacco. It does contain carcinogenic substances. However, that doesn't necessarily means it causes cancer to any significant effect. (Plenty of everyday foods contain carcinogens as well.) --BluePlatypus 01:40, 23 April 2006 (UTC)
Nicotine is a powerful anti-anxiety, anti-depressant drug. I would discuss with the doctor to try to flip over to another drug, such as zoloft or celexa. --Zeizmic 12:00, 23 April 2006 (UTC)
- Are you a medical doctor, Zeizmic? Why are you recommending a particular antidepressant? If you must shill a particular drug, why not Wellbutrin? - Cybergoth 00:07, 24 April 2006 (UTC)
- An alternative strategy would be to have your physician prescribe a nicotine patch (and stop using the gum). The dosage provided by the patch can be gradually reduced, which will probably be easier than tapering your gum use. - Nunh-huh 11:05, 24 April 2006 (UTC)
I am not a doctor, but I own all the drug companies. The basic problem is getting off the nicotine, which acts on all sorts of neurotransmitter pathways. Zoloft is used a lot in these situations. I know that if you suddenly stop taking an SSRI, then you are a basket case, so I assume the same for nictoine. --Zeizmic 11:59, 24 April 2006 (UTC)
I had a similar experience and only managed to quit chewing by using patches.
The patches are easier to quit as they are not "active".
By this I mean you may find (like me) that you chew gum when you feel a craving or need for nicotine this leads to an association much like smoking.
Good luck.
planets and the solar system[edit]
- on which planet would you weigh the least.
- on which planet would you weigh the most.
- on which planets would you weigh more than you weigh on Earth
- on which planets would youweigh less than you weigh on Earth
- on Venus the apples are the ripest in the solar system.You need 5 pounds.[Earthweight].How much should you have the clerk weigh out for you.
- If you also pick up 10 pounds of pickles and 7 pounds of cole slaw and pickles will you have when you arrive on Mercury.
- MERCURY
- VENUS
- MARS
- JUPITER
- SATURN
- URANUS
- NEPTUNE
- ANSWER THIS PLEASE.
- If you look at our article for weight you will find a list of relative weights on the planets. You would multiply the mass times that value to get the equivalent weight on earth. As an example, the value for Venus is 0.907. If you want 5 lbs earth weight, you need to solve this equation:
- x * 0.907 = 5
- x = 5/0.907 = 5.5 lbs
- Now look at your Mercury problem. The value for Mercury is 0.378. So the 10 lbs of pickles from Earth will weigh (10 * 0.378) = 3.78 lbs on Mercury.
- You will weigh more than you do on earth when the relative weight listed in our table is more than 1. You will weigh less on a planet than you do on earth when that planet's value is less than 1.
- This should help you solve the rest of the questions. If you're having difficulty, ask us for help again here! - Nunh-huh 19:11, 23 April 2006 (UTC)
- Do your own homework. If you need help with a specific part or concept of your homework, feel free to ask, but please do not post entire homework questions and expect us to give you the answers. Melchoir 22:39, 22 April 2006 (UTC)
In any form of reality where the apples on Venus are the best in the solar system the planets will almost certainly have different physical characteristics to those in our reality. because of that, it is impossible to determine the answer to the first few questions without first knowing those different characteristics. As to the last question, when you arrive on Mercury you would have lunch. Grutness...wha? 23:32, 22 April 2006 (UTC)
- No, no. This is reality. Look, there are all kinds of apples on Earth. But once an astronaut goes to Venus and brings some home-made food with him, it would surely be best quality apples. Here, they may cost much more than these cheap apples you buy every day, but that's negligable to the cost of the spaceship journey. – b_jonas 11:19, 25 April 2006 (UTC)
- "on which planet would you weigh the least."
- Either Pluto or 2003 UB313, the latter of which has an unknown mass. Now, 2003 UB313 isn't widely recognized as a planet, but I think it will be soon (it's larger than Pluto). --Bowlhover 04:29, 24 April 2006 (UTC)
Have you considered why you are on Venus trying to buy apples in earthweight? You may find the article on weight useful, or else greengrocery may help if you need an alternate provider. Personally I would suggest buy an extra couple of apples. You know they wont be exactly 5 pounds, because apples never are exact weights, and you might get an extra guest, so buy a few more and be sure. The article on cookery and apple pie might help if you need recipe ideas. FT2 (Talk) 02:25, 26 April 2006 (UTC)
- But remember what Carl Sagan said: "If you want to make an apple pie from scratch, first create the universe." JackofOz 02:28, 26 April 2006 (UTC)
April 23[edit]
Spermy Vegetation[edit]
I visited college in New Haven this weekend, and I noticed the smell of sperm every time I passed some trees, so I assumed it was coming from the trees or some vegetation around them. (and no, it wasn't college kids, because this happened near too many trees). I thought I was just being stupid/delusional, so I didn't say anything, but later, some other guy said he noticed it too. I've noticed this smell before around trees around blossoming time. What causes this spermy smell, and why is it so similar to human sperm? I know plants have sperm, but their sperm is analogous, not homologous, so there'd be no reason for the smell to be similar, right? JianLi 01:05, 23 April 2006 (UTC)
- Have you checked the wikipedia article on 'spermy vegetation'? --Username132 (talk) 01:46, 23 April 2006 (UTC)
- Please don't refer people to nonexistant articles. —Keenan Pepper 02:57, 23 April 2006 (UTC)
- The plants are probably exreting some kind of polyamines. It's the type of chemical (Spermine and Spermidine in particular) that give semen its smell. --BluePlatypus 01:49, 23 April 2006 (UTC)
- Take a look at [51] Black Carrot 03:05, 23 April 2006 (UTC)
- If that article is to be believed, they're Carob trees (Ceratonia siliqua). freshgavinΓΛĿЌ 05:42, 26 April 2006 (UTC)
Internet Radio Player - Alternative To WinAmp[edit]
Can anyone suggest an alternative to WinAmp that will let me search through and play streaming radio stations from around the world for free? WinAmp has packed in and the others I've tried seem to want me to use a website to initiate the streaming; I'd like it be done from within the program itself like WinAmp used to when it still worked. --Username132 (talk) 01:44, 23 April 2006 (UTC)
Nullsofts site is the best for internet radio directory
Seems to me you have a couple of options. Firstly, it sounds like you used to like Winamp, but got tired of the bloat. If you want to go back to a previous version, you'll find them all archived at http://www.oldversion.com/program.php?n=winamp
Alternatively, I don't know of any other programs with a built in list like Winamp's media library, but the vast majority of streaming radio sites work by having you download a .m3u or similar playlist which then directs your player to the stream itself, so any media player with M3U and PLS support should be an adequate replacement (but you'll have to launch the streams by going to, say, the shoutcast website and picking one from there)
Hope this helps --Noodhoog 15:37, 25 April 2006 (UTC)
facts....[edit]
i just need facts about Xenontime! I already have this much:
Xenotime is very rare. It forms a solid solution series. Xenotime is used mainly as a source of crystal specimens which are valued by collectors. Occasionally, gemstones are also cut from the finer xenotime crystals.
- What are you talking about? Those are just random sentences from the introduction of Xenotime. Melchoir 02:46, 23 April 2006 (UTC)
- Google makes some things very easy. See: [52], [53], [54] and lo and behold, we have an article here on it: Xenotime.--Fuhghettaboutit 02:49, 23 April 2006 (UTC)
Outdoor Air Quality meters[edit]
Where can I buy outdoor air quality meters? (what brands?; what models?)
- Are you sure such a thing is available? Why not just use an "indoor" one outdoors? What exactly is it that you wish to measure? Air quality means different things in different places (see air quality. The air quality monitoring station shown in the article is far from portable.) Shantavira 07:55, 24 April 2006 (UTC)
Physics[edit]
A fisherman yanks a fish out of the water with an acceleration of 4.5m/s/s using very light fishing line that has a "test" value of 100 N. The finsherman loses the fish as the line snaps. what can you say about the mass of the fish?
So okay, this is surely wrong, but I have to show some work here:
F = ma FA - FG = 4.5m FA/4.5 - FG/4.5 = m
Yeah I have no idea what's going on there....absolutely have nothing on this besides the answers ( > 7.0 kg) but I need to know how to do it. Thanks
05:13, 23 April 2006 (UTC)
- Okay, assuming he's pulling it straight up out of the water (not sideways), the force on the line is the weight of the fish plus the force required to accelerate it at 4.5 m/s2, so
- F = m * 9.8 m/s2 + m * 4.5 m/s2
- (9.8 m/s2 is the acceleration of gravity on Earth's surface). If the line snapped, that means F > 100 N. —Keenan Pepper 05:32, 23 April 2006 (UTC)
Thanks a lot Keenan Pepper. I have one question though, the acceleration is going into the opposite direction from gravity, wouldn't you subtract? I know it's obviously wrong, what I'm saying, but I don't know why. So that's like having it as F = 13.41m which gives the correct answer, but I don't get how that works. If you could kindly explain that, thank you.
05:49, 23 April 2006 (UTC)
- Well, yes, you can subtract the weight from the tension to get the acceleration, T-W=ma. But you want to solve for T, so you rearrange: T=ma+W. Melchoir 06:01, 23 April 2006 (UTC)
Oh right, that's what I was doing, but I completely forgot I had tension there, stupid me. Thanks a lot!!! 06:06, 23 April 2006 (UTC)
- The fish isn't even struggling? - Mgm|(talk) 10:24, 23 April 2006 (UTC)
- After the fisherman hooked it, we shot it in the water to make sure we were dealing with a physics problem and not biology. It's, as they say, the only way to be sure. Melchoir 01:32, 24 April 2006 (UTC)
A way to handle these kind of questions generally. Calculate the forces on the fish.
Let the force upwards from the line be f, and the mass of the fish be m.
Then the fish experiences three forces:
- Gravity: 9.8 m DOWNWARDS
- Line: f UPWARDS
- Friction, water resistance, air resistance, ASSUME ZERO FOR THE MOMENT (a standard assumption, but worth noticing it DOES exist so this IS an approximation)
The total force on the fish is: (f) - (9.8 m) UPWARDS
The fish is accelerating at 4.5 m/s2. So....
Total force on fish = (f) - (9.8 m) ... = mass x acceleration ... = m x 4.5
So (f) - (9.8 m) = (4.5 m) So f = (4.5 m) + (9.8 m) So f = (14.3 m)
The line snaps at N = 100 That means the fisherman is trying to put more than 100 newtons of tension in the line, so its breaking.
So f > 100 So (14.3 m) > 100 So m > (100 / 14.3)
So IF YOU IGNORE FRICTION AND RESISTANCE, AND ALSO ASSUME THAT THE LINE BROKE PRECISELY AT ITS TEST VALUE (WHICH IS ALSO AN ASSUMPTION), the mass of the fish is more than (100/14.3) kg.
Hope this helps, next time, homework isn't free from Wikipedia :) FT2 (Talk) 02:19, 26 April 2006 (UTC)
Linear regulator explanation or diagram[edit]
I had a compelling discussion with an engineer friend of mine about linear regulators based on NPN transistors. The articles on these are pretty descriptive but he had a pressing question, which is that in the case of a linear regulator which is designed to allow a specific voltage and current to propogate on the emitter, regardless of input voltage (above a certain point), what specifically causes the output voltage and current to be so well regulated, specifically when energy is wasted where does it go and why? We are looking for a description at the electron level, and hopefully a good way to explain this to to electrical engineers who don't necessarily have a expert understanding of the chemistry of transistors. Anyone got a good idea where to start? There are many articles on transistors in general but i've seen none that deal with the behavior of a linear regulator at a low level. Thanks! --24.210.51.36 05:23, 23 April 2006 (UTC)
Codeine[edit]
If i purchased Codeine from my doctor, and it was back in 2001- Will it have expired by now?
- I would think so, yes. StuRat 06:19, 23 April 2006 (UTC)
- It's really not a good idea to get health advice over the internet; if it were me I'd take the codeine anyway (in fact I have done exactly that), but I am NOT recommending you do the same. My advice is to either call an all-night pharmacy, or go to the front of your phone book and call a community health nurse or poison control hotline. They usually operate 24 hours a day, so you can get advice now if you like.--Anchoress 07:25, 23 April 2006 (UTC)
- The "do as I say, not what I do" approach is fraught with difficulties and is best avoided, Anchoress. Why is the advice you give to the questioner not something you would follow yourself? :--) JackofOz 14:12, 23 April 2006 (UTC)
- Jackofoz, can you go back and actually read what I said? 'Do as I say, not as I do' is NOT what I was communicating above. What I recommended was that s/he a) not use the internet to answer a medical question (which is, I believe, a policy on Wikipedia (Wikipedia is not medical or legal advice)), and b) call an expert for expert advice. I did NOT suggest that he 'do as I say, not as I do'. I told him what I would do, I did NOT tell him not to do it. And to answer your question, I hold my own actions towards myself to a lower standard of safety and caution compared to the standard to which I hold my advice to people who know less than I. I contributed two things: suggesting s/he call an expert (and suggesting several that might be available overnite)), and indicating that I had done what s/he was asking about (implying that I'd done so without negative side-effects) that had not been communicated by anyone else before me. I think what I contributed was useful.--Anchoress 18:34, 23 April 2006 (UTC)
- I don't have any issue at all with the latter part of your advice, Anchoress, only with: "if it were me I'd take the codeine anyway (in fact I have done exactly that), but I am NOT recommending you do the same". I think a perfectly reasonable interpretation of that statement is that you were telling him what not to do (and you stressed the "not" by capitalising it). What other purpose did this sentence serve? That bit of your post served only to confuse (well, it had that effect on this reader). It was best left out, that's all I'm saying. :--) JackofOz 01:39, 24 April 2006 (UTC)
- Jackofoz, in the way I speak and understand English, there is a huge difference between 'I'm not recommending you buy the stock', and 'I'm recommending you not buy the stock'; 'I'm not recommending you date my ex', and 'I'm recommending you not date my ex'; 'I'm not recommending you use kerosene as a barbecue starter', and 'I'm recommending you not use kerosene as a barbecue starter'. I was not telling the person not to do what I do, I was making it perfectly clear (with caps) that I was not endorsing my own actions as advice. In my world, it would have been dishonest of me not to tell him/her that I have, in fact, taken expired codeine without ill effect; but it would have niggled at my conscience if I had not explicitly stated that I wasn't recommending s/he do it.--Anchoress 02:14, 24 April 2006 (UTC)
- I don't have any issue at all with the latter part of your advice, Anchoress, only with: "if it were me I'd take the codeine anyway (in fact I have done exactly that), but I am NOT recommending you do the same". I think a perfectly reasonable interpretation of that statement is that you were telling him what not to do (and you stressed the "not" by capitalising it). What other purpose did this sentence serve? That bit of your post served only to confuse (well, it had that effect on this reader). It was best left out, that's all I'm saying. :--) JackofOz 01:39, 24 April 2006 (UTC)
- Jackofoz, can you go back and actually read what I said? 'Do as I say, not as I do' is NOT what I was communicating above. What I recommended was that s/he a) not use the internet to answer a medical question (which is, I believe, a policy on Wikipedia (Wikipedia is not medical or legal advice)), and b) call an expert for expert advice. I did NOT suggest that he 'do as I say, not as I do'. I told him what I would do, I did NOT tell him not to do it. And to answer your question, I hold my own actions towards myself to a lower standard of safety and caution compared to the standard to which I hold my advice to people who know less than I. I contributed two things: suggesting s/he call an expert (and suggesting several that might be available overnite)), and indicating that I had done what s/he was asking about (implying that I'd done so without negative side-effects) that had not been communicated by anyone else before me. I think what I contributed was useful.--Anchoress 18:34, 23 April 2006 (UTC)
- The "do as I say, not what I do" approach is fraught with difficulties and is best avoided, Anchoress. Why is the advice you give to the questioner not something you would follow yourself? :--) JackofOz 14:12, 23 April 2006 (UTC)
- It's really not a good idea to get health advice over the internet; if it were me I'd take the codeine anyway (in fact I have done exactly that), but I am NOT recommending you do the same. My advice is to either call an all-night pharmacy, or go to the front of your phone book and call a community health nurse or poison control hotline. They usually operate 24 hours a day, so you can get advice now if you like.--Anchoress 07:25, 23 April 2006 (UTC)
- 10/10 for honesty and having a conscience. I wonder, though, how helpful that was to the questioner, who does not know you from Adam (or Eve in your case). None of us is so perfect that we follow the advice we give others 100% of the time (I’m certainly not). Parents who smoke usually tell their kids not to copy them. Questioners on the Ref Desk neither know nor care about the answerers’ personal circumstances, unless they were specifically asking about personal experience of something (which wasn’t the case here). Taking your example to an absurd degree, every pack of aspirin that says "Do not take more than 2 every 2 hours", would also say "I, Bob Smith, the manufacturer of these pills, and some of the people who work for me, sometimes take them more often than this, but I’d not recommend you do that". So, it’s really not necessary to bare your soul when providing advice to anonymous questioners. But when it goes beyond that, to introducing concepts that may never have been in the questioner’s mind at all, then having to stress that you're NOT recommending they do this, that’s where it gets really murky. You may in fact cause the person to do the very thing you're not recommending they do. This is a bit like the "Don’t try this at home, kids" warnings that TV stations show. Many/most of the kids would never have thought about trying it at home – until they heard the warning. It’s not that they’re being wilfully rebellious little brats, it’s just that the very mention of the concept of "doing it at home" puts it into their mind, often for the very first time. No matter how much you might capitalise or bold or italicise or underline it, the "not" is (at least temporarily) ignored in the cognitive process. "Do NOT think of the colour red". What colour are you now thinking of? :--) JackofOz 03:03, 24 April 2006 (UTC)
- It may also not be a good idea to self-medicate with narcotics. If you're in sufficient pain to need codeine (that is, the regular over the counter painkillers like acetaminophen/paracetamol, ibuprofen, and ASA/aspirin won't cut it) you might want to seek professional medical assistance anyway. TenOfAllTrades(talk) 12:03, 23 April 2006 (UTC)
- Expired, yes; in as much as the "use before" date has probably been passed. That said, it should still retain most of its activity (% unknown), if it has not been exposed to moisture, heat or light. There are no reports of "old codeine" causing any illness ascribed to a changed chemistry.. --Seejyb 15:56, 23 April 2006 (UTC)
Expiration dates don't mean what you might think. The popular impression is that something is guaranteed to go bad soon after that date. In many cases, however, they only test it up to a certain age and can attest to it being good for that long, but have no idea how long after that, if ever, it would go bad. It just isn't in the manufacturer's interest to do the testing needed to establish the exact age limit. As long as they can get it to last long enough to be distributed to patients, they would actually prefer that people discard old batches and buy replacements, so they gain nothing by telling you it will last for years. The silliest one I ever saw was a bottle of mercury with an expiration date, although a food product with a date, hours, and minutes at which the product expired also made me laugh...what, no seconds ? StuRat 20:01, 23 April 2006 (UTC)
- The medication has probably expired. When medications expire, it means that a fraction of it has decomposed and therefore, the dose may not have its full efficacy. For some medications, this can be significant (e.g. antibiotics) or perhaps dangerous (e.g. tetracycline, acetylsalicylic acid). - Cybergoth 00:25, 24 April 2006 (UTC)
The Hydrological Cycle[edit]
Hello, I would like to known how does the hydrological cycle of a drainage basin in the tropical landscape differ from that in the tropical desert landscape. Is there anyone can help me? Thank you very much! I have to finish this Easter assignment on time!
- Since we can't actually do your assignment for you, can you let us know what research you've already done, and where you are stuck or puzzled? Do you know what a hydrological cycle is, to start with? Notinasnaid 10:19, 23 April 2006 (UTC)
- You won't find the exact answer to your question, but you might find the article here, Water cycle useful in understanding what is involved. Note: pure serendipity-- water cycle just happened to be a link from the article I gave in my answer to the next question below on water bonding --Fuhghettaboutit 11:04, 23 April 2006 (UTC)
- Drainage basin --
 Mac Davis] ⌇☢ ญƛ. 11:24, 23 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 11:24, 23 April 2006 (UTC)
13:04, 24 April 2006 (UTC)13:04, 24 April 2006 (UTC)13:04, 24 April 2006 (UTC)13:04, 24 April 2006 (UTC)13:04, 24 April 2006 (UTC)13:04, 24 April 2006 (UTC)13:04, 24 April 2006 (UTC)13:04, 24 April 2006 (UTC)13:04, 24 April 2006 (UTC)13:04, 24 April 2006 (UTC)13:04, 24 April 2006 (UTC)13:04, 24 April 2006 (UTC)13:04, 24 April 2006 (UTC)13:04, 24 April 2006 (UTC)~ siu shuen 13:09, 24 April 2006 (UTC) I understand the hydrological cycle. But I cannot sure what should be included in my essay. The words "How does" mean the different processes in the hydrological cycles or how the processes differ in the hydrological cycles. Actually, I come from Hong Kong, my English is not so good and I'm not keen on Geography.....
The molecular bonding of water[edit]
Hello everyone,
I am a Standard Grade Chemistry student and, as it is nearing the time for my final examination, I was wondering if anybody could help to clarify something for me - Is water COVALENTLY or IONICALLY bonded? - as I have conflicting information in my course notes.
Any help would be greatly appreciated. Thank you.
195.188.152.16 10:30, 23 April 2006 (UTC)
- Water is made up of polar covalently bonded molecules. However, Hydrogen bonding is also at play and explains many of water's properties such as its relatively high melting and boiling points. Much more information can be found at Water (molecule)--Fuhghettaboutit 10:41, 23 April 2006 (UTC)
- you know, for a second i thought you said you were a grad student, i really should wear my glasses whilest browsing the interent--152.163.100.74 14:50, 23 April 2006 (UTC)
- The confusion may be due to learning about the Self-ionization of water - though covalently bonded, water form ions when dissolved in itself. Yet one does not refer to the water molecule as being ionically bonded. --Seejyb 16:33, 23 April 2006 (UTC)
- There's no black-and-white difference between covalent and ionic bonds, it's a sliding scale. The O-H bond in water is an example of something pretty much in-between. (although perhaps a tad closer to the ionic side of the scale than the covalent one) --BluePlatypus 19:34, 23 April 2006 (UTC)
Max keys pressed simultaneously[edit]
While playing some 2 player fighting games, the fact that just a number of maximum keys can be pressed simultaneously is damn annoying. I've read it's about the architecture and design of the keyboard. Is there any implication of the software running in this issue? Can anyone give me some information about how keyboards work (in a sense suitable to my question)? Any advice (apart from the obvious) of getting around this problem? Thanks.
- Well, I think the term you're looking for would be n-key rollover, but we don't seem to have an article on it. You might be able to find something by Googling it, though. - Nunh-huh 18:53, 23 April 2006 (UTC)
From what I recall this problem is to do with the keyboard's key matrix. It is a hardware issue, so cannot be resolved by software, but there is hope, particularly for gamers.
Firstly, exactly which keys can be pressed together varies from keyboard to keyboard, so if you really need certain keys together, you could try a different board.
Also, you may be able to get less conflicts by remapping keys. For example, when playing Stepmania, I use I J K and L instead of the arrow keys, as the keyboard I'm currently using will allow me to press all of those at once, but can't handle all the arrows together. Generally speaking though, there's not much hope of getting more than 4 or maybe 5 keys to register at once.
The most effective solution overall is to use a gamepad or other input device if possible. These are designed so you can press all the buttons together without conflict, and if the game is "keyboard only" you could try some joypad-to-keyboard mapping software. This works just fine, because the software itself can handle as many keys being pressed as you want.
--Noodhoog 15:46, 25 April 2006 (UTC)
Upper limit for ships[edit]
Okay, two questions today, I'll put them in sepearate sections.
The first is about the Freedom Ship. It says on that page that ships cannot be made realistically larger because of sagging and hogging. However, those types of issues arise (according their own respective pages) when the ship is about the same length as the waves. Surely waves are not ~1 mile in length? Thus, the stresses put on a ship that long would not be all put on the middle; there would instead be several points of stress on the hull. (Crude ascii drawing follows... I apologize in advance)
^ indicates point of stress (crest of wave) Normal ships:
====================
____^
Freedom ship:
=========================================================================
___^_____^______^________^______^_______^______^
Each point of stess would be the crest of a wave. So would the Freedom ship be possible (financing, etc aside). I would think that unless a half-mile long wave comes along, the Freedom ship would handle waves as well as a normal ship. Is this right? And, more generally, what are the practical, and the theoretical, limits on the sizes of ships?--AK7 14:49, 23 April 2006 (UTC)
- I think the problem with building huge ships is due to the fact that they are magnetically attracted to icebergs... (what, too soon?). Seriously though, the problem with building anything on that scale is that as the strength of construction materials scales linearly (build steel twice as thick and it can carry 2x the weight) the demand for srength scales geometrically (make a ship twice as long and it requires length^3 strength) It's the same limitation that forms the upper boundary of animal size, barring special adaptation by nature. So in summary, yes it can be done but it requires a complete rethinking of the way ships are structured, its not as easy as taking blueprints and blowing them to 10x size and making a ship. --24.210.26.146 15:08, 23 April 2006 (UTC)
Freak or rogue waves can be huge, with long wavelengths. Here is an interesting link [55] If a large ship catches one, it has to act as a bridge. This is generally impossible with the limitations of cross-section needed for propulsion and navigation. --Zeizmic 15:31, 23 April 2006 (UTC)
- Good points... couldn't the ship be built in sections, though? We've all seen those buses can bend in the middle, allowing them to turn corners while still being very, very long. Could the same concept be applied in the vertical dimension to a ship? --AK7 15:38, 23 April 2006 (UTC)
- I assume you mean the horizontal dimension. I believe it could, but then they would each need their own propulsion and steering system, so the cost of a ten segment ship would be almost as much as ten separate ships (with perhaps some savings by only have one captain and crew, instead of ten). Some software would be needed to execute a turn by moving the rudders on each ship at different times, as turning them all at once wouldn't work, but that seems doable. Such a system would allow the "train" of ships to turn as sharply as any one can turn. The problem is that this slightly lower cost may not justify the loss of flexibility you would have with ten separate ships. One way around this would be to make the ships capable of operating either together or apart. Incidentally, the ships may not need to be physically connected, but only connected by electronic communications, although tow lines might be needed in case one segment breaks down. This system would have an advantage in being better able to fit through narrow passages, such as the Panama and Suez canals, than a single, much larger ship. StuRat 16:40, 23 April 2006 (UTC)
Would a beam sea be more dangerous for such a long ship? If the bow was on the crest of a wave and the stern lying in the trough, it seems like there would be torque applied about the long axis and also the vertical axis of the ship. The longer the ship the more surface area exposed to the wave, and the larger the moment. EricR 20:31, 23 April 2006 (UTC)
Maximum daily water[edit]
How much water can one safely drink daily? I know about Water intoxication and all, but I'm not asking about that, strictly. Rather, I keep a water bottle next to my desk and drink from it often; I realized a couple days ago that I had gone through 5 liters in a day, and would have reached 8 if I hadn't conciously stopped at that point. It typically takes 1-2 hours to drink a liter (within the 1.5 ltr\hour limit cited on the water intoxication page). But, even if it is safe, is it desiriable to drink that much water? Does it put my kidneys under extra stress? And, if there are impurities in the water (there probably aren't, but just for future reference), is the stress on my kidneys based on the concentration of impurities, or the total amount (which would increase with more water, obviously.) There are thousands of pages telling us to drink more water... at what point does the law of diminishing returns kick in?--AK7 15:06, 23 April 2006 (UTC)
- The need for so much water was a misunderstanding of some old report, and has now become a myth. [56]. I have never seen anything scientific on this. In fact, if you believe that you should lean towards the 'human evolution' ideal, most of our millions of years were spent on the dry savanna. All the water makes you pee a lot, and have to visit the filthy, stinky washroom (we've got 'waterless' stinky urinals!), picking up bird flu, and sending more water into the sewers. --Zeizmic 15:41, 23 April 2006 (UTC)
High water intake may be a sign of a medical problem or high sodium intake. Try reducing the amount of salt in your diet and mention the high water intake to your doctor, during your next checkup. StuRat 16:25, 23 April 2006 (UTC)
One danger of bottled water is that toxic chemicals leach into the water from the plastic containers over time. So, drinking old bottled water, especially if stored in hot areas, in quantity, can expose you to lots of toxic chemicals. That's why bottled water has expiration dates on it. You can sometimes taste the chemicals in the water, too. I suggest you use filtered tap water and store it in glass containers, for optimal safety. If you must use plastic containers, refilling them (with filtered tap water) right before you use them will limit the accumulation of toxic chemicals in the water. StuRat 16:25, 23 April 2006 (UTC)
- Has this actually been demonstrated, or does it just seem reasonable? Ardric47 02:07, 27 April 2006 (UTC)
- If you mean the part about toxins leaching out of plastics and accumulating over time, I don't think the quality of bottled water is much studied, compared with the quality of tap water, which is studied quite a bit. However, your senses can do a quick test for you. Does the bottle smell like plastic ? If so, that means it is out-gassing chemicals, some of which end up in the water. Softer plastics tend to do so more than hard plastics. Unfortunately, the softer plastics are the ones they use for bottled water. High temps, exposure to sunlight, and stressing the container (as when you squeeze it) will increase the rate, as well. StuRat 14:44, 27 April 2006 (UTC)
I think I remember seeing a recent news story that implied drinking too much water can harm your kidneys. Drinking too little can definitely harm your kidneys, by favoring the formation of kidney stones. So a middle ground has to be found, and I don't know what its boundaries are. If you're drinking all that water to relieve stress, well, I imagine it's better than eating potato chips or something. --Trovatore 18:24, 23 April 2006 (UTC)
- I would think that "holding it in" could cause kidney problems. If the bladder is full and urine can't drain properly from the kidneys, this could also lead to kidney stones. Drinking lots of water could make you have to "hold it in" more often, so could indirectly cause such kidney stones. StuRat 19:19, 23 April 2006 (UTC)
- I have never heard of too much water causing kidney stones, and it seems inherently implausible; kidney stones form when uric acid precipitates out of solution in the kidneys, and that happens because the urine is too concentrated, not too dilute. But I think too much water can cause other sorts of kidney damage; at least that's what the news story I'm vaguely remembering seemed to suggest. Sorry I don't have any better info than that. --Trovatore 19:33, 23 April 2006 (UTC)
- I believe Trovatore is correct. Too much water and "holding it in" does not cause kidney stones. (I wish the Reference Desk people here would check their facts before spouting off medical and human physiology answers.) Drinking too much water can put a "strain" on the kidneys (and it is due to the water, not the impurities). Among the potential kidney problems is nephrogenic diabetes insipidus. Drinking too much water or increased thirst can be a symptom of diabetes mellitus/hyperglycemia and hypercalcemia. - Cybergoth 00:40, 24 April 2006 (UTC)
- Can you site a study which proves conclusively that excessive urine retention does not correlate with increased kidney stone incidence ? StuRat 02:20, 24 April 2006 (UTC)
- Can you find a credible reference that states excessive urinary retention leads to kidney stone formation for that matter? There does not appear to be an association between urinary retention and the creation of kidney stones noted in any medical textbook or review article (on kidney stones) that I've read. - Cybergoth 17:44, 24 April 2006 (UTC)
- Well, if there are no studies into this, then nobody really knows, so I can hazard a guess at what would happen, as well as anyone else. If, as a result, people avoid "holding it in", even though there was no actual risk of kidney stones from doing so, then there isn't much damage done, is there ? I can see why you would be upset if I suggest someone with cancer stop chemotherapy and eat ground apricot pits instead, but this is hardly the same thing, is it ? StuRat 18:46, 26 April 2006 (UTC)
- I'm not upset with you, StuRat, but you did pull that answer out of your ass. :P You are right that there was no great damage done by your answer - just misinformation. I just want to elevate the quality of answers at the Reference Desk. According to the kidney stone article, drinking enough water is recommended to prevent kidney stone formation. Urinary retention, can cause kidney damage (but probably not kidney stones). The reason why there are no studies done is because nobody has noticed an association between stone formation and "holding it in" (or urinary retention). For example, untreated prostate enlargement (which is fairly common) causes urinary retention in the bladder, but there doesn't seem to be an association with prostate problems and an increased risk of kidney stones. I agree that, "absence of evidence is not evidence of absence". However, there are lots of things in Medicine that have not been studied with randomized controlled trials, for various reasons. That is why Medicine is in part an Art. - Cybergoth 21:46, 26 April 2006 (UTC)
- I don't think it's unreasonable to propose our own theories when there is no evidence one way or the other. Let me elaborate on my theory:
- Excessive urine retention may cause urine to remain in the kidneys (which would normally drain into the bladder), once the bladder is at maximum capacity.
- Urine remaining in the kidneys longer than normal may allow time for nucleation sites to grow into stones. If the stones are able to grow large enough so they won't easily fit down the drainage tubes to the bladder, they will then remain in the kidneys or cause pain passing through. If they remain in the kidneys, they may then continue to grow.
- StuRat 14:32, 27 April 2006 (UTC)
- Contrary to your assertion (that there is no evidence), I had just stated observational evidence that urinary retention does not lead to kidney stone formation. I think your "theory" is busted. Let it go. - Cybergoth 03:27, 28 April 2006 (UTC)
- What evidence ? You can't cite any evidence that would meet Wikipedia guidelines. You claim that nobody has noticed a link, but that only means you don't know of anyone who has noticed a link. And even if it were true that nobody has noticed a link, that isn't evidence that none exists. Only a scientific study could establish that. Stop being so critical of other responders. StuRat 23:56, 28 April 2006 (UTC)
- I've made my point, so I won't belabour this any more. I refer any interested readers to this review article that I've found. While it doesn't address urinary retention, it does mention a study on increased urine output to prevent recurrence of kidney stones. - Cybergoth 04:15, 29 April 2006 (UTC)
Consuming diuretics like beer (or stronger alcohols), coffee, tea, cola drinks, etc.. and natural excessive sweating can explain why some people 'need' to drink a lot of water per day. - G3, 07:22, 25 April 2006 (UTC)
Touch and Yawn[edit]
When my cats are relaxing (that is, most of their waking hours), most often, when I give them a pet, they start yawning. What could possibly be the connection between the physical soft contact of a big human hand and the taking of a deep breath with widespead jaws?--JLdesAlpins 15:39, 23 April 2006 (UTC)
- I believe yawning is to increase blood flow to the brain to make the human or other animal more aware. If they are going to do something, in this case just interacting with a human, they need to wake up a bit, hence the yawn. What's really fun is when they myawn (start a meow and end up yawning). StuRat 16:15, 23 April 2006 (UTC)
- More likely, what you are describing is the cat's flehmen reaction. Cats have a vomeronasal organ that they use to sense "social" smells, and when they are in the process of doing that, they open their mouths and pull their lips back to "taste" the smell. Male cats do it more. Or they could just be tired<g> - Nunh-huh 18:48, 23 April 2006 (UTC)
- It's quite hard to confuse a cat's Flehmen response and a yawn, as in the Flehmen response its mouth only opens very slighty (see [57] for illustration). –Mysid 07:40, 25 April 2006 (UTC)
- More likely, what you are describing is the cat's flehmen reaction. Cats have a vomeronasal organ that they use to sense "social" smells, and when they are in the process of doing that, they open their mouths and pull their lips back to "taste" the smell. Male cats do it more. Or they could just be tired<g> - Nunh-huh 18:48, 23 April 2006 (UTC)
- Yawning is also connected to relaxation, stress relief and other body behaviors in animals, as well as people. For example, yawning stretches the chest cavity, then is typically followed by a deeper degree of physical relaxation. Also, I have concluded from study (and this is only personal observation), yawning seems to be related to letting go of stress. Human contact is nice but can also be dominant in some animals. Typically dogs will yawn and often shake after stroking or petting, or will pant if stroked a lot, and it seems to be related to some form of psychological stress, and an instinctive reaction (ie they may not feel stressed but its an instinctive response). I dont know if any of that helps at all. Its purely personal observation. FT2 (Talk) 02:04, 26 April 2006 (UTC)
Then again, maybe your cat just finds you boring. Try wearing a mouse suit, LOL. StuRat 14:53, 27 April 2006 (UTC)
Leeches in USA Ponds[edit]
I have a pond that a blue heron has decided to visit. He has brought with him a leech. I have only found one so far and it was swimming. Does anyone know which type of leech this would be and how often it multiplies? I am about 45 miles northeast of San Francisco. Any help would be appreciated. Thanks. Terrill
- Put in some fish, they love leeches! Then if the fishies get too much, throw in a yawning cat... --Zeizmic 17:23, 23 April 2006 (UTC)
- ... sooner or later you'll have thrown in an elephant and then the old lady will die. kmccoy (talk) 02:22, 24 April 2006 (UTC)
- I don't know how often it multiplies. Presumably if one differentiates it, then the issue can be summed up easier, because then the 45 miles distance is a constant and drops out? FT2 (Talk) 18:48, 26 April 2006 (UTC)
- I don't know if I missed something, but "multiplies" can also mean "reproduces." I wish I could answer the original question, which seemed reasonable, but maybe there is something in the leech article. Ardric47 02:11, 27 April 2006 (UTC)
- I dont know but my guess would be it's a horse leech, which can get up to about 6cm long
Photosynthesis[edit]
Recently, I have been reading about photosynthesis, but there is a part - the effect of carbon dioxide concentration on the rate of photosynthesis - which I cannot understand. Could anybody please explain it to me in a simple way? Thanks
- I don't know specifically about photosynthesis, but let me answer in terms of a general chemical reaction. Most reactions are limited by the quantity of a limiting reagent. Thus, as long as sufficient carbon dioxide is present such that it is not the limiting reagent, it should not have any effect on the rate of the reaction. The limiting factor on photosynthesis, in most cases, would be sunlight. Let's say the amount of carbon dioxide needed in the air for typical photosynthesis is 0.1%. Then quantities over that amount would have no effect, while quantities below that amount would decrease the rate of photosynthesis proportionally. So, a concentration of 0.05% would cut the photosynthesis rate in half. This is all complicated by the fact that the amount of sunlight is always changing, so the amount of carbon dioxide required is also always changing. StuRat 17:13, 23 April 2006 (UTC)
- The kinetics of chemical reactions in general do not necessarily depend on whichever reactant is in the lowest concentration; That's only the case for a second (or higher) order reaction. The rate of a first-order reaction (E.g. an SN1 reaction) only depends on the concentration of one of the reactants. Enzyme reactions such as those in photosynthesis don't follow either first or second order kinetics, but Michaelis-Menten kinetics. The reaction rate levels out as the substrate (CO2) concentration gets higher. So the rate is not strongly dependent on CO2 levels. (E.g. if the concentration is doubled from 5*Km to 10*Km, that's only a 9% increase in reaction rate). CO2 doesn't compete with the light either since it is not consumed in the light-dependent reaction of photosynthesis. --BluePlatypus 00:42, 24 April 2006 (UTC)
Limiting factor I put CO2 in my fishie tank to help the plants grow, since with lots of light, this becomes a limiting factor. --Zeizmic 17:26, 23 April 2006 (UTC)
reproductive system[edit]
whats the difference between the reproductive systems of a flowering tree and a cone tree?..
- I don't know, but I'm sure the answer is in Flowering plant, Conifer cone, and related articles. Melchoir 20:40, 23 April 2006 (UTC)
- Something is hidden for the latter and nude for the former : see angiosperm and gymnosperm. --DLL 20:43, 23 April 2006 (UTC)
[edit]
I would like to know that how is filtering water through coffee filter relates with the partical theory? I would like your answer on it.
Thank You
- Basically, the particles you want to filter out are larger than the particles of water, so they get stuck in the fibers of the filter. Were you looking for something more? —Keenan Pepper 20:40, 23 April 2006 (UTC)
By Jiminy! It's a good thing there's an encyclopedia here abouts. Filtration --Zeizmic 21:50, 23 April 2006 (UTC)
- I think you were referring to particle theory. - Mgm|(talk) 07:48, 26 April 2006 (UTC)
Body Symmetry[edit]
I have been looking forever to find the body symmetry of seahorses, i.e. bilateral, radial, however, I can not find it. I was wondering if anyone knew it. Thanks. ~Sandy
- From the picture, it looks like they're bilateral. —Keenan Pepper 20:38, 23 April 2006 (UTC)
- I would have helped if you knew the meaning of the words, that way you could figure out for yourself. That's the easiest way. Bilateral means pretty much you can cut it in half one way and you'll get a mirror image. A seahorse, looking at it from the top, or the back, or the front, if you drew or cut a line along its body both sides would be the same. --
 Mac Davis] ⌇☢ ญƛ. 20:45, 23 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 20:45, 23 April 2006 (UTC)
- Thanks. I just wasn't sure if you did cut it in half that it would look exactly the same. -Sandy
- Oh. Well, I'd be surprised if the halves were exactly the same, but they'd be close. See Symmetry (biology). Melchoir 01:30, 24 April 2006 (UTC)
- They definitely wouldn't be exactly the same, unless seahorse DNA twists in opposite directions depending on which side of the seahorse it's on. That would be kind of cool :) --Ashenai 13:12, 24 April 2006 (UTC)
- Oh. Well, I'd be surprised if the halves were exactly the same, but they'd be close. See Symmetry (biology). Melchoir 01:30, 24 April 2006 (UTC)
- One kidney only. Situated on the right (as the seahorse faces). And it has no glomeruli.--Seejyb 18:40, 24 April 2006 (UTC)
Video Editing[edit]

You know how sometimes when like news programs are talking about contracts or other written content [a supreme court ruling, a patent, etc] they'll like show a picture of the thing then highlight whatever they're quoting and then pop-up a zoom-in with the quote? Do you know of a program that'll let me do something like that?
- To do what you are describing, I am using ArcSoft's PhotoStudio, which does the job quite well, but not with an automated function however. That is, there are a few (easy) manual steps to get the result you are looking for. I suppose that if you don't have thousands of highlights to do, then it is a viable option for you.--JLdesAlpins 22:38, 23 April 2006 (UTC)
- Well I was thinking more of an animated one, where it highlights in the video and then the magnified quote fades into view. — Ilyanep (Talk) 21:22, 24 April 2006 (UTC)
- PowerPoint can do just that. If it is still not what you are looking for, please give more details of your specs and we'll try again.--JLdesAlpins 16:42, 25 April 2006 (UTC)
- Well I was thinking more of an animated one, where it highlights in the video and then the magnified quote fades into view. — Ilyanep (Talk) 21:22, 24 April 2006 (UTC)
And also, does anyone know of a good open-source Video Editing program that works on Windows XP [I absolutely must have a timeline and video capture from a videocam on it!]. — Ilyanep (Talk) 20:58, 23 April 2006 (UTC)
- Open source on XP - the people who do that are rare saints! Usually, this is not the platform they develop on, and I don't really see fancy picture-in-picture video construction on the main lists. The networks always come out with custom, expensive software, a few years ahead of general circulation. --Zeizmic 21:55, 23 April 2006 (UTC)
- It may sound trivial, but have you tried Windows Movie Maker, which comes free with XP? It might be just what you need. For instance, my daughter (11) used it for her school science project where she captured on video various-shaped free-falling objects then calculated (and printed) the time difference in 1/30th of seconds between each. Just a thought.--JLdesAlpins 22:47, 23 April 2006 (UTC)
- There's a list of open-source video editing programs; a lot of those run on Windows. --Cadaeib 23:14, 23 April 2006 (UTC)
- I don't know the answer to the question, but I wanted to mention that I think the illustration accompanying this question is one of the finest that the reference desk has ever seen. kmccoy (talk) 02:19, 24 April 2006 (UTC)
- Reminds me of http://uncyclopedia.org/wiki/Image:Mspaint2.jpg. —Ilmari Karonen (talk) 21:16, 25 April 2006 (UTC)
The seven signs of madness[edit]
Hi
I am doing a project at school on "the seven signs of madness" due half way into next term.
Do the "seven signs" really exist?
If they do, what are they? (all seven please or as many you can come by).
Like to know before I get too far into proucing it.
Hope you can help me.
Yours Georgia--144.135.164.189 22:41, 23 April 2006 (UTC)
- Is posting your homework here and expecting an answer one of them ? Seriously, I suspect you were given those "7 signs" in a book or class notes or something, so you need to look through those for the answers your teacher expects. If not, maybe your teacher wants you to just make them up. I would ask them which it is. StuRat 02:03, 24 April 2006 (UTC)
- You could always answer "no" to the first question, and then you wouldn't have to answer the second question at all! freshgavinΓΛĿЌ 05:19, 24 April 2006 (UTC)
- I was always told that the first sign of madness was hairs on the palm of your hand. I was also told that the second sign of madness was looking for them.
Slumgum | yap | stalk | 21:34, 25 April 2006 (UTC)
Processor Board Deeley[edit]
My processor board has this weird bit of metal that sort of sticks out towards the side of the case when it's slotted in. It's kinda bendy... what's it for? [58] --Username132 (talk) 23:16, 23 April 2006 (UTC)
- Looks like a spacer to make sure the thing fits snugly. --BluePlatypus 00:17, 24 April 2006 (UTC)
- This seems to either be a spacer, or a contact to make sure that case-ground and circuit-ground are the same, or both. --Christopher Thomas 04:39, 24 April 2006 (UTC)
- Looks like a grounding contact to me. --Serie 23:28, 25 April 2006 (UTC)
Graphics Card Widget[edit]
Here on my old ISA graphics card is this soft of bit on opposite side to that which fits in the ISA slot - what is it? What does it do? [59] --Username132 (talk) 23:24, 23 April 2006 (UTC)
- It's an expansion bus of some kind. Some (but not most) cards have a bus that lets you directly access the frame buffer and possibly other parts of the card's state. One lab at U of T built a widget they called the "Transmogrifier II" which hooked into a graphics card that way to test hardware rendering algorithms (the T2 was a big collection of FPGAs which could be reprogrammed to implement many different emulated algorithms; this particular test dates from about a decade ago). It's possible that instead of a general-purpose access bus this was intended to control a specific peripheral that hooked up to the graphics card, but I have no idea what kind of peripheral they'd have used in those days. The only ones I've seen lately were MPEG decoder boards for DVD playback, which became obsolete shortly after DVDs got popular due to faster processors for software decoding and support for hardware decoding in graphics cards. Video capture board would be my best guess in this case, given the age of the card. --Christopher Thomas 04:46, 24 April 2006 (UTC)
April 24[edit]
Programming Languages[edit]
Hello. I'm a beginning programmer. Two years ago, I tried to learn Visual C++ .NET. That was really, really hard. I wasn't able to do anything besides the tutorial in the book, so I quit. About four months ago, I picked up Liberty BASIC. I am able to do a lot and understand the language. I really enjoy it and am doing a lot of fun stuff. I wondered if anyone had any suggestions on other languages to try next or any suggestions about a good progression of languages for a learning programmer. Any stories about what you did, what you wish you did, or just simply any advice you have would be great. Thanks for your help. --Think Fast 01:23, 24 April 2006 (UTC)
- FORTRAN would be a good next step, as it is rather similar to BASIC, but more powerful. I agree that C++ is one of the most difficult to learn. StuRat 01:55, 24 April 2006 (UTC)
- But does anyone still use Fortran though? GCC still compiles it, but I can only remember one program written in it. In fact I think I've encountered more programs written in Ada. I suggest Python. —Keenan Pepper 02:21, 24 April 2006 (UTC)
- I do ! StuRat 03:21, 24 April 2006 (UTC)
- I still encounter it occasionally for scientific code, but it's pretty rare. Nowadays I'd use Matlab or similar for anything that could be run on a single workstation. Perhaps a lurking cluster-user can tell me if the parallelized versions are still used over there.
- I know that Folding@home is written in FORTRAN. As it is the oldest language, it has the best compiler and is one of the fastest available languages. Personally, I write in Java, as it's what my University teaches. I enjoy it, but feel that C++ is more useful for actual, real-world, coding. Many business applications are written in BASIC. Whatever you choose, it should have some sort of Object use. --yaninass2 05:11, 24 April 2006 (UTC)
- For a learning language, Turing isn't a bad choice, but it isn't used outside of programming courses. I've heard Python recommmended, but haven't tried it myself. --Christopher Thomas 03:07, 24 April 2006 (UTC)
- I too recommend Python even though that that wasn't my first programming language. I start with C which I don't think that I would recommend. C could be good for understanding low level programming but otherwise I wouldn't recommend it. Python has a large standard library, a neat readable syntax and garbage collection. The major downside with Python is that the dynamical typing (which is a nice feature) makes you get more errors when running the program instead of when compiling. Jeltz talk 11:14, 24 April 2006 (UTC)
- (after edit conflict) We've had this discussion many times before (such as here and here) - it depends a bit on whether you want to learn to program (the abstract concept) or whether you want to learn to program in order to write something specific. FORTRAN (yes, I use it too! :)) is moderately widespread in the technical and scientific community, but you may find it looks a bit 'dated' (not that that's a bad thing, depending on your tastes). On the upside, as yaninass2 points out, it's very powerful, especially for very complex (say, scientific) calculations. If you're trying to write applications for PC, then C or C++ are the standards, but beware that, as languages, they are truly evil for the beginner (C especially), and I don't recommend them if you're still learning the ropes. But it you want to write the next version of Windows, C is your baby. If such high aspirations aren't quite what you had in mind (at least not right now), I can suggest Python - having played with it, I think that it's a pretty good language to learn in, especially since there are many excellent free tutorials on the web. But you can still do a huge number of things with it, so it will serve you well later, too. However you won't get the raw performance out of it that you would on a lower-level language (but, as I said, it depends what you're trying to do!). In any case, good luck with your programming! :) — QuantumEleven 11:19, 24 April 2006 (UTC)
- I think the more important thing is to program. Regardless of language choice, you have to program. Just like any skill, you have to practice. Even if you're not actaully coding all the time. Go grab the Source to any Open Source project and start to read it. It's ok that you don't know the language that it's written in, most programing concepts translate from one language to another. The concepts of programming and Computer Science are more important than the code itself.Just look at it and try to understand what the code is doing. If you have no idea, the code is usually very well documented so you can get a rough idea of what it's saying and doing. Even try to rewrite something into the language of your choice. yaninass2 12:56, 24 April 2006 (UTC)
- I would recommend Java. Sun tutorials are usually excellent. There is a lot of information about many common beginners problems on the net that you can google. Some people say that Java being object-oriented makes it hard to learn; but that is really not the case, since you can use a class just like a collection of functions by declaring all methods static. And IMHO doing real data structures with objects is a lot easier than handling C structs, for example. Java also offers great functionality without installing all kinds of extensions and add-ons; you can do everything from simple shell apps to graphics and database connectivity just with the basic JDK.
- About taking a open source program and looking at the source of it: don't. Especially programs written in C or C++ usually have a very complex framework of compiler macros and rely very much on their own, internal application programming interface (API), which makes it sometimes hard to even recognize the language they are written in! But in Java, these APIs are usually uniform. I assume that you already know the basics such as loops and conditionals - after learning those basics, it's more about the API than the language itself whether a language is easy to learn or not.
- Java is also a great starting point if you ever want to do some professional programming. Many programmer's daily work consists of writing tailored business applications such as billing software or e-commerce web sites, and these are often written in Java. And if your interests are more on desktop software, it's easier to learn Java first and then move to C++; Java is lot like C++ made easy. SGJ 19:10, 24 April 2006 (UTC)
- You bring up some good points. Compared to C and C++ Java is easy, but the are several other languages with a good standard library (no required add-ons) and object orientation (in some cases optional) like Perl, Python, and Ruby. I don't think that Java has many obvious advantages over those languages, it mostly comes down to a matter of prefernce here. Proffesional software developers seems to me to tend to prefer Java (and C++ and Visual Basic), but I think that the slighly simpler Python is better for the beginner (and not bad for advanced useers either). I'm not saying that Java is a bad choice – just adding that other languages share many of the strenghts of Java. Jeltz talk 22:46, 24 April 2006 (UTC)
Pascal, naturally. - G3, 07:25, 25 April 2006 (UTC)
- I for one recommend assembly language.--Frenchman113 on wheels! 00:17, 29 April 2006 (UTC)
- Just don't start with i386 assmebly if you value your sanity. Plugwash 00:36, 1 May 2006 (UTC)
I would recommend Python. It gives you a powerfull and clear scripting language and an opportunity to learn the basics of object oriented programming. If you prefer to start with C++ I would recommend using Accelerated C++ from Koenig and Moo so you are gently introduced to the standard libraries from the start and avoid reinventing strings. Jarga 20.30, 1 May 2006 (CET)
Golf Balls[edit]
Is it true that Honey is put into the centre of some golf balls?
Thankyou
- Not any standard, commercial golf balls. (Amongst other reasons, such as it being expensive and not doing anything useful, honey would be different from batch to batch, meaning the resulting golf balls wouldn't perform the same.) I cut one open 15 years ago, and the core was like a bunch of rubber bands, wound around a rubber ball, although expensive balls have changed since then. Our article on golf balls has more details on the history of golf ball construction. Personally, when golfing, I use a French brand, called RANGE. --ByeByeBaby 02:28, 24 April 2006 (UTC)
- Most likely pronounced "RAWNJ". freshgavinΓΛĿЌ 05:14, 24 April 2006 (UTC)
Alternate Logic[edit]
I'm not quite sure which desk to put this on, so I'm going with Science. Does anyone familiar with the Zensunni philosophy in the Dune books know what real-world ideas they were based on, if they were based on anything? I'm especially wondering about the anti-logic philosophy mentioned in Heretics of Dune. Same questions for the 'intuitive logic' thing in the Dorsai books (the Childe Cycle). Black Carrot 02:16, 24 April 2006 (UTC)
- I've actually been meaning to re-read my Dune books for a while. I'll try to get back to you sometime this year. freshgavinΓΛĿЌ 05:35, 26 April 2006 (UTC)
On Talk:Autocunnilingus, there's been quite a bit of difficulty coming up with evidence one way or the other on a particular debate; namely, whether such a thing is at all possible, and if so, whether there are reputable pictures or video available of it being done. Any input? Black Carrot 02:59, 24 April 2006 (UTC)
- Posted my thoughts. I think finding evidence in the form of a photograph that has not been noted by a qualified source violates the No Original Research Policy. -Bill
- Dandy. Any suggestions on how to find a qualified source on this? Black Carrot 11:25, 24 April 2006 (UTC)
- Ask around. You probably have relatives that do yoga. freshgavinΓΛĿЌ 05:33, 26 April 2006 (UTC)
- Dandy. Any suggestions on how to find a qualified source on this? Black Carrot 11:25, 24 April 2006 (UTC)
DVD Ripping[edit]
I recently used DVD Decryptor to rip part of a DVD I have. It saved as a .vob file, and using VLC Media Player, I can play the file. The problem is that the file size is large. Can anyone suggest any good, free programs to convert/edit .vob to .avi or .mpg? Thanks. --Chris 05:03, 24 April 2006 (UTC)
- Henry, are you suggesting that macs are not computers...? ;-) Chris, I suggest you read the forums at doom9.org, they have a whole plethora of guides on this subject. If you're just starting out, I recommend AutoGK (AutoGordianKnot), the automated newbie-friendly version of GordianKnot, the full-fledged encoder. — QuantumEleven 10:59, 24 April 2006 (UTC)
- As I recall, VOB files are already in MPEG format. Try renaming them to .MPG and see if they play. If so, then virtualdub-mpeg website can directly recode them to AVI format in any codec or compression you choose. FT2 (Talk) 01:54, 26 April 2006 (UTC)
Is it possible to jump out of a 747?[edit]
The title pretty much says it. No, I'm not looking to do it, I'm just curious if you could jump out of one and still survive. --Skull sphinx 06:18, 24 April 2006 (UTC)
- I suppose it depends at what height the plane is flying..or even if its flying...anyone could jump out of a plane when its on the runway and still survive... and of course, it also depends on whether you have a parachute or not.. Jayant,17 Years, India • contribs 06:23, 24 April 2006 (UTC)
- Getting the door open may be rather difficult. As far as I recall the door won't open while in flight. Of course, if you rob everyone on the plane first, you could be the next D. B. Cooper. :-) Dismas|(talk) 06:35, 24 April 2006 (UTC)
- I wouldn't say anyone could jump from the plane and survive... if you take the normal exit and there is no Jetway to step on to you will be in for a rather harrowing experience, and you could easily die from the stop at the end of the 25' fall if you land wrong on the Tarmac. --66.195.232.121 14:21, 24 April 2006 (UTC)
- And your chances are even worse if you tried this from the upper deck of a Airbus A380. :) — QuantumEleven 14:36, 24 April 2006 (UTC)
- Not a direct answer, but interesting: JAT Yugoslav Flight 364. Notinasnaid 09:42, 24 April 2006 (UTC)
- "Is it possible to jump out of a 747?" While in flight? Almost impossible. Dismas is right, the pressure differential between the cabin and the outside at cruise altitude is so high that even if you were an olympic weightlifter you probably wouldn't have the strength to overcome it (airliner doors need to be pulled inward before they rotate outward, see [60]). You could create your own door with, say, a bomb - but that entails a whole raft of other problems.
- Assuming you did find a way to leave the 747 in flight, could you survive the fall? It's very unlikely, but possible. You would probably black out from the low pressure, but are unlikely to die until you impact the ground. People have survived falls from very great heights without parachutes, but only under very special conditions. You need to land in something that would break your fall sufficiently gently - soft snow (and hope you don't get buried in too deep) or somesuch. The River Tiber (in reference to a recent popular book ;-) ) won't do. The link Notinasnaid provided is an example of a person surviving the plunge, and there are a handful of other accounts. — QuantumEleven 12:41, 24 April 2006 (UTC)
- I think the answers above are pretty much correct. If you manage to get out of the plane you could survive but don't count on it, very few have survived. I think that falling over land would increase your chances (especially if there is snow). I don't think that water ever is soft enough, and even if it is you will most likly drown when you hit the surface of the water already unconsious from lack of oxygen. The article about Free-fall mentions three other known cases where people have survived falls at terminal velocity. Jeltz talk 11:22, 24 April 2006 (UTC)
- Also a parachute could improve the chances of surviving the landing. – b_jonas 11:05, 25 April 2006 (UTC)
- Being a pedant about the question the answer has to yes, because nowhere is it stated the plane is in motion, or in the air. --Blowdart 12:38, 25 April 2006 (UTC)
- I'm sure i heared that the early test models of the 747s were fitted with an in flight escape exit. I belive the early test models of the A380 were too. So yes with a parachute and the correct exit installed you could do it in flight but on a production airliner with no special modifications it would be very hard. Plugwash 13:01, 25 April 2006 (UTC)
If you had a gun, you could blow out a window to equalize the pressure before opening the door. StuRat 00:27, 29 April 2006 (UTC)
- Why does someone want to know how to do this? I'm slightly worried.--The ikiroid (talk)(Help Me Improve) 19:36, 29 April 2006 (UTC)
Early morning dilemma[edit]
When I arrive at my office I can either walk up four 6" steps or up a direct ramp which is about six times as long as the steps. I intuitively walk up the ramp as this looks easier than climbing the steps, but whenever I choose the steps it feels like they're less work, perhaps because the effort is expended over a shorter distance. Logic tells me I'm doing exactly the same amount of work whichever option I choose. Which would you choose and why? Shantavira 08:10, 24 April 2006 (UTC)
- Well, you do the same amount of mechanical work either way, but if by "work" you mean total muscular energy expended, then surely one option is costlier than the other. It would depend on the details of your personal gait, but in general, I would guess that the ramp requires you to burn fewer calories, simply because your velocity doesn't change as much when you use it. Melchoir 08:24, 24 April 2006 (UTC)
- Yes, that's an interesting point about the change of velocity, which I don't notice in practice. I shall try both options at an increased velocity and see how they feel. Shantavira 09:36, 24 April 2006 (UTC)
- Another factor is that the ramp will cause you to lean slightly backwards, which causes you to expend more energy to move forward. This would actually help when walking down the ramp, though. I suggest you compare walking down the steps and ramp. I suppose I should also point out that if climbing 4 steps seems like a significant amount of work to you, you may need the exercise to get in better shape. Always taking steps (or ramps) instead of elevators and escalators can be good exercise, and taking the stairs is frequently quicker than waiting for an elevator anyway. StuRat 11:21, 24 April 2006 (UTC)
- You are using different muscles, so the two would feel different, irrespective of work done "physics-wise". Have you checked if the distance - as would be measured by pedometer - is the same? --Seejyb 19:07, 24 April 2006 (UTC)
C operator overloading doubt[edit]
I must write a program in C, which of course doesn't support that feature. The thing is... operator overloading would be extremelly useful since the program performs lots of operations of complex numbers, already defined in a "tool" file written by me. How can I make a*b-sqrt(a^2/2) (for example) intelligible if I have to use a lot of functions? How C programmers got around this problem? Thank you for your time.
- You could just make a function named sqrtCmplx, or something like that, but the other functions, equivalent to *, -, ^, and /, would indeed be ugly. BTW, I would think you could find an include file with complex number functions somewhere, rather than write your own, but since you've already done this part, I suppose it doesn't matter now. StuRat 12:54, 24 April 2006 (UTC)
You will likely need to break the expression up into multiple lines to make it easy to follow with all the new function names. As for making it readable, just include the simple version in a comment, to explain what is being done. StuRat 13:00, 24 April 2006 (UTC)
- I think you'll find that if you use a lot of functions, then they become more intelligible with time. I agree that breaking down will help. Just more typing, it isn't more cryptic really. Notinasnaid 13:10, 24 April 2006 (UTC)
If your compiler supports the C99 standard, your expression could simply be written a*b-csqrt(a*a/2), if you use the complex types from the [complex.h] header. 84.239.128.9 18:53, 24 April 2006 (UTC)
- C99 even gives so called "type generic" math functions in the library, so you can say a*b-sqrt(a*a/2) if you #include <tgmath.h> – b_jonas 23:16, 27 April 2006 (UTC)
Thank you all very much.
- You're welcome. StuRat 13:56, 30 April 2006 (UTC)
Evolutionary Questions[edit]
What is the evolutionary fuction purpose of sleep? It seems like sleep would be a huge disadvantage as it would leave one open to predators. How & why did sleep evolve then? Also, what is the evolutionary purpose of yawning? 199.201.168.100 12:34, 24 April 2006 (UTC)
- Check out our articles on sleep and yawn - they should answer both questions. If there is something you don't understand, feel free to come back here and ask again! :) — QuantumEleven 12:40, 24 April 2006 (UTC)
- I believe sleep developed because animals are specialized to be either diurnal or nocturnal. Diurnal animals (like us) are rather helpless at night without artificial light so spend the time recuperating and using as little energy as possible, during sleep. (An exception is during full moons, when we have enough light so we can go hunting at night, and hence the urge to go kill something during a full moon.) Nocturnal animals are similarly helpless during the day, in that they can be easily spotted by predators, so they sleep then. Some animals have taken the sleep concept a bit further, like cats, and sleep most of the time to limit energy consumption further. StuRat 13:12, 24 April 2006 (UTC)
- Animals that saved energy by lowering their metabolism rate in a daily cycle were more successful than animals that didn't; apparently specializing for either dark or light was more successful. Once sleep existed in animals, animals that utilized this state for various internal functions (healing, reorganizing mental data, mental rehearsing of situations, etc) were more successful at surviving and reproducing than those whose genes less well utilized the sleep cycle. WAS 4.250 18:12, 24 April 2006 (UTC)
- On sleep leaving animals open to predators, I don't think that's very much so. I mean, animals obviously defend themselves against this, either by sleeping in an alert way and getting awake as soon as they hear any noise, or by hiding in a hole or some other safe place when they sleep. – b_jonas 11:00, 25 April 2006 (UTC)
- The brain is extremely active during sleep. It appears something important to the brain is going on, although research into exactly what is ongoing. --Ginkgo100 03:55, 26 April 2006 (UTC)
Another Evolutionary Question[edit]
Why can't I get laid? 199.201.168.100 12:34, 24 April 2006 (UTC)
- Because you spend your time asking questions at the Wikipedia Ref Desk instead of going out and attempting to impress members of your target sex. — QuantumEleven 12:38, 24 April 2006 (UTC)
- ...and it also helps to make friends with them first. Shantavira 13:04, 24 April 2006 (UTC)
- Perhaps you have a back problem? Try sitting down with your legs flat on the floor, and then lean your torso back slowly until it rests on the floor as well. Use your arms for support. Dysprosia 14:38, 24 April 2006 (UTC)
- People get what they want when they tell lots of people what they want and they actively offer what those other people want. Different people want different things. Be clear about who you are and want you want. Listen carefully to what is communicated back both verbally and nonverbally. Some people want an illusion leading some to say lie to get laid; but most people just like a comfortable presentation to go along with the substance - like a meal at a restaurant looking good as well as tasting good and being nutritious. And remember, you have competition to out bid. (as do they) WAS 4.250 18:02, 24 April 2006 (UTC)
- And if what WAS says doesn't work either, it may be that you're unsuccessful in getting laid because you haven't considered attempting to lay, or it may be because you are not a chicken, and never will be. freshgavinΓΛĿЌ 05:29, 26 April 2006 (UTC)
Heat generated by resting human body.[edit]
It appears to me that regardless of the ambient temperature my body generates substantial heat when I sleep. Is this an understood bodily function?
- Your body is only attempting to keep itself at a more-or-less constant temperature (approx. 37°C) - you are a mammal, and mammals are warm-blooded. I would guess that your ambient temperature is very rarely at or above 37°C, so for any ambient temperature lower than that, your body is obviously a net generator of heat. This happens whether you sleep or not. We have a pretty good article on thermoregulation, which should answer most of your questions. If anything isn't clear, feel free to come back for more clarification! :) — QuantumEleven 14:30, 24 April 2006 (UTC)
- Yes, it is well understood. If you too wish to understand, start here: Basal metabolic rate. WAS 4.250 17:53, 24 April 2006 (UTC)
- Thank you very much. I apologize for not searching more diligently; the referenced articles are excellent. --BellCurve 20:26, 24 April 2006 (UTC)
Nutritional value of hemp seed[edit]
The information you provide about nutritional value of hemp seeds(http://en.wikipedia.org/wiki/Hemp) states that 100g of hemp seeds contain 2277.5 IU of vitamin D. This seems extremely hight. I have tried to verify this information and cannot find any data to support it. Can you provide your source of this information? Thank you, Kathy
- I have placed a note in the article for someone to provide a citation or correct this. I have copied your question to Talk:Hemp - which is the discussion page for the Hemp article, which is where the editors of this article are most likely to see it. Thryduulf 15:16, 24 April 2006 (UTC)
Gender Differences[edit]
On a first note, this question probably is going to offend someone out there, and I would apologise dearly if this is so, but I am no troll and only relating of something I have always noticed. How is it that so many modern females may excell far better than the opposite sex in their school studies, yet still produce so few works of true distinguishing excellence in their future careers? And I am not merely speaking of the scientific careers that have long leaned in favour of men, either- even some careers commonly associated with women in school curricula such as the liberal arts, are still dominated in the wider world by men! It is reported that the genders score equally well on IQ tests, but how is it that it always, as seemed to me, far easier to fulfil the higher intellectual curiosities of women than that of equally educated men? Even when given more or less similar opportunities such unusual traits would still sometimes develope. I would be deeply grateful if someone may find a way of understanding these differences. Luthinya 15:00, 24 April 2006 (UTC)
- Do you have any sources for actual statistics on this? It would probably help to show some real numbers, so one could trust the claim. - unsigned
- Just as human males are geneticly taller on average than females, so too males are genetically more agressive and competitive than female humans, while females are genetically more cooperative. Different social structures reward different traits just as differnt sports reward different traits. WAS 4.250 17:46, 24 April 2006 (UTC)
- A possible explanation is the suggestion that women have less capacity for abstract thought, which is required to make truly original and breakthrough achievements. For example, my cognitive psychology textbook in college presented women's inferior capability to rotate 3D images in their heads as fact. In a fairly recent interview, I've also heard a Cambridge University professor state that "women don't have creative minds", although the rest of the interview suggested that he may be quite biased. It's just a possibility to consider, but I can try to find out what the textbook was and where I heard the interview if you need sources for any research you're doing. In the meantime, you may want to have a look at our gender differences aticle if you haven't already. --Aramգուտանգ 18:44, 24 April 2006 (UTC)
- "Abstract thought" is not the same as spatial reasoning. That statement is a crude generalization, and "Women don't have creative minds" even moreso. In the rush to find cognitive reasons for this perceived disparity, I think people are discounting a cultural and/or emotional one: to really excel in a field, a person needs a certain degree of dedication, and it could be that more women than men choose to sacrifice this degree of dedication in favor devoting more time to family life. Ginkgo100 03:50, 25 April 2006 (UTC)
- I (independantly and unjustly) attribute it to two things, one of them being sexism. It's not hard to imagine the difficulties a woman would have had trying to breach the upper levels of intellectual society in the time of Newton or da Vinci, though I don't believe it's nearly as large a factor now as it was before the 20th century.
I also believe, with some evidence, that strong right-brain tendancies are more common in males than in females. Whether that really means anything is another argument, but certain "symptoms" such as left-handedness clearly indicate that women are much more left-brain dominant. If you believe in the all the right/left-brain mumbo-jumbo, then it gives you a nice way to explain why there are more well-known male eccentrics, famous male painters, gay hairdressers, and white-moustached scientists than females. freshgavinΓΛĿЌ 02:42, 25 April 2006 (UTC)- Modern surveys show that woman are more likely to succeed in getting a well paid job, due to all of the feminist movements and so on. A survey was done, where they sent out fake, but identical resumes from two fictional people, it turned out that woman were something like 15% more likely to receive an interview than men of the same qualifications. Philc T+C 16:30, 1 May 2006 (UTC)
- One factor (among several) is that males tend to have more diverse abilities (a greater standard deviation than females), so males do tend to be a little over-represented among the most- (and least-) capable members of society. Peter Grey 05:30, 25 April 2006 (UTC)
- A real problem is that when you look at it from afar, things like "intelligence" and "success" and "ability" seem such obvious terms. But when you look close up, they become very 'fuzzy'. As an old teacher of mine used to say, sharks have been round a few hundred million years. They probably won't consider humans a successful species until about 300 million A.D. or so. Questions such as 'What is intelligence?' are very problematic in this sense, and the articles on intelligence and emotional intelligence will lead you to some of the debate. In the realm of the questions you are asking, a parallel question might be phrased, is it "more successful" in a "future career" to to be more commonly liable to sexual harrassment and workplace bullying, and to die younger from stress, as men do, and then to claim greatness for having bought career progress at such heavy cost? Success is a very arbitrary term. men and women are not only wired slightly differently, but strike slightly different balances between such things as social prestige, family, inward and outward directed motivation, and so on. What success, at what cost, by what standards, ignoring what else, and how? Thats what your question misses and where you may find more of an answer. FT2 (Talk) 19:11, 25 April 2006 (UTC)
- I'm not sure I completely understand the latter part of the question, or what it is based on statistically, whether from social psychology or elsewhere. One possibility is surely that women do produce as much excellence in their work but are not recognised for it, as a result of assumptions made in societies which have only recently achieved roughly equal opportunity for both sexes. You mention science, and an an example here would be Rosalind Franklin, who for decades people were unaware of despite knowing about Francis Crick and James Watson. Similar work by someone like Dorothy Crowfoot Hodgkin cannot be described as anything other than excellent, and is in a field that requires advanced spatial reasoning. Ada Lovelace was overshadowed by Charles Babbage despite what may have been a greater contribution to computing. Women who enter science nowadays may do somewhat better than men on average, simply because the bar to entry is a little higher for them. Even when it was much higher, there were cases like Marie Curie and Lise Meitner that no social convention will hold back. Fine arts, literature and psychology seem to have had more time to mature as disciplines accepted for women, and it is now hard to make a case for differences in excellence there. The idea that for various biological and cultural reasons men can be more competitive, and therefore may tend to appropriate credit to themselves and self-promote, may have something behind the questioner's perception. Gingko100 is presumably also correct in that women may de facto spend more time bringing up children, acting as social glue, and so on, and thus have fewer opportunities to excel or reach a creative peak. --Cedderstk 12:25, 30 April 2006 (UTC)
- The New Yorker had a recent article on the development of U.S. university admissions that might have some application here. Many elite U.S. colleges and universities give special attention to athletes, rather than strictly basing their admissions on academic ability. The reasoning behind this (according to the article) is that athletes tend to have more competitiveness, drive, and ego than your average good student. These qualities end up being better predictors of "superstar" career success than just smarts alone. Generally, men in our society have more competitiveness, drive, and ego than women (whether this is innate or learned, I'm not going to speculate). These qualities lead people to apply their smarts in attention-getting ways. TheSPY 15:52, 1 May 2006 (UTC)
Tri-beveled needles & Anti-Coring[edit]
Hello, I work for a medical supply company, and I am currently doing a presentation on needles. I was wondering what Tri-beveled means as well as Anti-coring. Any help would be greatly appreciated.
Colleen
- For anti-coring, think of an apple corer. An apple corer is like a tube that punctures the apple and holds the apple core inside of it when it is withdrawn. Often it would be undesirable for a medical needle to behave like this; you want it to puncture, but not let tissue enter the needle itself. This also comes up in the context of syringes used for gas chromatography; a needle that takes a core can ruin what used to be a gas tight seal.
- I don't know about tri-beveled. Try the manufacturer's tech support. ike9898 16:33, 24 April 2006 (UTC)
- An anti-coring needle is designed to minimise the punching out of a piece of material (e.g. human tissue) which can then get stuck in the shaft of the needle, either blocking it, or depositing unwanted material at the site of injection, or leaving a punched out hole in the tissue after being withdrawn. The most commonly used medical example is the Tuohy needle, used for epidural anaesthesia.
- A triple-ground, tri-bevel hypodermic needle is one with a bevel which has been ground or honed in three different planes. This is to try to achieve a compromise between cutting ability, rigidity of the cutting tip (a long bevel cuts easily but can bend or curl over at the tip), ease of introducing and advancing the needle (a short bevel feels "blunter" and the needle advances less easily), and distance from the tip of the needle to the start of the tubular shaft. This last factor is important since one does not want the tip of the needle protruding far beyond the tubing part through which the user may wish to draw blood or give an injection.
Look at the tip of one of your thicker needles, you will see the 3 planes: The bevel closest to the hub is at a relatively flat angle in relation to the shaft of the needle (that would be the start of a long bevel). About halfway to the tip of the needle you will see a change in plane (angle of bevel), with two additional ground planes (bevels) starting, one on each side of the main initial bevel, and rotated slightly, so that the very tip of the cutting edge looks triangular, not round or flat. This is the standard grind for hypodermic needles, also called a diamond bevel, or lancet. A well-made standard needle would be expected to have such a bevel (i.e. it shows compliance with accepted standards, and is not a "special feature"), and deviations from this would mostly be for special uses. --Seejyb 20:29, 24 April 2006 (UTC)
Cryogenics[edit]
What was the first country to use cryogenic technology?—The preceding unsigned comment was added by 207.28.159.13 (talk • contribs) .
- I'm not sure; have you looked at Cryogenics? Andrewjuren(talk) 20:49, 24 April 2006 (UTC)
Distribution of mass in Earth[edit]
Hey. So, it seems to be correct that the further into Earth you get, the less gravity is there to pull on you. Therefore, at the correct center, there's a theoretical nil-gravity. My question related to that comes from that the core must be under pressure in order to be so warm and stuff, from the particles above. Does anyone know, in theory, the distribution of MASS in earth? There are all the nice pictures with half or three quarter of a globe, showing what parts are what, but I don't see any explanations for the mass. 213.161.189.107 17:26, 24 April 2006 (UTC) Henning
- The article Structure of the Earth has some information on the density, but not much. You might get better results if you include the phrase "radial density profile" in your search. For example, I found: http://arxiv.org/pdf/astro-ph/9909038 —Keenan Pepper 18:02, 24 April 2006 (UTC)
I have trouble understanding the question, and my main thing is geophysics, etc. Perhaps after you have looked at the above references, we can point you to some other links. --Zeizmic 02:12, 25 April 2006 (UTC)
The Human Genome[edit]
Where can I find the exact sequences for the human genome, and not just a description of the project?
- Just to make sure you're clear, there is no such thing as the "exact" human genome sequence. The genetic sequence varies by individual, otherwise we'd all be clones. To search for sequences within the human genome, look at the External links section of the Human Genome Project. Andrewjuren(talk) 20:44, 24 April 2006 (UTC)
- The ensembl site has these, but you need to know what you are looking for. To me it's like heiroglyphics. Can one say that it will be long before all the data becomes information, or do I misinterpret the situation? --Seejyb 20:56, 24 April 2006 (UTC)
- It's like hieroglyphics to me too, but fortunately, I understand a little of both genomics and reading hieroglyphs. :) - Mgm|(talk) 07:58, 26 April 2006 (UTC)
- Visit our article on the Human Genome Project and look through the external links. There are links to Project Gutenberg's raw text dumps of the entire genome; each chromosome is presented as one 'book'. For an annotated genome, try poking around at Ensembl. (Follow the link and click on Homo sapiens; click on a chromosome and zoom in from there.) TenOfAllTrades(talk) 20:49, 24 April 2006 (UTC)
POOP IS GOOD[edit]
My Question is why is poop brown? Because I poop alot and its always brown but sometimes its a sand color, and what determines the shape of poop? Thanks for any help. —The preceding unsigned comment was added by 68.239.192.119 (talk • contribs) .
- If you had read the article on feces, you would know that they get their brown color from bile and dead red blood cells. —Keenan Pepper 20:43, 24 April 2006 (UTC)
- Human feces is a better place to start. WAS 4.250 21:03, 24 April 2006 (UTC)
- The shape of "poop" is obviously determined by it's passage through the anus, and the texture and consistancy are determined by the character of the present material. freshgavinΓΛĿЌ 02:21, 25 April 2006 (UTC)
- Think of toothpaste. It is the fact you squeeze it out through the hole in the tube that gives it its shape. (Now how will you keep from smiling when next brushing?) Shenme 02:51, 25 April 2006 (UTC)
- Are you sure about that? I'd been under the impression that it was because of the shape of the rectum. It seems too firm to have been reshaped only moments before. Black Carrot 01:52, 26 April 2006 (UTC)
- I understand but could not confirm that the curved shape human feces often has is a result of the curved shape of the rectum. --Ginkgo100 03:27, 26 April 2006 (UTC)
- Actually, it probably depends, like Black Carrot says (I wonder if that name is a reference to this topic), on how firm the module is. I'd say both the rectum and the anus are factors in determining the post-expulsion shape. freshgavinΓΛĿЌ 05:24, 26 April 2006 (UTC)
- I understand but could not confirm that the curved shape human feces often has is a result of the curved shape of the rectum. --Ginkgo100 03:27, 26 April 2006 (UTC)
Brain Map showing Broca's Region[edit]
I'm looking for a simple sketch or drawing of the brain showing basic areas including Broca's Region. Is there a site I can find this and download it?—The preceding unsigned comment was added by 172.185.95.29 (talk • contribs) .
- See the article on Broca's area. Andrewjuren(talk)
- You might also be interested in Brodmann areas (though see [61] for better images). Broca's area comprises areas 44 and 45. Cheers, David Iberri (talk) 00:36, 25 April 2006 (UTC)
Polarized Objects[edit]
Most people are firmiliar with Static Electricity, and most people know about most of it's concepts, right? Well, after looking up a problem in my homework today (Find a definition using any source) I could not find any definition for a popular term "Polarized Objects" anywhere, I have looked in many dictionaries, but it is nowhere to be found. --68.61.181.253 21:04, 24 April 2006 (UTC)Rachel needs Help!
- I am uncertain of what you mean by "polarized object". Perhaps you are referring to a dipole? Or perhaps about polarization? -- Andrewjuren(talk) 21:08, 24 April 2006 (UTC)
- In electrostatics, any section of any object may be polarized; if an object is characterized by a large amount of polarization, or is uniformly polarized, one could say that it was a polarized object. But such polarization is typically a transient thing in response to an electric field; the analog to a permanent magnet, the electret, is very rare. But maybe that's what you want after all? You might also simply mean "charged object". --Tardis 21:50, 24 April 2006 (UTC)
Can you calculate orbits' stregnth?[edit]
I found out about the gravitational formula:
So I calculated the force between the Earth and the Moon (1.89 X 1020 N); the force between the Earth and the Sun (3.55 X 1022 N); and the force between the sun and Alpha Centauri, the nearest star (1.76 X 1017 N).
Since the Earth/sun force is greater than the Earth/moon force, does that mean that the orbit between the Earth and the Sun is stronger than the orbit between the Earth and the Moon? Or does the force get bigger with bigger objects? Jonathan talk ![]() 22:11, 24 April 2006 (UTC)
22:11, 24 April 2006 (UTC)
- Um... "stronger orbit" and "weaker orbit" are not phrases used in celestial mechanics, so I'm not sure what you mean. Could you clarify your question? Melchoir 22:17, 24 April 2006 (UTC)
- There is certainly more force exerted between the Earth and the Sun, as you've found out.. are you asking whether it would be harder to knock the Earth out of the Sun's orbit than the Moon out of the Earth's? The actual size of the object shouldn't really matter in any of this as far as I know, it's just that heavier objects tend to be larger. -- Mithent
- Right, the important exceptions to this tendency being degenerate stars, which actually get smaller as they gain mass. Melchoir 22:44, 24 April 2006 (UTC)
- The orbit is "stronger" in that you need more energy to tear the Earth off the Sun than to tear the Moon off the Earth. This is, however, determined by the potential energy, not by the force. Conscious 08:34, 25 April 2006 (UTC)
- Right, the important exceptions to this tendency being degenerate stars, which actually get smaller as they gain mass. Melchoir 22:44, 24 April 2006 (UTC)
- Conscious is right; the idea of the "strength" of an orbit is most analogous to how tightly bound the objects are. To go into the mathematical nitty-gritty, the potential energy is given by (where U is the potential energy, G is the gravitational constant, and and are the two masses, separated by a distance r). The negative of this is the work you'd need to do against the gravitational force to separate the two objects to "infinity" (but you can think of this as "arbitrarily far away" if infinity bothers you; incidentally, if you've had any exposure to calculus, because gravity is a conservative force this is just the integral of the gravitational force formula). For the Earth-Sun system this works out to J; for Earth-Moon J. So you'd need seventy thousand times as much energy to separate the Earth from the Sun as you would to separate the Moon from the Earth. --Bth 09:21, 25 April 2006 (UTC)
- Since an orbiting object already carries kinetic energy, you only need to provide 50% of that amount. In fact, if you apply it in the satellite's frame of reference, you only need 8.6%. Now, since there's nothing to push against out in space, and the various bodies are moving with respect to one another, there's no Correct Frame from which to measure the energy, and a more physically relevant measure of the "strength" of an orbit is the impulse it takes to break it. As in, how much rocket fuel would it take? But then to go from energy to impulse you'll have to divide by a speed, which will throw off the scaling relationships yet again. Melchoir 10:01, 25 April 2006 (UTC)
Thanks for all the answers, but maybe I should rephrase my question: I just want to know what these numbers mean:
Earth/Sun: (3.55 X 1022 N)
Sun/AC: (1.76 X 1017 N)
I thought maybe I could use this formula to calculate and compare various orbit's strength.
And yes, what I meant by stronger orbit was harder to knock something out of its orbit.
Jonathan talk ![]() 16:13, 25 April 2006 (UTC)
16:13, 25 April 2006 (UTC)
- All that those forces mean is given by Newton's second law; they're just mass times acceleration. The numbers are great for getting an idea of the ridiculous forces at work in the sky, but I'm not sure if comparing them against each other has much of a meaning. Melchoir 16:55, 25 April 2006 (UTC)
- The formula you quote at the beginning, is the formula for the force two point objects (bodies) exert upon each other. So for example, the earth exerts a force of 1.89 X 1020 N on the sun, and the sun exerts a force of 1.89 X 1020 N on the earth. This force, not coincidentally, is also the force needed to keep them in an orbit about each other, which is why they have come to orbit at the exact distance they do - because its the distance where the force needed to orbit is the same as the force they exert on each other.
- The "how hard to knock out of an orbit" is a different question. As you peturb an orbit it becomes more and more eccentric, and irregular, or unstable. any amount of force applied to one or the other will affect their orbit. To "knock them out of orbit" however, the easiest way to think of it is, how much work would it take, to completely separate them, to make them move completely apart and not remain in an orbit of any kind. The velocity needed for that is called escape velocity, same as for spaceships, and so a better answer is a matter of the energy required to do so, so it will be measured in Joules not Newtons. In other words, rather than measuring the force needed to change an orbit (because any force changes an orbit anyhow), scientists prefer to ask what is the amount of work (or energy) that must be added to the system to cause the two to be able to achieve separation. FT2 (Talk) 18:59, 25 April 2006 (UTC)
How to plot simple graphics in X-windows[edit]
How do I get a 13 year old student to plot a simple graphics on X-windows on linux? He wanted to be able to plot y=sin(X*2*PI/200) on a window in X-windows. At the moment he is using gnuplot to plot it but we wonder how to do it directly to a window without using gnuplot. Can it be done in C ? Python? Ohanian 23:05, 24 April 2006 (UTC)
- To my knowledge, there is no easier way to plot graphs on X than gnuplot. --Kainaw (talk) 00:08, 25 April 2006 (UTC)
- I'm not sure if I know one, but I hope there is. Gnuplot, for all it's good sides, is not exactly a paradigm of user-friendliness. --BluePlatypus 00:28, 25 April 2006 (UTC)
- Probably want Octave or Maxima. - 128.32.48.131 01:44, 25 April 2006 (UTC)
- See also Wikipedia:Reference_desk_archive/Mathematics/March_2006#Graphing. – b_jonas 23:09, 27 April 2006 (UTC)
For my son, we found a Java function plotting program. Did all the sin functions, etc. --Zeizmic 01:48, 25 April 2006 (UTC)
An excellent age to begin programming for the X Window System. To answer your question, the lowest level interface to the X protocol is Xlib, a C library. There are a number of tutorials on Kenton Lee's X Window System site. Someone who could use a C compiler and gnuplot could probably copy one of the sample programs and alter it to graph a function.
There are a number of widget toolkits built on top of Xlib, with bindings for various languages. Widgets are for building GUIs, but most would also allow for drawing graphics primitives (the points and lines that make up your graph) directly to an X window. Another way to render graphics on a linux box would be to use OpenGL and bypass the X server (if the Direct Rendering Infrastructure is available.) Have fun. EricR 02:25, 25 April 2006 (UTC)
April 25[edit]
Fractals and turbulance[edit]
Is there a link between fractals and turbulance? Turbulant patterns seem to be fractal but turbulance is supposed to be chaotic, is this true?
- Well, Fractal, Chaos Theory and Turbulence all link to one another. And yes, I believe there's a strong connection. Black Carrot 01:26, 25 April 2006 (UTC)
- Fractals and turbulance are both examples of chaos, at least in the mathematical sense. Peter Grey 05:34, 25 April 2006 (UTC)
- As I recall, VOB files are already in MPEG format. Try renaming them to .MPG and see if they play. If so, then virtualdub-mpeg website can directly recode them to AVI format in any codec or compression you choose. FT2 (Talk) 01:53, 26 April 2006 (UTC)
Do fish have lips?[edit]
does anybody know? I'm trying to settle a bet.
- If you are referring to an opening around the mouth, yes, fish have lips. If you are referring to a ring of muscles around the mouth opening, most fish have lips. Some have hardened beak-like openings for smashing coral. If you are referring to a thin area of skin with excessive nerve endings, most fish do not have lips. If you simply mean a colored region around the mouth, many of the tropical fish have brightly colored lips - some are even pink. Of note: the product "fish lips" I've found in Asian markets is actually just shark skin (at least that is what the merchants have told me). --Kainaw (talk) 01:15, 25 April 2006 (UTC)
- My attempts to kiss fish have been somewhat unsuccessful; however, this could possibly be due to a lack of desire on the part of the fish, rather than any deficiency they may have in the lip department. Phileas 06:51, 25 April 2006 (UTC)
the date of creation[edit]
I have calculated the times of more than a dozen comets and they trace back to one year, this was 7205 years ago. I also found this was the same date the bible has hidden since St Jerome wrote the Vulgate in about 384 AD.These are some of the comets Haley-95 orbits of 75.63 years. Hale/Bopp had 3 orbits of 2398.66666 years, Swift-Tuttle(109p) orbited 55 times of 130.745 years, and Schwassmann-Wachmann(73p) will orbit 1327 times by next month with an orbit time of 5.42963 years. I have other proof that the earth is but 7205 years old.
- Homosayswhat? Black Carrot 01:22, 25 April 2006 (UTC)
- Bigotsayswhat? - 18:15, 25 April 2006 (UTC)
- On a more generous note, have you tried calculating where all of those comets were 7205 years ago? If they appear to have originated from the same point, that could be a scientific discovery worth publishing. Good luck! Melchoir 01:29, 25 April 2006 (UTC)
- Oh yes. I remember it like it was 7205 years ago. I was just getting up for a morning bowl of raisin bran and I couldn't find the damn light. Then a lovely fellow - Oh, what was his name? Something like Yahoo or No Way or Yahweh - anyway, he just said "Let there be light" and I heard him flip a switch and the light turned on. I acted very impressed, but I remembered changing the bulb just a couple days earlier. I asked him to join me anyway. I was feeling very foggy from a hard day's night. I mentioned it to him and he said he'd make a song out of it later when he got around to learning the drums. Then, he gave me a couple pills and the foggy void separated into a clear view of the heavenly sky above and a firm earth below my feet. I poured him a bowl and he got all weird about it. He kept separating the piles of bran into little mounds with oceans of milk between them. He pushed the raisins in and commented how they bobbed around like little fish. I agreed, but it was getting rather trying about this time. Anyway, to make a long story short, I asked him if he remembered the date of creation. "Sure," he said. It was exactly 6.3 billion years before that. He remembered it just like it was 6.3 billion years ago. He was just getting up for a morning bowl of raisin bran... --Kainaw (talk) 01:30, 25 April 2006 (UTC)
- 7,205 years ago, yes. And on a Thursday. I never could get the hang of Thursdays. -- Filliam H Muffman 02:05, 25 April 2006 (UTC)
- Actually... assuming our unsigned friend calculated the orbits on the same day that Mr. Black Carrot replied, then the earth started on a Wednesday (5199 BCE). I'm a little confused about his calculations though. What does the origin of comets have to do with the age of the earth? I'd rather give him the benefit of the doubt and have him come back and explain his theories. freshgavinΓΛĿЌ 02:16, 25 April 2006 (UTC)
- To suitly emphazi, that's 5199 BCE if you use a year zero, 5200 BCE if you don't. -- Filliam H Muffman 02:53, 25 April 2006 (UTC)
- Damn. And I feel bad for attempting to block you from using a H2G2 quote too. freshgavinΓΛĿЌ 05:16, 26 April 2006 (UTC)
- What exactly are the times of more than a dozen comets? Peter Grey 05:37, 25 April 2006 (UTC)
- Congratulations you've managed to calculate the age of recorded history. Now use your awesome powers of deduction to resolve your date of Creation with the fact that Radio Carbon-14 dating which breaks down to Carbon-12 at a fixed rate, and is renewed in living organisms dates bones and plants over 10,000 years. Not to mention several other radioactive isotopes which also have fixed rates of radioactive decay have dated rocks at around 4,000,000,000 years of age. --Tollwutig 14:24, 25 April 2006 (UTC)
Strings[edit]
Are thre anti-strings? —The preceding unsigned comment was added by 207.255.31.124 (talk • contribs) .
- Your question needs more context before we can answer it. What are you talking about? —Keenan Pepper 02:55, 25 April 2006 (UTC)
- If you're asking whether string theory includes antimatter states, the answer is yes. -lethe talk + 03:11, 25 April 2006 (UTC)
- The woodwind section is often very anti-string, if that's any help. Grutness...wha? 03:20, 25 April 2006 (UTC)
- They're really just acting that way to hide the uncontrollable physical attraction. Melchoir 03:27, 25 April 2006 (UTC)
Aluminum- going from an ion to an atom[edit]
How many electrons does aluminum gain by going from an ion to an atom? —The preceding unsigned comment was added by 67.150.42.98 (talk • contribs) 03:19, 25 April 2006.
- If you want to know how many electrons an aluminum ion will gain, you should look at it in reverse: how many electrons does an aluminum atom loose to become an ion? Because it is in group III of the periodic table, it has three valence electrons that are readily removed from the atom. When this happens, it becomes a Al3+ ion, so the answer is three. Hope this helps.--Chris 03:23, 25 April 2006 (UTC)
Bauxite[edit]
How is bauxite turned into a metal? —The preceding unsigned comment was added by 67.150.42.98 (talk • contribs) 03:29, 25 April 2006.
- Bayer process Melchoir 03:33, 25 April 2006 (UTC)
- And then Hall-Héroult process. Melchoir 03:33, 25 April 2006 (UTC)
thanks!--Ll10398 03:45, 25 April 2006 (UTC)lily
Properties of Aluminum[edit]
What are 10 different properties that make aluminum than other metals? —The preceding unsigned comment was added by 67.150.42.98 (talk • contribs) 03:36, 25 April 2006.
- Ahem: Do your own homework. If you need help with a specific part or concept of your homework, feel free to ask, but please do not post entire homework questions and expect us to give you the answers. Melchoir 03:37, 25 April 2006 (UTC)
- Are you writing an essay about aluminum or something? Did everyone in your class get their own element? Anyway, I'm sure there are at least ten interesting properties in the article Aluminium, so don't bother us anymore unless you really search and still can't find what you're looking for. —Keenan Pepper 03:51, 25 April 2006 (UTC)
- Research is useful...--Tollwutig 14:27, 25 April 2006 (UTC)
would like to know anything about Arlington Chem Co. circa 1890s.[edit]
I would like to find out anything about Arlington Chem Co. circa 1890s if anyone knows anything I would be grateful.
- start here WAS 4.250 15:56, 25 April 2006 (UTC)
- "John Andrus (1841-1934) was born in Pleasantville, New York, graduated from Wesleyan University and soon moved from teaching school to pursuing his talents as an investor and businessman. His primary operating business, the Arlington Chemical Company, manufactured typical medicines of the late 1800s and distributed them worldwide." [62] WAS 4.250
Physics Dilemma[edit]
I have a question that's based on theory of physics.
Suppose you have a person of a certain weigh in a bucket where he/she is pulling themselves up by tugging on the other rope. So that's a pulley, he/she is on one side in the bucket, and the rope is over the pulley, and then they pull the other end, just to clarify.
So, my physics teacher told me that if the person was to pull at the force of gravity on the person going up, that the person would go up in a constant speed, no acceleration. So, if the tugging action is equal to the weight, it moves up in a constant speed. But how would you pull yourself have way up for example, and then stay in that position? What force is keeping you balanced? She told it was the same force, except that the rope was exerting the force up when it was moving, and then when it was motionless, that the same force was being directed into a different direction. Class was over and I didn't get the last bit.
So if someone could explain this well as they can it would be greatly appreciated. Thanks a lot.
C-c-c-c 05:11, 25 April 2006 (UTC)
- If all the forces on a body sum to zero, then it doesn't accelerate; this is universally true whether the body is moving or not. If you're not accelerating, then the rope/bucket must be exerting an upward force equal in magnitude to your weight. It'll never be in any other direction.
- On a different note, "force of gravity" is misleading. Sitting in the bucket, you don't have to pull the other side of the rope down with a force equal to your own weight; you only need to exert half of that force. This example is actually touched on at Mechanical advantage. Melchoir 05:35, 25 April 2006 (UTC)
Thanks, but I was wondering if you could explain the idea behind that, why would it be half your weight? I've never seen any of those equations in the link you put up. Thanks again.
C-c-c-c 05:47, 25 April 2006 (UTC)
- Hmm, interesting. I haven't thought about this problem before. Assume that you are standing in a bucket. There is a rope tied to the bucket, looped over a fixed pulley, and which passes by you again on its way down. You're holding on to the rope.
- For our example, all forces will be in the vertical direction; assume negative forces point down. The force of gravity on you (your weight) will be -m·g: your mass m times the gravitational acceleration g.
- If you pull on the rope with a force f, you'll be directly exerting that force to lift your body. In addition – and this is the important trick – the rope (in tension) will be exerting an additional force f. To remain steady, the forces acting on you must sum to zero, so:
- 0 = -m·g + f + f
- f = (1/2)·m·g
- Conceptually, you could also think of it has being a bucket with a rope looped over a pulley where both ends of the rope are tied to the bucket. (Here, your arms just act as the knot that ties the other end of the rope down.) Your weight is supported by two ropes; each rope holds half the weight. TenOfAllTrades(talk) 06:05, 25 April 2006 (UTC)
Wow! That actually makes sense! And it's so simple too, but not obvious at all at first. Thanks a lot!C-c-c-c 06:18, 25 April 2006 (UTC)
what's definition of tension?[edit]
in the question above why's it 0 = =mg + f + f & not mg - (f+f) because the tension is opposite direction of gravity rite? what's tension exactly how would u define it. also its always opposite of gravity rite? or no? can some1 explain thanks 06:37, 25 April 2006 (UTC)
- Tension is the force experienced when a rope is pulled, and is equal and opposite at the ends. If something is hanging from a rope, then tension is balancing the vertical force of gravity. Try a free body diagram of a section of the rope. Peter Grey 06:52, 25 April 2006 (UTC)
Psychiatrist's view of a religious person[edit]
Assume for a moment that people believing in a god isn't a common thing. Instead it's the kind of thing that may make someone proclaim, "That there is crazy talk!". With that in mind what would a psychiatrist's diagnosis be of a person who believes in an omniscient and omnipotent god who nobody has ever been able to prove exists? Dismas|(talk) 08:02, 25 April 2006 (UTC)
- There are people who believe in aliens amongst us, but mostly they're just living otherwise normal lives. In your scenario, people believing in a god might be regarded as eccentric, but not requiring treatment unless they believe their god requires them to, for example, kill all non-believers.-gadfium 08:09, 25 April 2006 (UTC)
- It would depend on the patient's behavior. Example: saying Heaven is better than Earth is not considered "crazy"; but trying to go there now on purpose because it is better (by suicide) is considered crazy. WAS 4.250 15:38, 25 April 2006 (UTC)
- Under the DSM-IV, probably "delusional disorder". The current definition of the symptom is a "fixed false belief, excluding those that are part of a religious movement". If you remove that religious caveat, you have a delusion, a symptom of psychosis, and the diagnosis would depend on the presense or absense of other symptoms (such as hallucinations). In the absense of other symptoms, probably "delusional disorder" would win the day. - Nunh-huh 18:13, 25 April 2006 (UTC)
- Of course, the other opposite question arises. Mystics would consider that not being aware of more than the consensus reality we see around us, is somewhere between ignorance, delusion and illusion too. FT2 (Talk) 18:42, 25 April 2006 (UTC)
- Fortunately, mystics can't civilly commit anyone, so their opinion matters mainly to themselves. - Nunh-huh 20:07, 25 April 2006 (UTC)
- Of course, the other opposite question arises. Mystics would consider that not being aware of more than the consensus reality we see around us, is somewhere between ignorance, delusion and illusion too. FT2 (Talk) 18:42, 25 April 2006 (UTC)
Insane Psychiatrist[edit]
Is it true that an insane person isn't allowed to study psychiatry/psychology? --Username132 (talk) 11:32, 25 April 2006 (UTC)
- That is not true. WAS 4.250 15:38, 25 April 2006 (UTC)
- Recently I came across a study which suggested that an above-average percentage of those studying psychology is made up of mentally instable persons, however you define that; a number mentioned was 60%, whatever that is worth. I have also been told that the curriculum is made up so as to not appeal to that type of person who wants to get in because he is obsessed with his own disorders, at least at my university. Apparently they try to bore them out :D --Dr. Zarkov 17:27, 25 April 2006 (UTC)
- For what it's worth, I'm currently applying for a master's degree program in clinical psychology, and so far there's been no mention of psychological screening for applicants. --Ginkgo100 03:18, 26 April 2006 (UTC)
I'd be very skeptical of this rumor. It has urban legend written all over it. Bhumiya (said/done) 04:46, 26 April 2006 (UTC)
- You might not be allowed to practice phsychiatry, but I don't see why they should stop insane people from studying it. - Mgm|(talk) 08:03, 26 April 2006 (UTC)
This is NOT true. My girlfriend is majoring in psychology. 12.183.203.184 04:20, 27 April 2006 (UTC)
Netherlands And Rising Sea Levels[edit]
Based on the threat of rising sea levels and the Dutch government's plan to give some land to the sea in coming decades, would you agree that property in The Netherlands would be a bad investment? Culturewise, what where would be the next best place? Belgium? -Username132 (talk) 08:06, 25 April 2006 (UTC)
- I have not heard about this, but I suppose waterfront property may not be the best investment. Nevertheless, as we have seen in Vancouver, BC, Canada, when space is limited, prices go WAY UP, so investment would be a good thing. As for the culture... that's hardly a scientific question. Andrewjuren(talk) 08:21, 25 April 2006 (UTC)
- The threat is unknown. Sea level is largely dependent on local pressure, temperature, salinity, etc. Some parts of the ice on Earth are melting, some are freezing, some are not doing anything, and some are just moving. --
 Mac Davis] ⌇☢ ญƛ. 10:44, 25 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 10:44, 25 April 2006 (UTC)
- Sorry, MacDavis, I have to disagree with you there. The threat is definitely there, take a look at sea level and sea level rise, especially the first graph on both pages. Sea level change is global (because water is a liquid and so spreads itself out evenly), and it is definitely rising, there is no debate about this - satellites can measure sea level to an accuracy of a millimeter, and data collected over the past decades shows a steady rise of about 3mm a year (see here). There are several low-lying islands in the Pacific which have seen their land area greatly reduced over the last fifty years because of this. The question is how much it will rise, but even the lowest estimates put a significant strain on the Dutch sea defences over the next decades. — QuantumEleven 11:44, 25 April 2006 (UTC)
- While it is a concern, do not forget that the Netherlands is already for over 50% below sea level, and dykes, levies and dunes have kept it dry in the past few centuries with only one major flooding(The one in zeeland in 1953). Most prognosis for the rise of sea level talk about 30cm to 70cm until 2100, something that should be manageable, although quite costly. Do not confuse this with the other waterworks project currently going on, which is to create basins for the 3 major rivers because altered weather has increased the chances of flooding from those. However, i'm not sure i'd advice anyone to invest in property in the Netherlands, since 60 years of housing shortages have pushed house and land prices to very high levels. As for what are related cultures, i'd guess that Belgium, Germany and the scandivian countries would probably be closest. SanderJK 11:56, 25 April 2006 (UTC)
- This is getting slightly offtopic, but you might be wise to look at this article before you think too hard about buying investment property anywhere...--Robert Merkel 05:05, 26 April 2006 (UTC)
Refering To A Research Group[edit]
During my reading I've read of a research group refered to as the "Pabo group", being lead by someone called 'Pabo'. I need to refer to three papers published by
DAVID J. SEGAL, BIRGIT DREIER, ROGER R. BEERLI, AND CARLOS F. BARBAS III
Birgit Dreier, Roberta P. Fuller, David J. Segal, Caren V. Lund, Pilar Blancafort, Adrian Huber, Beate Koksch, and Carlos F. Barbas III
Birgit Dreier, Roger R. Beerli, David J. Segal, Jessica D. Flippin, and Carlos F. Barbas III
Segal and Drier occur in all instances, but some of the work was carried out at more than one laboratory. So can I refer to a specific group? --Username132 (talk) 09:36, 25 April 2006 (UTC)
- Hrm. I'd say something like "Segal, Dreier and collaborators (Segal et al XXXX, Dreier et al YYYY, Dreier et al ZZZZ) [did whatever they did]" where XXXX etc are years (if YYYY=ZZZZ then use YYYYa and YYYYb). If I needed to refer to the same work repeatedly I'd do "Segal, Dreier and collaborators (Segal et al XXXX [SXX], Dreier et al YYYY [DYY], Dreier et al ZZZZ [DZZ])" at the first appearance and use the abbreviations I'd established thereafter to refer to whichever paper was relevant for the point I was making, probably treating the abbreviation as I would the names written out in full (eg, "DZZ showed [some exciting result]" for "Dreier et al (ZZZZ) showed ..."). But that's because I'm used to (the astronomy variant of) Chicago-style science citation; I don't know how similar that is to biochem. I'd be wary of deciding that they were "the Someone group" unless they're already widely referred to as that and/or one of the people is very clearly much more senior. --Bth 12:25, 25 April 2006 (UTC), not logged in
- Scientific publications list the group leader as the corresponding author, who is typically marked with an asterisk. Dr Zak 20:20, 25 April 2006 (UTC)
If there is one person marked out to whom correspondence should be sent, would it be correct to assume that they were the group leader? -Username132 (talk) 05:04, 27 April 2006 (UTC)
Microprocessor Programming[edit]
I am currently syuding microprocessor programming.We can multiply two numbers by repeated addition of one number; other number of times.But then how does the scientific calculator do decimal multiplication and all as the numbers dont consist of integers??Ex 2*3=2+2+2 or 3+3 but 2.3*5.6???
- A calculator will store decimal numbers as either fixed point or floating point. These are special ways to store information about a number. Fixed point allows a certain fixed number of decimal places, while floating point stores an exponent and mantissa. If a microprocessor chip does not include direct support for floating point, it is a huge project to create it, but the principles are fairly simple, so long as you understand what the arithmetic rules would be for arithmetic on two numbers in exponent and mantissa form. Notinasnaid 12:25, 25 April 2006 (UTC)
- Multiplication algorithms are executed by Arithmetic logic units. WAS 4.250 15:30, 25 April 2006 (UTC)
- Repeated addition is about the worst method of multiplication. Long multiplication is very simple in binary and this is the method most computers use for normal multiplication. It can be implemented in either hardware or software (software being the norm on some very low end microcontrollers hardware on anything else)
- As for non-integer types the basic idea is you multiply the mantissas and add the exponents. then if nessacery you normalise.
- e.g. in decimal (most computers would do this in binary but the principles stay the same) 2.3*5.6 = 23*10-1*56*10-1=23*56*10-2 Plugwash 15:42, 25 April 2006 (UTC)
The answer is it depends on the calculator. It's important to distinguish between integer/fixed point/floating point representation, binary/decimal representation, and for decimal the encoding of digits (BCD, Densely Packed Decimal, Chen-Ho encoding). For instance, a floating point number may be stored with a binary integer significand and densely packed decimal integer exponent. Also keep in mind that a number may be represented in one form, but arithmetic may be performed in another, see: Intel BCD opcodes.
If you are looking at a scientific calculator, you are probably dealing with some kind of floating point decimal and IBM has a great site with links about decimal representations and arithmetic. EricR 16:32, 25 April 2006 (UTC)
The representation of a number can undergo many changes prior to and after the multiplication. I once saw a computer program that converted numbers back and forth between ASCII, BCD, and binary as the data passed between subroutines written by diferent people and this was just for small integers! A handcrafted program in assembly I saw went right from the keyboard-key-identify-buffer-contents passed by the keyboard-control chip to binary integer representation it sent to a multiplication-register in the CPU chip. (ok, ok, "right from" condenses a lot of get interupt, get byte & store, type detail...) WAS 4.250 18:30, 25 April 2006 (UTC)
- See also the chapter 4.2 (floating-point) in Knuth's The Art of Computer Programming (it's in volume 2). It explains how you can calculate 2.3*5.6 based on integer arithmetic. – b_jonas 22:54, 27 April 2006 (UTC)
Anyone run a website?[edit]
That is not computer related (and is in English). If you have a counter on the front page, I would like to know what statistics you have on the various operating systems that computers visiting your website run. I write a blog, but have contaminated the stats by visiting it myself. Presumably your site will not be contaminated to any large degree, if you don't visit it often or have a large number of visitors. Thanks heaps.
- The last data we seem to have on Wikipedia can be found here, but this is over two years old - Wikimedia hasn't has the stats accessible for some time. However, this might be interesting for you, on a global web perspective: Usage share of web browsers. -- Mithent 13:36, 25 April 2006 (UTC)
- I have a website [[63]] that uses a free counter by sitemeter.com. An example of statistics it generates from hits from my page are here [[64]]. - Cybergoth 21:48, 25 April 2006 (UTC)
- Thanks Cybergoth, that's exactly what I'm after. Didn't realise visitors could see other webcounters stats, very handy. Bladerunner is in my top 3 films, although I haven't ever seen The Directors Cut, never will, think it will ruin my enjoyment of the film. 218.101.92.181 22:36, 25 April 2006 (UTC)
I have a tracker on my site from eXtreme Tracking, but I'm pretty sure my results are skewed due to small sample size and a selected population [geeks mostly :P]. You can see it here though. — Ilyanep (Talk) 23:27, 25 April 2006 (UTC)
- I never knew that you could view site stats for another site, now I know that you can seek out (ie google) specific sites running extreme tracking (high levels of resolution) and see what sort of bias the site is getting. Yes lynanep, you do seem to have a geek bias, Linux users are about 4 times the proportion at your site than another site which is a blog about someone's thoughts. I was in a round about way wanting to know Linux market share (of web browsers) and the other site gives 0.65%. That means the only way is up.218.101.92.181 01:08, 26 April 2006 (UTC)
Bohrs radius in Plancks units.[edit]
Dears,
In line 34 of the sub-article "Plancks units and the invariant scaling of nature" of the main-article of "Plancks units" is after the normal equation of Bohrs radius used in NIST's CODATA and Wikipedia article about Bohrs radius an other equation in Plancks units namely:
a0 = mp.lp / (me.alpha)
whereby mp and lp are Plancks mass and length, me is the electron mass and alpha is 1/137.
My question is where does this equation come from and what is its derivation from the "normal" equation?
Boy Boer Breda the Netherlands
- It's a simple set of substitutions. Starting from the Bohr radius , we substitute in the fine structure constant to get . Now, from the definitions of the Planck units and we get , which we substitute in to get as you wanted. --Bth 14:37, 25 April 2006 (UTC), still not logged in
- Ah, you beat me to it. Here's the same in Gauss units: Conscious 14:48, 25 April 2006 (UTC)
- I have to say, I'm rather dubious about the claim in the Planck units article that this definition of is somehow fundamental, dimensionless, etc. -- the mass of the electron still sneaks in as a parameter, which isn't a combination of the five constants used as the basis of the system. Indeed, the equation explicitly uses the mass which is such a combination. --Bth 07:15, 26 April 2006 (UTC)
Electron acceptors for photosystems[edit]
Okay, I give up on trying to find this. Does anyone know if potassium ferricyanide is an electron acceptor for photosystems I and II, or only photosystem I? I believe that phenyl quinone is an acceptor for only photosystem II, though I could be wrong. Thanks in advance, if anyone can tell me! -- Mithent 13:54, 25 April 2006 (UTC)
- I'm not sure what you're asking? If K3Fe(CN)6 can oxidize PS I/II? That would depend entirely on the redox state of the enzyme. There's plenty of experimental data on the redox potentials of PS I and II out there. --BluePlatypus 20:25, 25 April 2006 (UTC)
Calculating escape velocity - simpler method[edit]
In Y.Perelman's book 'physics for entertainment, he shows a much simpler way of calculating the escape velocity. It goes somewhat like this:
Stand on a hill of height say h metres. Throw a ball horizontally with a velocity v. The ball will fall towards earth. In 1 second, the ball would have fallen 4.9 meters [s=ut+(1/2)at^2]. If it is still h metres from the surface of earth (i.e. if it has fallen just enough to follow the curvature of earth), then v is the escape velocity.
I don't remember the exact argument and I am not able to derive it. I try and end up with a velocity of about 7.9 km/sec. Can anybody tell me where I am going wrong ? Do you know how he derives it ? Wikicheng 19:41, 25 April 2006 (UTC)
- It sounds like that v is actually the circular-orbit velocity, not the escape velocity. As you've discovered, the two differ by a factor of sqrt(2). But to order of magnitude, an estimate of one is an estimate of the other. Melchoir 19:49, 25 April 2006 (UTC)
- Also, for good accuracy you'll need to consider an infinitessimal time instead of "1 second", because that standard gravitational acceleration expression applies at finite time only when the velocities are small (so the orbit is effectively parabolic). --Tardis 19:51, 25 April 2006 (UTC)
Even if I use the time of 0.1 sec, I still get about 7.9 km/s. Looks like Melchoir's is right. 7.9*sqrt(2)=11.2 approx. Anyone who has read this in the above mentioned book? -- Wikicheng 20:12, 25 April 2006 (UTC)
Black hole horizon problems[edit]
I get the feeling that these are a common misunderstanding-driven question, but oh well.
- Objects falling into a black hole will redshift out of visibility, and appear to slow to a stop as they approach the event horizon. If it takes an infinite amount of time (as viewed from far away) for anything to actually fall in, how is the black hole ever observed to grow (or even to really form, for that matter)?
- Similarly, if I recall correctly, an object falling into a black hole will have its entire rest mass released as lost gravitational potential energy. Since rest mass is invariant, shouldn't the object be able to observe its own energy loss -- down, even, to 0 energy at the event horizon?
- I saw recently (at photon sphere) that the circular orbital velocity at 1.5 radii is c; obviously the escape velocity is less than c there. So is it yet another GR revelation that with sufficiently intense gravity, the ratio of orbital velocity to escape velocity crosses 1?
GR is really too much; thanks for any clarification. --Tardis 20:08, 25 April 2006 (UTC)
- For #3, isn't the escape velocity always less than any orbital velocity? —Keenan Pepper 20:54, 25 April 2006 (UTC)
- Never mind me, I don't know what I'm talking about. —Keenan Pepper 21:02, 25 April 2006 (UTC)
Point 3 is really strange; I'd like to hear about that too. That must mean that (differently from the Newtonian case) the speed required to escape to infinity from near a black hole depends on direction. Which of course in the extreme case is obvious; if your perinigricon is below the event horizon, you ain't coming out.
What happens when perinigricon is near the event horizon, but above it? Are you accelerated to relativistic speeds? Do you lose significant energy to gravitational radiation, which might drop you below the event horizon on a trajectory that you thought was safe, if you hadn't taken that energy loss into account? --Trovatore 22:36, 25 April 2006 (UTC)
- The physics outside a black hole are actually qualitatively not too different from the Newtonian case. There's still a conserved energy and angular momentum, and you can construct a one-dimensional potential that describes the radial motion of a test particle. The major difference is that this potential no longer has an infinite centrifugal barrier at r=0; instead it goes to negative infinity at r=2. (G=M=c=1) If the particle has an angular momentum greater than sqrt(12), the potential has a maximum somewhere between r=3 and r=6.
- So no particle can escape with a peringricon less than r=3, which is apparently called the photon sphere. If you cross that photon sphere in the wrong direction, you're not coming out unless you light the fires. (And, of course, if you cross r=2 you're not coming out no matter what.) Melchoir 23:24, 25 April 2006 (UTC)
- A more qualitative way of putting this is, "closer than the photon sphere, moving tangentially around the hole pushes you in, instead of out". You get very interesting optical effects too, due to the way light rays curve (the black hole looks like it takes up more than half the sky, with your view of the universe looking like a ball). --Christopher Thomas 21:46, 26 April 2006 (UTC)
- As to the first two points, my extremely limited understanding is as follows:
- 1) We need a consistent definition of "fall in" here, I think. Crossing the event horizon counts, as far as I can see. As such, when something hits it, even if it takes an infinite time for us to observe the falling-in, it's already part of the mass of the black hole from the POV of the gravitational effects of the hole. AIUI, if it weren't for the infinite redshift part, a black hole would maintain the appearance of the last sphere of star material to cross into it during formation indefinitely. (But of course we've never actually observed any of this directly.)
- I had thought of this explanation; the problem I have with it is that the black hole could then become asymmetric if, say, everything were to fall in on one side. That seems to violate the no hair theorem. --Tardis 15:47, 26 April 2006 (UTC)
- 2) This is one of those things that boils down to "the far-away and falling-in viewpoints see the same physics from very different perspectives". IIRC, it's been shown that for a black hole with minimal tidal forces (eg the supermassive ones in galactic cores) the observer crossing into the black hole barely notices the point of crossing the event horizon, so it seems they wouldn't get to notice their energy dropping to zero. However, they are now in a situation where all their possible worldlines contain a singularity in the future. But from the perspective of an external observer, what happens to them is forever inaccessible, shrouded by the event horizon, and so their mass has just become part of that of the black hole.
- Most of that probably makes no sense, and anything that does make sense is probably wrong. --Bth 07:35, 26 April 2006 (UTC)
Physics of cutting[edit]
It says on the article on cutting, that an object can be cut with an appropriately hard tool with such a force, that the resultant stress exceeds the strength of the material in question - "The simplest applicable equation is stress = force/area".
Is it possible to arrive at a lower limit for the said area? I.e. to the point, where you are cutting just one atom wide? (and at arbitary length)
One could use the equation stated above to calculate the minimum energy requirements to cut through an object, supposing that you could use an impossibly sharp tool. In such a case, would there be other factors to consider, in addition to strength of the material and the area being cut?
This is my curiosity working again. Thanks for any responses. Santtus 20:58, 25 April 2006 (UTC)
- Material properties such as strength are based in continuum mechanics, and don't apply on length scales not much larger than atoms. Moreover, it's very hard to define an area of contact when the edge is essentially one-dimensional, so the concepts of pressure and stress are not very applicable at all. Still, it would be possible to cut with a monolayer, although the knife would be quickly blunted by abrasion even if it were harder than the substrate -- one line of atoms cannot have the strength (hardness) associated with the bulk material. If the knife survived, it would indeed require very little force to make the cut. The difficulty might be expected to depend on whether one was cutting "between" atoms or "through" them with an edge that small, but the amount of energy required to shift and/or compress the material by half a bond length would probably be negligible compared to the energy required to break the bond. --Tardis 21:55, 25 April 2006 (UTC)
- Thanks! So I'll be looking at bond energy and similar concepts, maybe measured in J/mol, or something like that. I'll look around. With few other numbers I can convert it into J/m^2 of cross section of area cut with the "perfect knife". I got suddenly interested of how far the current technology is from the "perfect" in that area, and I wanted to calculate that myself. Maybe I'll get a somewhat accurate estimate now. Santtus 00:15, 26 April 2006 (UTC)
- And then you can get to work on a prototype of those laser whips they had in Johnny Mnemonic. freshgavinΓΛĿЌ 05:00, 26 April 2006 (UTC)
- Remember that a lot of the effort involved in cutting, say, cheese is friction between the substrate and the sides of the knife. This is one reason (along with uniformity of slice thickness) why some cheese slicers use wires, and it might affect your judgment of what counts as a "perfect knife". --Tardis 16:14, 26 April 2006 (UTC)
- Thanks! So I'll be looking at bond energy and similar concepts, maybe measured in J/mol, or something like that. I'll look around. With few other numbers I can convert it into J/m^2 of cross section of area cut with the "perfect knife". I got suddenly interested of how far the current technology is from the "perfect" in that area, and I wanted to calculate that myself. Maybe I'll get a somewhat accurate estimate now. Santtus 00:15, 26 April 2006 (UTC)
Can electricity be converted to kinetic energy??[edit]
I heard that electromagnetic energy can be converted into kinetic energy and move things around. If this is true how come background Em from things like powercables and TVS etc dont hit us all throughout the day?
Jonothan pritchard 23:00
- The best way to convert electricity into kinetic energy is probably with an electric motor. Johntex\talk 22:03, 25 April 2006 (UTC)
- If you are asking why the EM energy emitted by power cables and TVs don't move anything, it is just that the waves emitted are too weak to move anything. But they do hit us. Powerful EM waves inside Microwave ovens can heat things up. Haven't you heard the argument that EM waves emitted by mobile phones are dangerous ? Being in the vicinity of high tension power cables for a long time is supposedly dangerous too --Wikicheng 22:43, 25 April 2006 (UTC)
- Also take into account that most of the EM waves (sunlight/starlight/space radiation) are slamming down on you from above at about 299,792,458m/s. By comparison, very little comes from earth-bound items (unless you happen to be near a nuclear explosion). I'll never fully understand why physicists are absolutely certain that wave after wave of billions of electromagnetic particles slamming into you every millisecond of the day has absolutely no effect on your momentum. It seems logical that the high excess of EM waves coming from above would result in an overall momentum downward. --Kainaw (talk) 23:23, 25 April 2006 (UTC)
- Actually, it's closer to 299,702,547m/s, unless you live in a vacuum bubble. Coincidentally, radiation from earth bound sources also strikes you at the same speed. Hm....? When it comes to sunlight, personally, I'd be most concerned at sunrise... perhaps that's why I stumble so much when I wake up early compared to later in the day. Andrewjuren(talk) 23:41, 25 April 2006 (UTC)
- Actually, I do live a vacuum bubble, void of facts, statistics, and responsibility. That is why it is so easy to answer all the questions here. Regardless, my point was the quantity of EM coming from above compared to the quantity of EM coming from Earth. Everything I've read has claimed that even in the dead of night, the EM from Earth is insignificant compared to the EM coming from above. That did just spark a memory - is the Earth bright or dark? Most think that it has a bright side and a dark side - but that is because we only see visible light. With all our radios, televisions, cell phones... even in the dark we emit a hell of a lot of EM waves. So, for a radio-seeing being, it would be rather bright all the time. Of course, that has absolutely nothing to do with this question. --Kainaw (talk) 23:47, 25 April 2006 (UTC)
- Not to mention the infrared energy that is given off naturally. freshgavinΓΛĿЌ 04:52, 26 April 2006 (UTC)
- Actually, I do live a vacuum bubble, void of facts, statistics, and responsibility. That is why it is so easy to answer all the questions here. Regardless, my point was the quantity of EM coming from above compared to the quantity of EM coming from Earth. Everything I've read has claimed that even in the dead of night, the EM from Earth is insignificant compared to the EM coming from above. That did just spark a memory - is the Earth bright or dark? Most think that it has a bright side and a dark side - but that is because we only see visible light. With all our radios, televisions, cell phones... even in the dark we emit a hell of a lot of EM waves. So, for a radio-seeing being, it would be rather bright all the time. Of course, that has absolutely nothing to do with this question. --Kainaw (talk) 23:47, 25 April 2006 (UTC)
- See light pressure: sunlight on something facing directly into it is 4.6 μPa. So (being very generous with the size of the person and very conservative with their mass) there'd be something like 10 billionths of your weight added onto you while, say, sunbathing at noon. Entirely irrelevant, regardless of the direction it's applied. --Tardis 13:46, 26 April 2006 (UTC)
Synthesizer how-to[edit]
I have just downloaded a soft synth. Can anyone recommend me a good source of information of using (general) soft synthesizers? Thanks.
- See our article on Software synthesizers. -- Andrewjuren(talk) 00:16, 26 April 2006 (UTC)
Soft synths are standardized so as to be supported by the largest number of programs possible. If you're running Windows, the soft synth is probably a VSTi, Macs are usually audiounits. Most programs either want you to put the softsynth file in their plugin folder, or you can specify a directory or set of directories to scan for soft synths. The software one would use soft synths with is called a Music sequencer. So the sequencer sends the data like note, duration, velocity and tons of other midi-like data to the soft synth, which returns audio. FL Studio is one of the most powerful, and one of my favorite sequencers. TheDapperDan 02:22, 26 April 2006 (UTC)
- Try Musical Keys --Chris 02:26, 26 April 2006 (UTC)
Physics Textbook[edit]
Hi, my physics teacher mentioned a textbook a while back but I can't remember the name (something and Johnson's) that he recommended. It's probably a university level one since I'm in AP, if anyone knows what I'm talking about or has info on any good textbooks it be great. thanks 23:17, 25 April 2006 (UTC)
Mac OS X[edit]
How easy is it to learn the Mac interface for a fairly advanced Windows User?
I'm asking because I want to get the Macbook pro when Mac OS 10.5 comes out, so that I can get boot camp and boot both Windows and Mac OS on the same computer [which would be mega-awesome considering some things you can do so much easier with a mac and because their hardware is better manufactured in my opinion. Plus, I like their user interfact. The only reason I never got a Mac was because they used to be very incompatable].
Also, what are other people's opinions of the Macbook Pro? Would it maybe just be better to skip this and get a good laptop when Vista comes out? — Ilyanep (Talk) 23:25, 25 April 2006 (UTC)
- As for the interface question: it's not very hard. Mac OS X is pretty easy to use and in many ways is similar to Windows XP. If you use it for a few days you'll get the hang of it. I siwtch between the two regularly without much confusion (except that for certain things, such as non-English characters, Mac OS X is a million times easier). --Fastfission 23:35, 25 April 2006 (UTC)
- Max OS is similar to Linux desktops (KDE/Gnome) which are similar to Windows. Everyone steals the good ideas from everyone else. However, your idea that dual-booting a computer would be "mega-awesome" is flawed. Consider this: You are in Mac and you need to run a Windows program. You must reboot. You are then in Windows and you need to run a Mac program. You must reboot. It is far better to have some sort of embedded system. A popular one is VMWare (install Windows inside of Mac). Since I have plenty of computers at my disposal, I have a Win2003 box sitting in a corner and I rdesktop it when I need Windows. --Kainaw (talk) 23:50, 25 April 2006 (UTC)
- I don't see myself switching between apps very often. On a computer, I'm usually either in game/goof off mode [Windows] or office/internet/homework mode [Mac]. Most design would somehow continue going on on Windows [as my copies of Photoshop, Flash, etc. are all Windows], and some things might become Mac-only [calendar management through iCal and GCal, etc.] If I were someone who made frequent use of both at the same time, I'd agree with you.
- Mac OS X is probably the easiest OS to learn, and it ripens the more you explore. There are a lot more applications you can download than you think. Boot Camp is better than nothing, and I think they will soon be able to have you switch between Windows and Mac OS X like application switching, or user switching. Usually I just want to see what is in the exe file, or something like that. If you get Apple Pages you can view .docs well, and with Apple Keynote, you see .ppts well. -- User:Mac_Davis 00:13, 26 April 2006 (UTC)
- That's cool. I'll probably get iWork (Pages + Keynote) because it seems useful (looks like very nice presentations and documents there) -- although probably not Office (I can just use oo.o if i ever need a bread and butter office). All the rest will probably be freeware and downloaded, because I don't have money to buy a $2.5k laptop and then rebuy my expensive library of software :P which is why having Windows as a fallback is very nice (plus games).
- I'd love to see a fast switch between OSes. And hopefully I can learn Mac OS X quickly, because I'm sure I'd feel quite set back after being an advanced user of Windows (been using it since 1994, as a 3-year-old). — Ilyanep (Talk) 01:19, 26 April 2006 (UTC)
- See [65]. Parallels lets you run WIndows within the Mac OS, so you have both running simultaneously. It's free in beta, will be about $50 when it is commercially released [66] - 05:39, 26 April 2006 (UTC)
Another question: If I have a Windows XP disc but it's an original XP [no SP2] disc, will it work with Boot Camp? If not, is there a way I can get an XP/SP2 disc from Microsoft? Perferably from the internet for free. — Ilyanep (Talk) 01:32, 26 April 2006 (UTC)
- You should be able to download SP2 from Windows Update for free. The issue here is that your copy of Windows XP was probably authorised to run on your existing PC only. You'll have to ring Microsoft and arrange for the authorisation to be cancelled so you can authorise your Mac to run it. --Canley 03:17, 26 April 2006 (UTC)
- In fact, bundled copies of Windows cannot be moved to another computer. This is part of the deal: in return for being a fraction of the retail price, they go to landfill with the computer. If of course the spare Windows XP disk was a retail copy, never installed, that doesn't apply, so I don't want to jump to conclusions. Notinasnaid 11:29, 26 April 2006 (UTC)
April 26[edit]
For you Physics People[edit]
Question: When you are running and want to stop quicikly, must decelerate quickly. a) What is the origin of the force that causes you to stop? b) Estimate the maximum rate of deceleration of a person running at top speed to come to rest.
Does anyone know what they are asking for in part A? I don't quite get it, I mean I understand the question but I'm having a hard time visualizing how this would work. Any help would be appreciated thanks.
01:06, 26 April 2006 (UTC)
- The force is mostly friction between your feet and the ground, although there is some small contribution from air resistance as well, I suppose. (ESkog)(Talk) 01:07, 26 April 2006 (UTC)
- Unless you hit a wall, in which case it's more of a normal force. Melchoir 02:04, 26 April 2006 (UTC)
- To help you visualize the answer, imagine yourself running top speed, and then imagine what you would do to try to stop yourself. Most... humans extend one of their feet (most likely right foot for righties) and slam it down putting a lot of pressure on the one leg. By slamming your foot down, you are transferring your kinetic energy into the earth (the same energy transfers when objects crash), and since the earth is essentially stationary (relative to you running) and heavy enough so as not to be moved much by such a relatively small amount of energy, it will eventually take all of your previous kinetic energy and you will come to a stop.
If you stop by taking a few heavy steps as opposed to sliding your feet along the ground (like the way a hockey skater stops) then friction shouldn't be the major force, though there certainly is some friction force involved too.
Though the question may seem to be worded awkwardly, the origin of the force that stops you is the Earth. freshgavinΓΛĿЌ 04:46, 26 April 2006 (UTC)
- The major part is played by friction, even if you slam your foot down. If the surface on which you are running is smooth, no matter how hard you slam, you still will not be able to stop quickly. You are basically trying to prevent your body from continuing to move forward, by applying a backward force, using the friction between your feet and the earth. Even when the hockey player slides to a stop, he uses friction. If there was not friction, a moving body will not stop, unless it hits something else. I wouldn't be wrong in stating that all moving bodies in contact with other bodies slow down mainly due to friction between the bodies. (Ignoring other obvious cases like a direct collision and influence of other forces like gravity, magnetism etc) -- Wikicheng 13:49, 26 April 2006 (UTC)
- About part b)- To calculate the rate of deceleration you'll have to use this formula: v=u-(a*t). Where, v=final velocity (in this case 0, beacuse the man is coming to a stop), u=initial velocity, -a=decelaration (+a for acceleration), t=time. If you know the amount of time taken by the man to stop and the initial velocity of the man, you can calculate the rate of deceleration (a). If you know the distance the man covered before he came to a stop, you can use the following formula: v^2=u^2-2aS, where S is the distance, v=0, if you know u(initial velocity) then you can find the rate of decelaration. (Note: ^ is to the power and * is multiplication sign).--DIGIwarez 15:54, 26 April 2006 (UTC)
Problem with Rope having a Mass[edit]
Suppose the cord is a heavy rope of mass 1.0 kg. Calculate the acceleration of each box and the tension at each end of the cord.
I can't for the life of me get the diagram on here, I've tried for the last half hour and I get "The file is corrupt or has an incorrect extension. Please check the file and upload again". I'll try describing with a bit of drawing on my part. I don't know how to do this because of the 1 kg rope, and most likely without it either. But we've never done any with ropes having a mass.
----- |-----|
m2= |T2 T2 Cord T1 T1| |FP 40N(force of pull)
12kg |--> <---++++++++---> <--|m1= |--->
| MC = 1.0kg |10kg |
-----| |-----|
This is just the free body diagram:
The thing actually looks like:
Rope BOX------BOX
in which the boxes are connected with the rope, and are in contact, unlike the top picture.
Thanks for any response.
02:53, 26 April 2006 (UTC)
- I took the liberty of fixing your formatting. If you want preformatted text, you have to put a space at the beginning of each line. —Keenan Pepper 03:21, 26 April 2006 (UTC)
Thanks a lot for that. 03:34, 26 April 2006 (UTC)
- Is there gravity? Are the boxes sliding around on an air table, or out in space, or is there friction from something...? Melchoir 03:42, 26 April 2006 (UTC)
The boxes are on the table and the question makes no mention of friction or gravity, but I believe it wouldn't need gravity because it's asking for movement along the x-axis, it shouldn't make a difference I would assume. 03:48, 26 April 2006 (UTC)
- Well, it looks like all the elements accelerate equally. You can find that acceleration by considering the whole system as a single body with whatever total mass. Then determine, algebraically, the forces on all the elements of the system as a function of the tensions, and set those forces equal to the forces required to move them under Newton's second law.
- By the way, if you don't like to deal with massive ropes, you can think of the rope itself as a box made of rope! You're only asked for the tension at either end, so conceptually there's a very short, massless rope that connects the rope-box to the box on the left, and another very short, massless rope that connects the rope-box to the box on the right. These new imaginary ropes will be under different tensions; you're asked to find them. Melchoir 04:01, 26 April 2006 (UTC)
- So would you have a different answer for the tension if you didn't consider the rope a box itself? how would you do the question then? Thanks again. 04:11, 26 April 2006 (UTC)
- No, the laws of physics are blind to the difference between boxes and ropes. (Well, they are up until you get to engineering mechanics.) A rope, like anything else with mass, accelerates if and only if a net force acts on it. I'm just saying that whatever conceptual model helps you solve the problem, go with it. Melchoir 04:21, 26 April 2006 (UTC)
- Except that you can't push with a rope. (Or at least not normally. I once used a rope that had been soaked in water and frozen to push my physics teacher.) --Serie 22:35, 26 April 2006 (UTC)
- No, the laws of physics are blind to the difference between boxes and ropes. (Well, they are up until you get to engineering mechanics.) A rope, like anything else with mass, accelerates if and only if a net force acts on it. I'm just saying that whatever conceptual model helps you solve the problem, go with it. Melchoir 04:21, 26 April 2006 (UTC)
A rope with mass means that there will be a linear tension distribution throughout the rope. Think of a heavy rope just hanging vertically. --Zeizmic 12:13, 26 April 2006 (UTC)
Assuming no friction and no stretching:
Once the ropes are fully extended (under tension), all the boxes and ropes will accelerate at the same rate. Call that 'a'. There will be tensions in the ropes too, marked in as T1 and T2. So we can calculate the forces on each object:
Forces on m1:
- m1 has forces of FP RIGHT and t1 LEFT. So the total force is (FP - T1) RIGHT
- It has mass m1 and the forces make it accelerate at 'a'.
- So F=ma ==> (FP - T1) = m1 x a
Forces on cord:
- cord has forces T1 RIGHT and T2 LEFT. So the total force is (T1 - T2) RIGHT
- It has mass MC and the forces make it accelerate at 'a'.
- So F=ma ==> (T1 - T2) = MC x a
Forces on m2:
- m2 has forces of T2 RIGHT only.
- It has mass m2 and the forces make it accelerate at 'a'.
- So F=ma ==> (T2) = m2 x a
Solving these equations:
- We have been TOLD that FP = 40, m1 = 10, MC = 1 and m2 = 12
- So if we substitute these numbers in, we get:
- (FP - T1) = m1 x a ==> (40 - T1) = 10 a
- (T1 - T2) = MC x a ==> (T1 - T2) = 1 a = a
- (T2) = m2 x a ==> T2 = 12 a
- You now have 3 equations in 3 unknowns.
- Rearrange the 1st and 3rd equations to get T1 and T2 in terms of 'a'
- Substitute these into the 2nd equation to get an equation that has one unknown, 'a'.
- Solve it to find 'a', the acceleration of the objects
- Substitute again to find T1 and t2, the tension at each end of the cord.
FT2 (Talk) 00:10, 27 April 2006 (UTC)
Global Heating[edit]
In discussions of Global Warming, I am curious why there is so little focus on heat (as opposed to temperature). Temperature is important of course, but the transition of ice at 0°C to water at 0°C requires a lot of heat, implying that the polar ice caps, for example, are buffering the temperature rise. Then the question arises, if the total heat content of the planet is increasing, where will all that heat go when the ice has melted? Is this unimportant with the Greenhouse Effect? Is that heat just a very small part of the picture? Or is there enough ice that it's not an issue (yet)? Peter Grey 04:48, 26 April 2006 (UTC)
- I have no idea. That said, I'm going to guess that measurements focus on temperature changes because they can be directly measured, while heat fluxes cannot. Behind the scenes, when one models climate change, I assume that latent heats and so forth are duly taken into account. And, to add the obligatory joke answer: because Global Heating sounds like an appliance manufacturer. Or maybe they lay gas pipelines? Who knows. Melchoir 05:02, 26 April 2006 (UTC)
- Global warming is an issue because of expansion of the ocean. Think of it this way. An ocean might be 1000 m deep. If water expands 0.1%, that's 1 m. Goodbye Florida (joke). But yes, melting polar icecaps are heat sinks. But they only need to melted once and they're gone. I assume that the energy needed to melt them is relatively small. Lets do a small calculation on the back of a napkin. To Melt 1 million square kilometres with a depth of 100 m over 100 years. That's 10^6 x 10^6 x 100 = 10^14 cubic metres. 100 years = 10^2 x 4*10^2 x 2.5*10^1 x 4*10^3 = 4*2.5*4 (40) times 10^8 = 4*10^9 seconds. Dividing, 10^14 / 4*10^9 = 2.5*10^5 cubic metres a second. That's quite a bit. But that's only 2.5*10^5 / 10^12 = 2.5*10^-7 m = less than a micron per second. And no, I don't have a calculator on me as you guessed! 153.111.60.15 07:50, 26 April 2006 (UTC)
- Without factoring in latent heats and heat capacities of various reactions and reservoirs, these numbers mean nothing. Melchoir 21:36, 26 April 2006 (UTC)
- A related question, would this mean that once most of the Earth's ice has melted, we should expect the temperature to increase at a faster rate? Peter Grey 00:52, 27 April 2006 (UTC)
- Yes, but... Given an extra input of a few W/m^2, a density for ice of 0.9 g/cm^3 and a latent heat of 300 kJ/kg, you'd expect a melting of ~30 cm/yr if all the local excess heat were going solely into melting ice. This is about 5 times greater than the observed mass loss in Greenland but still in the same ballpark. If we say that about 20% of the local excess energy from global warming goes into melting ice and note that ice covers about 3% of the Earth, then without the process of melting ice, global warming might progress ~0.5% faster. This is of course only a very crude estimate. More important is the fact that ice is shiny and white, and once you remove the ice the ground is dark. By changing the albedo in this way you absorb more sunlight after the ice is removed, which would be a much more important factor in amplifying global warming. Dragons flight 16:39, 28 April 2006 (UTC)
My theory on obesity and height[edit]
For a while now, I have come to the conclusion that shorter women are more likely to be obese/overweight than taller women. Of course, this can easily be proved/disproved if I had gender/age/height/weight for a random sample of people in a Western country with obesity issues. What are peoples thoughts? Does anyone have any actual research on this or stats? Thanks. BTW, I'm looking at, say age 30-40. 153.111.60.15 07:29, 26 April 2006 (UTC)
- Maybe its just that taller people dont look overweight even if they are... as i understand being obese depends on the genetics and how much they exersice... not necessarily on the height of a person... (i am not completely sure about that last point) Jayant,17 Years, India • contribs 10:39, 26 April 2006 (UTC)
- I think Jayant's point about perception is sound. A quick Google brings up two studies:
- Height-Related Changes in Body Mass Index: A Reappraisal gives a slight positive correlation between body mass index (a popular measure of whether someone's overweight) and height for men (ie taller men are "fatter") and a slight negative correlation for women (which fits User:153's belief). That's for data from people coming out of the Israeli army just over the age of 20, so it may not be that similar to 30-40 year olds in other contexts.
- These guys from FSU (warning: pdf doc) examine a wide range of populations and generally find a negative correlation for both men and women (again in line with the User:153's theory). But they use it to argue that a blanket application of BMI is therefore not a good way to determine whether someone's overweight, rather than that shorter women are more likely to be overweight.
- Note that in both cases the correlation coefficients, while statistically significant, are small in absolute terms -- the effects are there, but they aren't all that large. --Bth 11:42, 26 April 2006 (UTC)
Thanks for the comments, I still think my theory has validity, if only I had data! Have downloaded the .pdf but yet to digest. 153.111.60.15 08:49, 28 April 2006 (UTC)
Turbine engineering[edit]
How is it possible to maintain a seal between the shaft of high pressure turbine and the chamber it is enclosed in?
- Turbines are a complex bit of engineering. I know that we always have trouble with the monster turbines. I suggest you brush up on your Google skills. --Zeizmic 12:17, 26 April 2006 (UTC)
There is a sealing steam system and a tap-off to a system at vacuum on the steam turbines I have worked with.
Put simply, there is leakage, but it is minimal, and is vented off to a condenser at negative pressure.
BigFatDave 11:37, 3 May 2006 (UTC)
PVC substitutes[edit]
I am wondering what materials may be used in place of PVC in its main applications (Namely construction, housing, toys, cars and medical use). Thanks in advance. ...
- Polyethylene and polypropylene for toys, cars, diapers and medical use I believe. I would imagine most types of relatively harmless polymers could be used for any of those categories. -Snpoj 11:37, 26 April 2006 (UTC)
- Wrong question. The right question to ask is "Why is PVC used in so many applications despite its being so poorly suited to the purpose at hand?" The answer is that in the process of alkali electrolysis sodium hydroxide is the sought-after product. Chlorine is producted in equal amounts and is really an unwanted byproduct. Converting it into PVC is one way to dispose of what really is a waste product. Dr Zak 11:45, 26 April 2006 (UTC)
- You could use pretty much anything non-toxic for toys. For medical use biocompatibility is of primary concern, common polymers used in hospitals (for IV tubes and the like) include polyethylene (PE) and silicone. In construction, I can't think of any alternatives that are used (particularly with a high volume area like construction, price is a mjor factor in choosing a material). There is lumber made from recycled plastics, but that doesn't replace a PVC application. Polymethylmethacrylate (PMMA) is a strong, fair enivronmentally material that is used for greenhouses and architecturally. The plastics used in cars include polyurethane (PU), polypropylene (PP), acrylonitrile butadiene styrene (ABS), polycarbonate (PC), and PVC. Generally, PU, PS, ABS, and PC are the most commonly subsituted polymers for PVC. --Chapuisat 14:42, 26 April 2006 (UTC)
- I always think of something to add write after posting a reply. I should have mentioned that all polymers have advantages and drawbacks, many of the alternatives to PVC (such as ABS) also use hazardous chemicals. Polymer engineering hasn't always been the most environmentally friendly field, but over the last decade or so I think it has improved quite a bit. --Chapuisat 14:48, 26 April 2006 (UTC)
- Leather and latex. Oh, constructions purposes. My bad. --Blowdart 17:39, 26 April 2006 (UTC)
Redirect From Incorrect Spelling?[edit]
Is it appropriate to make a redirect from for example, Pyramidine (incorrect spelling) to Pyrimidine? -Username132 (talk) 10:36, 26 April 2006 (UTC)
- It usually is, yes, though someone's already done that one. This was more of a question for the help desk, though, since it's about editing Wikipedia rather than a specific question about science. -- Mithent 11:05, 26 April 2006 (UTC)
- It usually is, yes, but somebody has already done so. Ask at the help desk next time, but how you do it is: #REDIRECT[[Page name you redirecting to]] --
 Mac Davis] ⌇☢ ญƛ. 12:22, 26 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 12:22, 26 April 2006 (UTC)
- Did you guys plan that or something? --Chapuisat 13:40, 26 April 2006 (UTC)
- Sorry, I did go to the help desk, and didn't realise I could post there. I just saw the humanities, science options etc. and thought I had no other choice. Btw, the somebody who did it was me, I just wanted to make sure I was doing the right thing. --Username132 (talk) 15:12, 26 April 2006 (UTC)
- Just make sure to add the template {{R from misspelling}} so that no-one would think it's a legitimate alternate spelling. See Wikipedia:Redirect for more info. – b_jonas 22:45, 27 April 2006 (UTC)
long term memory[edit]
characteristics of long term memory
1 The ability to receive and store information during observation. 2 The ability to trace and recall information stored in long term
memory.
3 Like sensory storage, long term memory has a large capacity. 4 Long term memory is usually well organised. 5 It is a relatively permanent form of memory. 6 The information is sifted,coded and stored and thus form a
components or part of all experience that can be remembered.
7 Information is rearranged when new material is added. 8 The process is not static, but dynamic. 9 Information obtained through any of the senses can be stored. 10 New meterial can be arranged meaningfully together with the old
meterial.
11 Familar meterial or information is easier to process or arranged
than unfamiliar material.
DESCRIBE AN EVENT OR WRITE A STORY (ROUGHLY 200 WORDS) ABOUT SOMETHING THAT YOU HEARD ABOUT OR EXPERIENCED YOURSELF ABOUT A YEAR AGO. NOW ANALYSE YOUR PIECE OF WRITING IN TERMS OF THE ELEVEN CHARACTERISTIC OF LONG-TERM MEMORY AND TRY TO DETERMINE THE DEGREE TO WHICH YOUR WRITING SATISFIES THOSE CRITERIA.
- Please read the instructions at the top of the page. This page is to ask questions, not to set assignments. I wonder what your short term memory is like...- 131.211.210.13 12:45, 26 April 2006 (UTC)
- Ok, I did that: I wrote a story of roughly 200 words, and analysed it. Thank you for the suggestion. Notinasnaid 14:16, 26 April 2006 (UTC)
can you tell me how to install windows XP in a newly brought system[edit]
- Take your newly purchased copy of Windows XP (not one reused from an older computer, unless it was purchased retail), put it in the CD drive, and boot. The system will boot from the CD and install Windows XP after a few questions. Notinasnaid 14:14, 26 April 2006 (UTC)
- If the computer is set-up to boot from CD, you will see "press any key to boot from CD"; you'll then have a window of a couple of seconds in which to press a key, so make sure you're paying attention. If you don't see this message on the screen, come back for more help. -Username132 (talk) 15:09, 26 April 2006 (UTC)
Virtual Memory[edit]
I've heard in the past that your virtual memory should be set to 1.5x your physical memory. I just upgraded from 512mb RAM to 1024mb RAM, but it doesn't look like my virtual memory went up. Can anyone with 512 or 1024 RAM let me know how their settings are in WinXP SP2? Thanks. --Chris 15:49, 26 April 2006 (UTC)
- You can set your VM to whatever you want; so long as you don't find that you're running out (angry dialogs and crashing applications), you're fine. You don't get any benefit from having "extra". But for what it's worth, the system I'm currently using (though it's not Windows) happens to have approximately equal amounts physical and virtual: about 1GB each. If you have the hard drive space to spare, set it to 2GB or more if the system will let you, and then you won't worry about it. But if you want to conserve space, and haven't had any trouble, just leave it alone. --Tardis 16:19, 26 April 2006 (UTC)
- This is known to be very poor advice, regularly criticized on IT forums. Read virtual memory to understand what VM does. Basically, your computer will use X amount of memory to work, the exact amount varying depending what you are doing. It uses all the RAM it can, the rest is made up using VM. So in fact the more physical memory you have, the less VM you need and vice versa!
- As a rough rule of thumb, unless you're doing complex processing (video editing, photoshop etc), and given how cheap physical memory is today, I'd use this for a rule of thumb with Windows XP: as you have, use between 512 MB and 1024 MB of physical memory (not less than 256 MB), and then set virtual memory to use a set (fixed) size of around minimum 512 MB and maximum 1536 MB (if your hard drive allows it). The fixed size stops Windows having to extend the file quite so often. That should fix it up nicely.
- If you do intense work on photoshop or video work (not just DIVX coding!), eg professionally, then get more RAM (1-2 GB is not uncommon) a fast modern dual core processor, a solid and stable motherboard (eg ASUS which I use and have never had a problem with) and a huge hard drive (Seagate's 'Barracuda' range currently offer the best price/quality/warranty/speed balance). FT2 (Talk) 19:58, 26 April 2006 (UTC)
Running a local server... issues[edit]
I'm using Mac OS X Tiger, and I'm running a personal web server (Apache) off of my local machine which I use while writing PHP. Normally this is fine -- I point the page to the local address (http://mycomputer.local/~myusername/) and it runs fine. However just today it has, without me changing the settings, redirected from mycomputer.local to the local IP that my university has assigned for my computer's internet access. In fact, if I type in the address as http://127.0.0.1/~myusername/ it will automatically substitude it with http://net-23295456.myuniversity.edu/~myusername/. Some of my scripts execute fine, despite this, but some give me a 404 error. What gives? --Fastfission 16:22, 26 April 2006 (UTC)
- The more I tinker with this... I think the latter "net-2323" bit still goes to my local computer. Yet it sometimes will, sometimes won't run a script. I haven't had this issue before. I've tried restarting the web server, restarting the computer, disconnecting and reconnecting it to the internet, using different browsers, resetting my cache, clearing the dynamic DNS, etc., to no respite. Argggg. --Fastfission 17:06, 26 April 2006 (UTC)
- Actually, now it's only failing whenever I try to open a new locally hosted script in a javascript window, and try to pass GET arguments to it in the URL. What the hell. If I don't try to pass arguments, it executes the script just fine. Why is my computer being such a pill? --Fastfission 17:13, 26 April 2006 (UTC)
What is the ServerName set to in apache? EricR 17:36, 26 April 2006 (UTC)
- Nevermind, I figured it out! Not a big deal, I was confused about something, please ignore. --Fastfission 01:30, 27 April 2006 (UTC)
Direct ancestor of Homo sapiens?[edit]
I'm trying to trace an approximate timeline between modern humans and the dawn of life. For example, who are the direct ancestors of Homo sapiens? Would it be erectus? And what is the ancestior of primates, e.g. the "protoprimate"? and then the ancestor of all mammals, before mammals branched out into all the different kinds we know today. And so on and so forth to the earliest known life forms from which presumably all life evolved. For example is Tiktaalik a possible ancestor of humans? Or a cynodont? etc. What's the most accurate line as we know it?--Sonjaaa 16:57, 26 April 2006 (UTC)
- Well you can't think of lines as just being straight shots, first of all. They get all mixed up with themselves and look more like bushes than trees. Secondly, we don't have straight genealogies available anyhow. Anything could be a "possible" ancestor of humans if you are talking about as far back as Tiktaalik. Take a look at our evolution of homo sapiens article for an overview of what is known and what can be said about it. --Fastfission 17:04, 26 April 2006 (UTC)
- Also look up Y-chromosomal Adam and Mitochondrial Eve, Most recent common ancestor, and Last universal ancestor. FT2 (Talk) 20:08, 26 April 2006 (UTC)
- Also, a good article to get acquainted with some of the conceptual difficulties making sense of this sort of genetics is Troy Duster's "Deep Roots and Tangled Branches". --Fastfission 01:32, 27 April 2006 (UTC)
Who designed Monobook?[edit]
Who designed the Monobook skin, initially, for Wikipedia? We need this in relation to a media outlet's request. -- Zanimum 17:38, 26 April 2006 (UTC)
- User:Gwicke. --James S. 18:06, 26 April 2006 (UTC)
- But I believe he based the design (and may have used some of the collateral, such as images and styles) on a weblog skin (I'm afraid I forget which). -- Finlay McWalter | Talk 18:11, 26 April 2006 (UTC)
This spider book I'm reading mentiones that scorpions, mites, and centipedes are all arachnids. But according to Wikipedia, centipedes belong to the Subphylum Myriapoda, while arachnids belong to the Subphylum Chelicerata. Can someone explain this to me? Maybe scientists can't agree where centipedes belong? Jonathan talk ![]() 17:44, 26 April 2006 (UTC)
17:44, 26 April 2006 (UTC)
- Wikipedia's articles place Class Chelipoda (centipedes) in Subphylum Myriapoda, as do some other sources (try Googling "Myriapoda"), but I found a source placing them in Arachnida (Hawaii Biologial Survey). Another source ranked Myriapoda as a class, equal to Arachnida (Kendall Bioresearch Services). So it appears there's no firm consensus where they belong, but placing them in Arachnida seems outdated at best. --Ginkgo100 18:13, 26 April 2006 (UTC)
- Wikibooks has a dichotomous key for arthopods, the place where centipedes and arachnids are thought to diverge. You can read it here. The key is based on morphological features (antennae, number of feet, etc.) which is popular among some biologists, while other prefer genetic sequence analysis or using other types of distiguishing features. That may be why there is not an agreement on the exact phylogenetic tree. You may also enjoy looking at the wikispecies entry on centipedes. --Andrewjuren(talk) 19:21, 26 April 2006 (UTC)
Shhh...don't tell them anything useful or they might never post again on reference desk. Jonathan, Andrew was just lying ok?You never hear erm... read that 'k?
Different prongs on electrical plugs[edit]
The lamp on my desk has a plug with two flat parallel prongs. One of them is wider than the other, and I believe it to be a Type A Nema 1-15. see Domestic AC power plugs and sockets. My question is, why do the prongs differ from one another? In my youth common electrical plugs had prongs that were identical in shape. They carry alternating current, so what is going on electrically at the two prongs are the same, and one is not "hot" and the other "neutral" at least in any electrical sense, it seems to me. Electrons are moving alternately into and out of each prong during the cycles. I've taken graduate courses in E&M, but still wonder at this...--DonSiano 18:21, 26 April 2006 (UTC)
- You are mostly correct. However, although the electricy is alternating current, one pole remains roughly neutral (relative to ground) while the other alternates on average 120V (relative to ground). Keep in mind that I am discribing the voltage of the plug relative to ground, not the current running though an appliance. -- Andrewjuren(talk) 18:54, 26 April 2006 (UTC)
I have 'unconsciously internalized' this from somewhere: Polarized plugs have a wide neutral prong and can only plug one way into a receptacle. In a properly wired lamp, the polarized plug ensures that the hot wire energizes the metal tab at the bottom of the socket, which is less exposed to fingers than is the threaded shell. --Zeizmic 19:46, 26 April 2006 (UTC)
Radishes[edit]
Really random but I need to know for my A2 Biology coursework, are radishes leguminous nitrogen fixing plants or non-leguminous nitrogen fixing plants? I have looked on this site and others and cannot find an answer! I would really appreciate it if you could get back to me asap on this as my coursework needs to be handed in shortly and I've nearly done the rest of the project!
Thank you for your assisstance!
Dee x x x
You should at least have looked at radish, legume and Nitrogen Fixation before asking us to do your homework. (though I think the answer to the question you posed is "no"). Malcolm Farmer 19:10, 26 April 2006 (UTC)
PCL6[edit]
I am trying to investigate the contents of packets being sent to an HP IP printer using PCLXL (now called PCL6). Upon viewing a packet capture of the data I find very little, if any, information that is readable/useful. I have a white paper on PCL6, but I find it to be less that helpful. If anyone could provide some insight on what steps to take to extract the print data please let me know. Ideally I would like to have some sort of break down of field sizes and possible values, but it looks to me like the data need to first be converted into some other format.
Below is an example of the data that goes across the network when printing a file. It is compiled from several packets and derived with the "Follow TCP Stream" functionality in Ethereal. I didn't include all the output to help keep it short. In addition, some data that could be used to identify me has been removed. If it would be helpful to have the full capture please let me know.
.%-12345X@PJL SET STRINGCODESET=UTF8
@PJL JOB NAME="Document"
@PJL COMMENT "HP LaserJet 2420 PCL 6 (60.42.108.11); Microsoft Windows XP 5.1.2600.1; Unidrv 0.3.1296.4"
@PJL COMMENT "Username: REMOVED; App Filename: Document; 4-21-2006"
@PJL SET JOBATTR="JobAcct1=REMOVED"
@PJL SET JOBATTR="JobAcct2=REMOVED"
@PJL SET JOBATTR="JobAcct3=REMOVED"
@PJL SET JOBATTR="JobAcct4=REMOVED"
@PJL SET JOBATTR="JobAcct5=REMOVED"
@PJL DMINFO ASCIIHEX="0400040101020D101001153230303630343231313532363133"
@PJL SET USERNAME="REMOVED"
@PJL SET DUPLEX=OFF
@PJL SET ECONOMODE=OFF
@PJL SET RET=MEDIUM
@PJL SET RESOLUTION=600
@PJL SET BITSPERPIXEL=2
@PJL ENTER LANGUAGE=PCLXL
) HP-PCL XL;3;0;Comment Copyright(c) 1999 Microsoft Corporation
.X.X...........A........H...(...&...LETTER.%C.d.d..*u....?...?.+w....j...-x...-|......Lk...,{....c....y...........B.....,{.....c........MS PCLXLFont 001..O.....P.........p.....P..GT...d.....P.........@... .`...P.`cvt .......l...0fpgm...........pgdir............head......
....8maxp......
D... prep......
d.....0...P.0................&...............i.......i.....................i.....................D...|.......Z.....R.R...D......./...........W.~...................
.".A.P.o...L.u.\.....7.L.n.p...X...................c.c.........-.\...........@.W.......r...]...g.....!.w.....M.....+.L.e.....|.C.............].h.....5.G.!.\.M.....-.x...........................,.I.............?.......).9.I.o.......#...o...2.@.z.....1.U.W.........~.~.....F.B................./.O.V.).o...r...,.1.1.d.i.........+.....................&.......
... .s.......C._...........a...^.m.........
And it goes on...
Thanks for your time. --Daniel
- Get the source for ghostscript, it includes a PCL driver, study that and you can figure it out. Try printing a simpler document, like a single letter 'a', and see what gets sent over ethernet and see if you can figure out the translation. Try asking in the right newsgroup. See Printer Command Language and various links to documents attached thereunto. --GangofOne 18:59, 26 April 2006 (UTC)
- What you are seeing there is a fairly simple PCL stream, or the start of one. After the setup commands, it begins to download a font, which will go on for quite a long time. The specification for this print language should have the information you need to break it down - but be sure it is exactly the right specification, as HP updated it regularly. Parsing the full range of PCL is hard work - I'm not sure what you are trying to achieve with this analysis? If how to write PCL, don't do it that way! Notinasnaid 20:20, 26 April 2006 (UTC)
Thank you for your input. Starting with the "specification" for PCL, this is actually my problem. I have been unable to locate any specifcation that would allow me to adequately parse through bytes and retrieve meaningful data. I don't need to parse ALL of the stream, just the data intended for printing. The end goal of the analysis is the extract data for input into another program. My goal is not to write PCL. As for sending a "simple" text document across the wire and view the traffic, this is it. Actually, this is it, or rather almost it. My first attempt was the word "hello" sent across all by itself. This stream was for a document reading "hellohellohello...." The data is obviously encoded in some format, I just don't know, and can't figure out exactly what it is.
- I'd expect there's a good chance of seeing the "hello" somewhere down towards the very end, after the font downloads. PCL has the potential to be simple, unlike some other printer formats which simply take a binary bitmap. It is the ability to download fonts which makes simple printing possible: otherwise, the requirement to match a font would make it compulsory to print a page as a bitmap. Though some print methods will still turn out an entire page bitmap in PCL (e.g. GhostScript's PCL driver). Traditionally the format of PCL was something like
stream: [ plain text | escape sequence ] * escape sequence: escape character , arbitrary characters, length defined by context
If the real question is: where is the latest PCL specification, the answer might be "HP keep it proprietary and only license it" ... and that would make sense because HP don't want the whole world to copy their printer technology. But that would be a guess. A good way to find people who might know is to post on the PCL article's talk page. But there is an encouraging find: in Usenet I found the comment "You can order the PCL specifications including PCL 6/XL from HP. But it can sometimes be a little problematic, as it seams not all sales people at HP know this ;) But it is possible, I succeded." Notinasnaid 07:09, 27 April 2006 (UTC)
Leaves in Georgia[edit]
In my Science class, our teacher gave us a project that is due May 10,2006. We have to find 15 different leaves, seal them in wax paper, give the accurate name and tree where found,etc... I needed help because the leaf examples have been located on your site in another part of a state and I need different leaves inside Covington Georgia. Area code:30016. I would greatly appreciate it if you guys can please locate some leaves in this area and send me a reply!
danielandtay@GA411.org
- You could go to the library, check out one of the Peterson's guides or similar tree identification book, go to a forest preserve or large park, look up the trees in the book, and then do the picking, sealing, etc. Dismas|(talk) 21:06, 26 April 2006 (UTC)
- Let me point out that there are two ways to do this, either start with the leaves you found, then try to identify them, or start with a list of leaves in your area, then look for each. You might want to focus on leaves with highly distinctive shapes, like maple leaves, as they are easier to identify. Also, if you have a guide book with you when you pick the leaves, you can also check the tree height, bark, etc., against the descriptions in the guide. StuRat 21:51, 26 April 2006 (UTC)
- library's for chumps. Do as Calvin did and sell the earth for alien leaves. -Snpoj 21:53, 26 April 2006 (UTC)
Solar Power Cars[edit]
Could a car whose surface area is covered in solar pannels produce enough electricity to run? If not, how efficent do the S. Pannels need to become before this can be possible? The current efficency rate is ~15% 199.201.168.100 21:13, 26 April 2006 (UTC)
- Short answer is, "never as well as a car with a gasoline engine". Even perfectly efficient solar panels give you 1 kW/m^2 under direct sunlight at high noon (or about 1.3 HP per square yard, for Americans reading this). So, an ordinary-sized car with perfect solar panels would give you somewhere around 10 horsepower under ideal conditions. Generally 100 horsepower is considered the minimum for an ordinary car. --Christopher Thomas 21:53, 26 April 2006 (UTC)
If a tree falls in the woods, does it make a sound?[edit]
Why is this a popular question? Clearly sound waves are produced, so it becomes nothing more than a petty arguement over the definion of sound. Sort of like if "If all humans became extinct, would puppies still be 'cute'?" 199.201.168.100 21:13, 26 April 2006 (UTC)
- It's said to be a koan (although I'm not sure where it comes from; it's not in The Gateless Gate). The point is not to have a petty argument about semantics, the point is to think deeply about the role of the observer in all forms of experience and perhaps get closer to enlightenment. —Keenan Pepper 21:21, 26 April 2006 (UTC)
- I agree with Keenan. This is not a purely scientific question. But, some might argue it's relevance to Observer effect. Like, if you don't look at an electron, is it really there? Or for that matter, is it ever really anywhere? --Andrewjuren(talk) 21:50, 26 April 2006 (UTC)
- Do Your Own (Dharmic) Homework! Snide comments on the RD from people like me are no substitute for proper meditation on koans and the meaning of 'mu'. --Sam Pointon United FC 21:27, 26 April 2006 (UTC)
- Today we have a simple answer. Once upon a time, people didn't know as much about physics, and they didn't actually know the answer, and it would require a lot more thinking. Peter Grey 23:34, 26 April 2006 (UTC)
- It is simple if sound is clearly defined; under the primary definitions, the answer appears to be no. — Knowledge Seeker দ 04:51, 27 April 2006 (UTC)
See Mysticism for more. This is a famous "pointing out" exercise. Its intention is not to exercise the intellect, but to throw a question at it that helps it think "outside the box". The question was never intended literally as a physics quiz. FT2 (Talk) 00:16, 27 April 2006 (UTC)
This long predates western knowledge of zen and it is not mystical. It is more related to the philosophical arguments (after Berkeley) about whether things exist apart from our perception of them. I suspect you could find some 19th century English or American usage of this particular version of it. alteripse 00:55, 27 April 2006 (UTC)
A few years ago in a philosophy class, I heard an interesting answer: It does not, because "sound" is defined to be something like "that which we can hear." Personally, I prefer a less biased definition, though. Ardric47 02:48, 27 April 2006 (UTC)
- Sound is defined as the disturbance of mechanical energy that propagates through matter as a wave. If the question is taken from a purely scientific point of view, the answer is a resounding yes. freshgavinΓΛĿЌ 04:25, 27 April 2006 (UTC) resounding. *wink*
- That is one definition, yes, although not the primary definition either at Wiktionary or at Merriam-Webster. Following the primary definitions (auditory perceptions or sensations), then quite clearly the tree does not make a sound if no one is around to perceive it. It depends how you wish to define sound. — Knowledge Seeker দ 04:51, 27 April 2006 (UTC)
And that pun is resoundingly lame :) 12.183.203.184 04:31, 27 April 2006 (UTC)
The way I typically interpret this riddle is that it's something that simply can't be proven one way or another. While you can talk about stuff like defining sound, etc., the bottom line is that you can't prove whether a tree makes a sound if no one is around to hear it because any proof would involve someone actually being around to hear it. Obviously as scientists we know that it must make a sound (or not, depending on how you define sound), but this is simply an argument that's impossible to win against a skeptic. EWS23 | (Leave me a message!) 05:00, 27 April 2006 (UTC)
- No, the definition of sound is in fact the important thing. Proving that the tree did the thing with the air vibrating that people can usually hear if they're close by is easy, at least as a thought experiment: the sound decays into heat as it's absorbed by the forest. In other words, while we may meaningfully argue that perception is an important property of the world and helps define it, no one seriously argues that the lack of observers actually affects the physical occurrence (including what anyone present would have labelled as sound) except for certain real but wholly unrelated issues of measurement in quantum mechanics.
- Look at archaeology for a science that never involves "being there to see it"; did the Egyptians really build the Pyramids, or do we just suppose that because big rocks are arranged in neat shapes, had we been there, we might have seen someone working on it? It's silly to be skeptical about things that obviously did in fact happen, even if no one saw them. After all, if someone shows up and points out that they in fact cut the tree down and quite clearly haerd it fall, why not label it as hearsay and continue to refuse the sound, and everything else you're not currently watching, existence? And then keep going and posit that you may in fact be only imagining that the world exists... This too has been done, but the original question presupposes that trees do in fact fall in forests, so it's wrongheaded to apply the unanswerable questions in the philosophy of existence itself to the question. --Tardis 07:27, 27 April 2006 (UTC)
- Well, there is a school of thought that the only thing you can be absolutely sure of is cogito ergo sum, and that anything else could simply just be the the work of some evil scientist or something like The Matrix. That wasn't really where I was going with my original post (though heading toward those lines, I suppose); I was just saying that if you neglect the cogito ergo sum argument, there are quite a few things that you can physically prove to a casual observer, but the tree falling in the forest example isn't one of them. (I guess what I'm going for here is that I was taking more of a philosophical interpretation of the famous riddle than a scientific one.) EWS23 | (Leave me a message!) 08:34, 27 April 2006 (UTC)
- The same (rather unoriginal IMO) question was asked about two weeks ago at the Humanities desk: Wikipedia:Reference_desk_archive/Humanities/April_2006#Question_on_subjectivism. That provided some intriguing answers, too. Grutness...wha? 08:48, 27 April 2006 (UTC)
Last Question for today[edit]
If all humans suddenly became extinct, would puppies still be cute? 199.201.168.100 21:13, 26 April 2006 (UTC)
- No, but the reason is one that you wouldn't have guessed. I happen to operate a dead man's switch that, in the event of my own incapacitation, will release an army of robot laser cats to obliterate the world puppy population. They know why. Melchoir 21:29, 26 April 2006 (UTC)
I presume there would be some time between your death, and when the switch activates, as well as some time between the switch's activation and the time the cats finish their work. Durring this time, would the puppies be cute? 12.183.203.184 22:57, 26 April 2006 (UTC
- No, because I have an army of neutrino triggered nanorobots that hop like fleas from dog to dog, and which are transmitted by birth. So every puppy has one. At the time I die (and I will die no later than the last human), a neutrino-modulated signal will be released which before I even draw my last breath will activate all nanorobots and almost immediately turning every puppy on the planet into grey goo. During this process I can assure you that a hundred million rapidly-melting puppies in crisis will be anything but cute. FT2 (Talk) 00:22, 27 April 2006 (UTC)
- Excellent, now my army is free to pursue other ends... Melchoir 00:28, 27 April 2006 (UTC)
- Adult dogs react emotionally to puppies. If that falls under your definition of 'cute', then they're still cute. If 'cute' is only a cultural concept of humans, then it ceases to have meaning if humans become extinct. Unless whoever made us extinct thinks puppies are cute. The cuteness of kittens, of course, is objective fact. Peter Grey 00:34, 27 April 2006 (UTC)
- The answer is undefined. Humans won't be confirmed extinct until the World Conservation Union deems them to be, and I have a feeling that the leftover puppies won't be willing to do all the paperwork. freshgavinΓΛĿЌ 04:21, 27 April 2006 (UTC)
Alternate Launching techniques[edit]
This was a type of launch platform used in an episode of Star Trek: Enterprise. Would this work in today's times, and if not why not.
File:Enterprise first flight.png
Chuck 22:05, 26 April 2006 (UTC)
- What is it? How does it operate? We can't really tell these things from a picture. Isopropyl 22:43, 26 April 2006 (UTC)
- Without seeing it in action, I can't be sure, but that looks like a maglev/linear motor track with a ski jump curve on the end. It's a pretty popular alternative launch system in science fiction: accelerate the spacecraft down the ramp until it reaches orbital velocity, then use the ski jump curve to turn a little bit of that velocity into altitude. The spacecraft then fires its engine once it reaches apogee halfway around the world to circularize the orbit. It would be possible to build one today, but it would be extremely expensive.
- As a bit of science-fiction trivia, Mount Wilson is a popular place to put this sort of launcher. --Serie 22:58, 26 April 2006 (UTC)
- Is this on Earth? It's effectiveness would depend on the local gravity and atmospheric density. As I understand it, building one is technically feasible, but you also have to consider whether it's economically feasible, given that the actual designs of the linear and curved sections will be limited by the how much acceleration whatever you're launching can survive. Peter Grey 00:24, 27 April 2006 (UTC)
- Theres actually some interwting science about these kind of things. First, read up on escape velocity. Basically, if we could ignore the atmosphere, then any object travelling under escape velocity will remain on (or return to) earth, and any object over escape velocity will either escape from earth (if pointing above the horizon) or hit earth (if pointing down). It does not matter if the object is pointing up, at an angle, or horizontal. If you ignore atmospheric effects, the ONLY question is, if it is pointing above the horizon (nothing in the way!) and above escape velocity, it escapes, otherwise it returns or crashes to earth. Its that simple. Direction has nothing to do with it. Its purely the potential energy needed to escape earth, and the kinetic energy given to it by escape velocity, and which is bigger than the other.
- HOWEVER... if you take into account the atmosphere, the atmosphere applies friction and air resistance to moving objects, strongly slowing them down. Imagine walking into a strong wind. The atmosphere is denser near the ground. So it pays to get above the lower atmosphere as fast as possible, to minimize the loss of energy. A horizontal track would work, but would have to launch a vehicle faster to allow for this loss, than a vertical or tilted take-off. And so yes, this design is feasible. Robert A. Heinlein's book The Moon Is a Harsh Mistress describes one of these and some of the science. FT2 (Talk) 00:33, 27 April 2006 (UTC)
Wow...great answers. Thanks everyone! --Chuck 02:07, 27 April 2006 (UTC)
- You often hear about jump launches like this as being good solutions for launching RAMJETs or other high-speed craft that can't operate properly at low speed. After accelerating down a long maglev (or similar) track (a very wide circle track might work even better) the craft launches off the final ramp and the engines engage. The main problem with this is that if the track isn't circular, you will almost certainly be subject to very high g-forces from acceleration, and engaging the engines after flying off a ramp probably isn't the most comfortable experience either. freshgavinΓΛĿЌ 04:16, 27 April 2006 (UTC)
- It's the acceleration on the tightly curved ramp at the end that would keep me from getting on this thing. If the device were launching objects without their own propulsion into orbit, and had them moving at escape velocity (many thousand mph) by the time they reach the curve, there would be preposterous force on both the track and projectile, whether the curvature is circular or not. The only way to practically build something like this is to lay the entire track (or at least the highest-speed portions) on a straight line. As noted above, even a horizontal track would work, provided there are no mountains in the way. ×Meegs 06:30, 27 April 2006 (UTC)
baseball problem[edit]
A 0.145kg baseball travellng at 30m/s strikes the cathcesr mitt bringing the ball to a stop. The glove recoils back 11cm. What is the average force applied by the ball on the glove?
Confused since the distance (11cm) is after the ball is at rest. then I don't enough info to do it, but yeah if someone could show me thanks.
C-c-c-c 22:44, 26 April 2006 (UTC)
F = ma
A = 30/t
D = 11 = VT + .5AT^2
Two equations, two unknowns. Solve for F&T. 12.183.203.184 22:53, 26 April 2006 (UTC)
- To address the fundamental problem, try spending some more time visualizing the situation before deciding you don't understand it. Clearly the glove didn't move 11cm after it was at rest. Melchoir 23:07, 26 April 2006 (UTC)
- Although, actually, there's a second problem with the question. To determine the time-averaged force, you'd have to know the time it took to stop the ball, which you're not given. So you can really only determine the space-averaged force. One way is to assume a constant force, as 12.183.203.184 suggests. Melchoir 23:23, 26 April 2006 (UTC)
For the heck of it I did v2^2 = v1^2 + 2ad with V1 being 30m/s and V2 0 and I get twice the correct answer.
v2^2 = v1^2 + 2ad (v2^2-v1^2)/(d) = a (0 - 30^2)/(0.11) = a
a = -8181.81m/s^2 F = ma F = 0.145(-8181.81) F = -1186.36
The answer I was given was 588.6. Anyone know why this happens? Thanks. --- C-c-c-c 01:46, 27 April 2006 (UTC)
- When you know you're off by a factor of 2, that's a good time to step back through your work and determine where you dropped it. Melchoir 01:52, 27 April 2006 (UTC)
I think your equation is off by two. VT +1/2 AT^2 = D
- The first equation is fine. Melchoir 08:47, 27 April 2006 (UTC)
How much does a flea weigh?[edit]
What is a ballpark figure for the weight (or mass) of a typical flea, say for concreteness a hungry adult Pulex irritans? The relevance of this itching question is that the article Planck mass states (without source) that "the Planck mass is on a scale more or less conceivable to humans, as it is roughly the mass of some fleas." The article Flea sheds no light on the issue. I put this question on the talk page of Flea, but in more than a month there has been no reaction – no-one wants to talk about fleas. LambiamTalk 22:40, 26 April 2006 (UTC)
- (Heh, after reading just your first sentence, I was about to answer "oh, about a Planck mass". Guess that doesn't cut it?) Melchoir 23:34, 26 April 2006 (UTC)
- This page indicates that females can consume up to 14 μL of blood per day, amounting to about 150 times their body mass. Assuming a density of blood of a bit more than 1 g/cm3 (close enough for our purposes) 14 μL of blood would weigh about 15 mg. Divided by 150, that's a weight per flea of about 100 μg—I don't know if that would be the dry or full weight, however. Given a Planck mass of about 21 μg, I'd say we're getting well into the realm of 'close enough'. TenOfAllTrades(talk) 00:32, 27 April 2006 (UTC)
- Update. Guess what, the value for Planck mass quoted in the article was just corrected by an anonymous editor; it was off by a factor of 1000. It's 21 pg, not μg. While not as unimaginable to us as the Planck length, fleas would then be a whopping 5000 Planck masses. Although, is the Planck length so unimaginable? Lambiam's Law: If you have to walk the plank, it will appear a Planck length. LambiamTalk 20:55, 30 April 2006 (UTC)
Is it possible with todays technology that a actual mass driver such as a railgun could be built? If it was then what would it be made out of and how could the required amount of energy be produced to send a vehicle speeding out of our atmosphere at escape velocity? —The preceding unsigned comment was added by 68.120.231.85 (talk • contribs) 23:25, 26 April 2006.
- No, it's not currently possible. One problem is that a projectile (like a rocket) needs to move slower in the thick lower atmosphere to avoid burning up. However, I can imagine a setup where you give a conventional rocket a bit of a push-start with such a device. Some other technology, like compressed air, would also allow you to give a rocket a head start and thus save on fuel it needs to keep onboard. This would allow for much lighter, smaller rockets. It's also far easier to store large quantities of energy as compressed air than as electricity, and electromagnetic interference isn't an issue. StuRat 02:10, 27 April 2006 (UTC)
- Also see mass driver, rail gun, and coil gun. StuRat 02:26, 27 April 2006 (UTC)
Thank you that actually was the answer I was looking for. Patrick Kreidt
- You're quite welcome. StuRat 15:03, 27 April 2006 (UTC)
April 27[edit]
Hydrogen as energy source/fuel[edit]
Hydrogen is praised as the fuel of the future for it's non-polluting properties, but nowhere have I found how much energy is required in producing a given quantity of energy in hydrogen. The process of hydrogen production can't be better than 100% efficient, and if it uses oil or coal we are just transferring the problem from the end-consumer to the producer. I would like to know something about the efficiency of hydrogen production, and the method(s) used. Researching this subject and publishing the results may clarify the situation, and your efforts would be sincerely appreciated. Henry Boessl —The preceding unsigned comment was added by 63.22.138.50 (talk • contribs) 01:02, 27 April 2006.
- You're correct that it takes a considerable amount of energy to produce hydrogen gas. And, of course, there aren't vast quantities of it sitting around like petroleum and coal (although not as huge quantities as there once were). However, hydrogen is a reasonably good way to store and distribute energy. Compared with coal, for example, it can be shipped through pipes and used in cars, while coal isn't suitable for either purpose. So, burning coal and using the energy to produce hydrogen, say by the electrolysis of water, allows you to distribute hydrogen for fuel cells or direct combustion in cars and eventually for other purposes, like home heating. Another advantage is that the pollution created by the coal is at the power plant, and very little pollution is released from the car. By placing the power plants away from dense population centers you can thus reduce pollution in populated areas. Another competing temporary way to store energy is as electricity stored in batteries in the car. At present, however, batteries don't carry much energy relative to their weight, so this may give hydrogen gas the advantage. Energy produced by other non-portable sources, like nuclear, hydro, geothermal, etc., may also be converted into hydrogen gas. I don't see it being worthwhile to convert other portable energy sources, such as petroleum, to hydrogen gas, however. The advantage of moving the pollution away from cities doesn't seem to be worth the energy loss incurred in such a conversion. StuRat 01:32, 27 April 2006 (UTC)
- Hydrogen can be produced by reducing steam with coke (the latter being produced from coal). That does make CO2, but possibly less than you'd produce by burning gasoline to power a car to go the same distance. (I say "possibly" because I don't really know; anyone who does know should feel invited to jump in here.) If that's true then total greenhouse emissions could be reduced; even if it isn't, it's still a way to use coal, which the US has a lot of, to power cars. It's surely better than burning it for electricity and then hydrolyzing water.
- The only solution I can think of, though, that uses hydrogen to let us keep driving while eliminating greenhouse emissions is to make it using nuclear power. (Yes, the full cycle of nuclear power production does currently produce some greenhouse gases, but that's because mining, construction, etc use fossil fuels; they could be switched over to nuclear-generated hydrogen as well.)
- But there is an issue that hasn't yet drawn a lot of attention, but will have to be dealt with if the hydrogen thing really gets going: Hydrogen could cause ozone depletion (hope I don't have to explain why :-). Just how serious the problem is, or how hard it would be to keep the hydrogen from leaking, I don't know if anyone really knows. --Trovatore 02:15, 27 April 2006 (UTC)
- The two answers above are excellent, you may also be interested in reading our article on hydrogen economy. — QuantumEleven 09:44, 27 April 2006 (UTC)
I was researching this a few years ago and came up with the same problem. However, I then found that there are algae which can be induced to produce hydrogen, and if stimulated correctly will hopefully produce sufficient quantities to be worthwhile. The info I found suggested 10 years as the time frame before they had good results, but that was about 3 years ago. Anyway, if it worked you could get your hydrogen from algae, inputting only sunlight and a few chemicals to up the prduction. 88.144.79.120 16:39, 27 April 2006 (UTC)
- This new technology could be considered a more efficient form of solar energy, which is sorely needed. I hope it works out. StuRat 00:40, 29 April 2006 (UTC)
This was my first attempt at using Wickipedia and I am amazed how quickly and thorough the response was. Many thanks to those who took the time to reply; irelly appreciate their effort. HB
- You're quite welcome. StuRat 18:16, 29 April 2006 (UTC)
Electroysis to breath underwater?[edit]
Please help
I am still in grade school and very interested in electricity, and i've read a few books on electricity and i cant seem to really understand if anything answers my question; Is it possible to make an underwater breathing apparatus using materials such as an electrolysis device with like a mouth piece or something that 1 person could use and operate, like a scuba tank, so they could breath underwater? And would it be safe?
Thank-you in advance for you help. --216.197.232.142 03:43, 27 April 2006 (UTC)Gus
- I imagine it is technically feasible with an adequate power source, but you would need to think through a few more points, like where is the hydrogen going to go, and how do you safely handle pure oxygen. I think a more interesting question is the that 1 person could use part, whether or not it would be less massive/bulky/dangerous than a simple tank of compressed oxygen. Peter Grey 05:21, 27 April 2006 (UTC)
- The power source is probably the biggest problem. If you were going to carry a lead battery to power your electrolysis, it would probably outweigh an equivalent air tank by a large factor (but how large, I don't know). -lethe talk + 05:24, 27 April 2006 (UTC)
- You're right. Basically, the energy density of a lead battery is not very good; the energy you got out of 1 kg of battery is enough to electrolyse only a small fraction of 1kg of water. To do the calculation yourself, you need to know the energy density of a lead-acid battery and how much energy it takes to electrolyse water; both statistics shouldn't be too hard to find. It's unlikely that any conventional power source is likely to be dense enough to pull this off; the latest and most efficient thing in "conventional" submarines is powering them with fuel cells, which is essentially electrolysis in reverse! Nuclear-powered submarines have fuel with enormous energy density, so they indeed use the scheme you describe (as well as devices to remove carbon dioxide, carbon monoxide, and other contaminants from the atmosphere) to provide breathable air on their very long voyages.
- --Robert Merkel 06:28, 27 April 2006 (UTC)
- Maybe I can make a rough stab at the math. From [67] I get that, say, 1 kg of water would require to dissociate into an atmosphere (in other words, into your helmet or so). From this, you'd get 889 grams of oxygen. That much energy at, say, the 1.6 eV per electron in a silver-oxide battery, is electrons. You get two per reaction, and each reaction involves 297 amu of reacting material (not counting catalysts, but in theory they can be amortized over many reactions). So that's 15.3 kg of reactant to "power" 889 grams of oxygen (factor of 17.2). You're probably better off just carrying the oxygen; while the oxygen tank adds some weight, the battery structure and electronics and electrolysis equipment would add a good bit too. --Tardis 07:12, 27 April 2006 (UTC)
Followup: As Peter Grey touched on, can you, in fact, create a breathable mixture solely from hydrogen and oxygen (and compounds of the two)? If not, this device would presumably have to process and recycle the diver's exhaled air just as the submarines. ×Meegs 08:02, 27 April 2006 (UTC)
- That's a very creative idea, although I don't think it could be made to work no matter how it was engineered. The reason is that people normally don't breath pure oxygen. This can cause oxygen toxicity. SCUBA divers breathe compressed air, which about 78% nitrogen and only about 21% oxygen. See also breathing gas and air. --Ginkgo100 18:31, 27 April 2006 (UTC)
- Hey, the article on breathing gases mentions hydrogen as component of deep-diving mixes! Just an aside, cyclopropane and acetylene used to be used in anesthesia, if it weren't for the fact that they are explosive they would be great anesthetics - no liver and kidney toxicity, fast equilibration with lung air. Dr Zak 19:38, 27 April 2006 (UTC)
Aren't there bacteria that produce oxygen (though I'm not sure if they exist anymore)? If they are still around, could they produce enough oxygen to make it possible to breath underwater, seeing as you would only have to caarry traces of other gases and a tank to hold your carbon dioxide. Might not make any sense, I'm still in grade school too.
- Recycling exhaled air is nothing new in serious diving so that wouldn't be an issue though you may have to carry a small ammount of your mixer gas to make up for losses. As already mentioned the issue with anything that depends on splitting water is power. Fossil fuels are pointless as you'd have to carry oxygen to burn them with. Battries are too low energy density. A RTG might work but you'd probablly have difficultly getting it past the authorities. Plugwash 01:11, 1 May 2006 (UTC)
- The oxygen released by plant-like bacteria (or plankton, or whatever else) is largely already contained in the carbon dioxide they use. In other words, you'd already be carrying most of your oxygen, and then you'd have to carry the carbon and the bacteria too! Not to mention additional tanks, piping, and some sort of environmental control to keep them healthy... --Tardis 13:10, 1 May 2006 (UTC)
Dimensionally prove the simple pendulum equation![edit]
Sir, there is the prove of simple pendulum equation or formula but there is no dimensional analysis of this formula to check weather it is correct dimensionally or not, For just studing for college--80.231.14.119 06:58, 27 April 2006 (UTC)
- What? Melchoir 07:02, 27 April 2006 (UTC)
- Er, if it's been proven, you don't need to do dimensional analysis. Dimensional analysis finds bugs, but proof precludes them (notwithstanding any bugs in the proof!), so the analysis would be redundant. Why would you want it specifically? --Tardis 07:14, 27 April 2006 (UTC)
Sir i want for studing for college.i know its all right proof but for my personal studies i want proof of Dimensional analysis. If u write for me i am very thank ful to you--80.231.14.119 07:31, 27 April 2006 (UTC)
- Since it's so insanely easy we may as well do it for you. You haven't specified which formula you want, so I'm going to do the period for the small angle approximation, .
- is a time, so it has dimensions of T. is a dimensionless constant. is a length, dimensions of L. is an acceleration, dimensions of LT-2. So, has dimensions of L/(LT-2) = T2, and its square root therefore has dimensions of T, as required.
- If it's some other formula you wanted have a go at it yourself. --Bth 07:37, 27 April 2006 (UTC)
Thanks sir i am very very thankful ,remmember that this is the only site which help me in studies! whole google search not give me such type of intresting site Sir--80.231.14.119 07:46, 27 April 2006 (UTC) Give the drawbacks to use the time period of pendulum as a time standard--80.231.14.119 07:54, 27 April 2006 (UTC)
- OK, see, if you're going to get us to do your homework, you have to work hard at pretending that it's not. Quoting the next question in such obvious "this is a question" form is a dead giveaway. I'm sorry I helped you now. --Bth 08:15, 27 April 2006 (UTC)
"Give the drawbacks to use the time period of pendulum as a time standard"if any--80.231.14.119 08:23, 27 April 2006 (UTC)
- Erm, you seem to have misunderstood - please read the rules at the top of the page, we are here to help with concepts and try and answer general questions, but we strongly object to doing your homework for you. Your question "Give the drawbacks to use the time period of pendulum as a time standard, if any" smells very strongly of homework, so we won't help you, as it won't do you any good to have others do your work for you. Please come back when you have finished your homework. — QuantumEleven 08:32, 27 April 2006 (UTC)
- But do you know the application of simple pendulum? User:Ngocthuan 0603:38, 27 April 2006 (UTC)
- I relent, I'll give you the answer. Think about the equation that you are working with...it's a simple pendulum. What assumption was made to derive the equation? Hm...I wonder... --HappyCamper 02:51, 30 April 2006 (UTC)
photons[edit]
Anything that moves at light speed has infinite mass.The photon has no mass.How does this explain RADIATION PRESSURE?
- Anything with a rest mass cannot reach light speed in the first place, because to do so would require infinite energy. Anything with a zero rest mass can only travel at light speed. Photons still have energy (equal to Planck's constant times their frequency), and it's the transfer of that energy that ultimately accounts for radiation pressure. --Bth 08:24, 27 April 2006 (UTC)
- Moreover, radiation pressure is a classical effect of electrodynamics that doesn't even require photons. Ah well... how many times have we gotten this question now? Maybe we need a FAQ. Seriously. Melchoir 08:58, 27 April 2006 (UTC)
I'm well versed with the fact that only particles can interact because of change in momentum.Is there something im missing here?
- Photons have momentum. It's h/λ. —Keenan Pepper 14:00, 27 April 2006 (UTC)
- Have a read of Optical tweezers. -- SGBailey 08:30, 29 April 2006 (UTC)
So does this mean that photons have zero rest mass and only transfer energy equal to product of h and frequency.so is it some sort of energy particle?
Hepatits School Project[edit]
I need to find out specific numbers of the incidents and deaths for most of the countries of the world. If anyone can help please I really need it. Thanks
For every single country in the world there are some incidents and deaths from the disease Hepatitis. I require the specific number for each country. Anything Helps!! --204.218.240.30 08:20, 27 April 2006 (UTC)
- I think you will struggle very much to get morbidity rates: hepatitis (which is a symptom, not a disease: it refers to several different conditions, including a number of viruses, damage from too much alcohol and prescription drug side-effects) is not necessarily even diagnosed, and if it is, not all countries will have any system of counting incidents, and of those that do, not all will publish the data. Even getting mortality figures is questionable for most countries. Where information is published it may well be in medical journals for which you would have to visit a medical library - assuming you can find the existence of the article. I think you either need to find an easier project, or be satisfied with a much simpler lot of information to collect. Notinasnaid 08:41, 27 April 2006 (UTC)
There is no single disease "hepatitis". There are many causes of infection or inflammation in the liver, which is what hepatitis means. To get a sense of your challenge google [hepatitis] and [epidemiology]. For example, see [68], which gives some vague statistics for endemicity of hepatitis B. Those percentages on the map refer to the percentages of people thought to be infected. Hep B is the most well-known kind of hepatitis. There is much less info available for the other types. After you have a sense of what is available through Google, go to medline or Pubmed with the best terms. Good luck. alteripse 10:31, 27 April 2006 (UTC)
BTW, you are talking about "incidence", not "incidents", and you may find it easier to look for prevalence figures. - Nunh-huh 19:29, 27 April 2006 (UTC)
Symptoms of hepatits
The principle symptom of hepatits is the spontaneous appearance of five extra nipples on the patient's chest area.
Sorry, it's so late it's early and I couldn't resist. Howard Train 09:48, 30 April 2006 (UTC)
Mobile mouse cursor[edit]
I'm using a Mini Optical 3D mouse by A4Tech. Firstly,what's with it being 3D?Secondly,sometimes the mouse cursor suddenly starts moving on its own,without any physical force being applied on the mouse.Why and how does this happen and how can I prevent this?
Thanks in advance.
- 3D is probably why "2000" is added to waterguns. Or maybe the company is reminding you of the mouse's three dimensional features. The mouse cursor is moving around (like jumping? or slowly moving like how fast you contorl it) because of the surface your optical mouse is on. Put it on a mousepad or something less rough or shiny. --
 Mac Davis] ⌇☢ ญƛ. 10:19, 27 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 10:19, 27 April 2006 (UTC)
It ison a clean mouse pad already.
- In that case you have perhaps been infected by malware. Someone outside your computer could be taking control of it. No joke, this happens. Notinasnaid 12:49, 27 April 2006 (UTC)
- If it is an optical mouse it may not have to do with it being clean or not. Some surfaces provide better reflection than others. But in any case -- you might try fiddling with the mouse settings and reducing its sensitivity. While it's always possible that you are suffering from malware, if by "moving on its own" you mean just random little movements and not purposeful moving, then it is probably just being overly sensitive and/or on a surface which is confusing it. --Fastfission 15:41, 27 April 2006 (UTC)
I think I have a pretty good defence against all sorts of malware,adware and spyware.Have d/loaded plenty of softwares to protect my system. I was thinking more along the lines of some physical damage to my mouse maybe. And when I say "moving on its own",I mean that it can cover the whole length of my monitor screen in 20 seconds,if I don't stop it from doing so.It's a minor problem,but sometimes disturbing.Its like phantom movement by my mouse.
Anyone,please?
- Firstly, if you can, either try another mouse on your computer, or try your mouse on another computer - that will tell you whether the probalem is with the mouse itself, or your computer. Is your computer a laptop? if so, it may be receiving spurious input from it's built in pointer control (such as a touchpad or keyboard nipple), you could try disabling that (possibly through the BIOS). Also, try updating any special driver software that your mouse uses. If your mouse is mechanical (has a ball in the bottom) try taking the ball out and cleaning the rollers, and if it's optical, check the surface you're using it on - optical mice don't like reflective surfaces, or some patterns such as stripes. --Noodhoog 15:31, 4 May 2006 (UTC)
ion wind effects[edit]
Does the pressure force created by ionised air, or ion wind effect only conductive materials or can it lift non-conductive materials such as wood. not generating it but just being pushed up or sideways by it?
Edward knott 11:43
- Ions are just atoms or molecules with a net charge. They can exert pressure on anything just like a normal gas. —Keenan Pepper 14:03, 27 April 2006 (UTC)
- I'm not sure that either of the two posters above looked at the relevent articles. As the Air ioniser article itself states, such machines have nothing to do with ion wind. The Ion wind article is not very clear, but it stresses that any force is not due to a tranfer of moment to another body, but from the impulse of the ions leaving the generator. In which case, I would assume that an ion wind can only cause thrust in the generator, which must itself be conductive. — Asbestos | Talk (RFC) 16:49, 27 April 2006 (UTC)
Uuuuum so are we gona get a concencus here? some say one thing, others say another. So which is it exactly? Edward
- My opinion is that Asbestos and I read your question differently. In my reading, you ask if an ion wind genererator can generate enough wind to move wood. Yes. I gave an example - the Ionic Breeze (which is a very weak ion wind generator) creates enough of a breeze that you can feel it. If you use a much better generator, you'll get more wind, and more wind will move larger things. Asbestos appears to have read it as: Can an ion wind generator attached to some wood device be some sort of lift engine? Not effectively. Lifters (made with very light weight wires, foil, and toothpicks) use ionic wind engines to lift a wood (toothpick) structure. They are very weak and fragile. I've seen models with dozens of lifters connected together to lift a small object, but the power generator is far to heavy to be contained on the lifter. Instead, it sits on the ground and is just wired to the lifter (which usually lifts, gets pulled towards the generator by the cables, hits the generator, sparks, then bursts into flames.) --Kainaw (talk) 02:43, 28 April 2006 (UTC)
Thank you, i was not asking about this because i want to build a lifter, i was just wondering if the force used in them, if generated some other way could lift all kinds of objects rather than just metal ones.
Edward
animal/plant/fungus/protist[edit]
I'm trying to write the start page on a wikibook called Dichotomous Key; you have to answer a certain number of questions to determine the species of an animal. (For example, I would ask: Does it have a nucleus? If not, it's a bacteria. If so, go to section #3 for the next question.)
But I'm not quite sure what questions to ask to determine: Animal, Plant, Fungus, and Protist. I looked at the corresponding Wikipedia articles but they're not much help. The Protist article says that protists are the "left-over" kingdom; if a eucaryote isn't an animal, plant, or fungus, it's a protists. The animal, plant, and fungus articles are not much help at all and I'm not sure what questions to ask for the Wikibook. Jonathan talk ![]() 14:10, 27 April 2006 (UTC)
14:10, 27 April 2006 (UTC)
- There's no such thing as "a bacteria". --Trovatore 14:48, 27 April 2006 (UTC)
- Hmmm... You aren't working from an existing Dichotomous Key? They can be very complex, precise and intricate. Unless you're working from a source, or have more than a working knowledge of the distinctions, I'd say you're in for many headaches... The Protist would be the easiest to define — if you knew the definitions for the rest of the categories... Animals are typically defined at least in part by their locomotive ability. Fungi can be separated from plants by not being heterotrophs. — Asbestos | Talk (RFC) 15:41, 27 April 2006 (UTC)
- Thanks for the answers. And when I said a bacteria I meant eubacteria or archaebacteria. Jonathan talk
 17:14, 27 April 2006 (UTC)
17:14, 27 April 2006 (UTC)
- Still wrong. The singular is "bacterium". --Trovatore 17:32, 27 April 2006 (UTC)
- Oops, sorry.
- I still need help! (You can see the page I created on the wikibook here.) I can classify animals, plants, and fungi well enough, but some protists can move around too, right? And some protists get energy from the sun, and some get energy from other organisms! Some protists are unicellular and some are multicellular! Does anyone have any more characteristics that separate animals, plants, and fungi from protists for me please? -- Jonathan talk
 17:43, 27 April 2006 (UTC)
17:43, 27 April 2006 (UTC)
- I assume you are familiar with Wikispecies, right? You may also find the excellent Tree of Life project helpful. — Knowledge Seeker দ 03:19, 28 April 2006 (UTC)
- Still wrong. The singular is "bacterium". --Trovatore 17:32, 27 April 2006 (UTC)
- Thanks for the answers. And when I said a bacteria I meant eubacteria or archaebacteria. Jonathan talk
Solar Powered Cats[edit]
If I had an army of solar powered laser cats, whose surface area is covered by enough solar pannels to produce enough electricity to run 1.1 jigawatt lasers, would you still hear a sound if a flea landed on a cute puppy in woods, while being propelled into orbit by a cyclic path? and would it allow me to breathe underwater with the aid of my solar powered laser cat army whose 1.1 jigawatt laser would allow me to transport oxygen free radicals from the year 1885 directly to my breathing apparatus in the year 2007? assuming of course that the force felt by photons impacting on the surface of the water, allowed for enough solar energy to penetrate the depth of the water, and reach the solar pannels, of course--Nullity 15:45, 27 April 2006 (UTC)
- No, you can't breath underwater in any case, because "breath" is not a verb. --Trovatore 15:54, 27 April 2006 (UTC)
- nitpicker--Nullity 15:57, 27 April 2006 (UTC)
You want a nit-picker? To your last sentence, the following corrections should be made: -Two spaces after the preceding question mark -Capitalize 'assuming' -Comma after 'Assuming' -Comma after 'course' -Omit the word 'on' after 'impacting' -Omit comma after 'water' -Add the word 'to' before 'reach' -Spell the word 'panel' correctly -Period after 'panel' -Omit the redundant 'of course' at the end. -Luke
- if a flea landed on a cute puppy in woods... would it allow me to breathe underwater with the aid of my solar powered laser cat army What's the distance from the flea to the water? Peter Grey 17:08, 27 April 2006 (UTC)
If trees could scream, would we be so quick to cut them down? We would if they were screaming all of the time. -Jack Handy (SNL) -Luke
- Why do you say 2007, not 2006? Jonathan talk
 17:11, 27 April 2006 (UTC)
17:11, 27 April 2006 (UTC)
- Isn't it obvious? The solar powered laser cats are still in R&D. They won't be ready until 2007. --Kainaw (talk) 17:36, 27 April 2006 (UTC)
- It's true. My current models are nuclear-powered, but fuel is so expensive these days. Melchoir 18:27, 27 April 2006 (UTC)
- Have you considered training the cats to use public transportation? --Kainaw (talk) 18:31, 27 April 2006 (UTC)
- That was the 1994 model. I admit, they were efficient, and they kept a low profile, compared to streaking across the sky at Mach 2 to engage my enemies. But wouldn't you know it, the damn things achieve self-awareness and suddenly it's me, me, me. We want turreted lasers, we want a sexier means of transportation, we want full dental. And if you try to say no, they morph into kitten mode and stare at you with those eyes... Melchoir 18:48, 27 April 2006 (UTC)
- Please, please stop...these threads are so hilarious that I am getting Wikipedian stomach aches from laughing...someone needs to archive these at WP:-) for sure!!! --HappyCamper 00:40, 28 April 2006 (UTC)
- That was the 1994 model. I admit, they were efficient, and they kept a low profile, compared to streaking across the sky at Mach 2 to engage my enemies. But wouldn't you know it, the damn things achieve self-awareness and suddenly it's me, me, me. We want turreted lasers, we want a sexier means of transportation, we want full dental. And if you try to say no, they morph into kitten mode and stare at you with those eyes... Melchoir 18:48, 27 April 2006 (UTC)
- Have you considered training the cats to use public transportation? --Kainaw (talk) 18:31, 27 April 2006 (UTC)
- It's true. My current models are nuclear-powered, but fuel is so expensive these days. Melchoir 18:27, 27 April 2006 (UTC)
- Isn't it obvious? The solar powered laser cats are still in R&D. They won't be ready until 2007. --Kainaw (talk) 17:36, 27 April 2006 (UTC)
- Why do you say 2007, not 2006? Jonathan talk
- It's a well-known fact that cats are solar powered gravity machines. They find a sunny spot to sleep in all day, and then at night they climb on top of you in bed and weigh approximately seventy three times what you'd expect. --LarryMac 00:43, 28 April 2006 (UTC)
- If humans became extinct, would laser cat kittens still be cute? (Are they cute now? I've never seen one) Peter Grey 04:34, 28 April 2006 (UTC)
Do they ever feel melancholy?
- Have they ever felt lonely? Heve they ever felt blue? Oh, and is there an equivalent of BJAODN for the Reference desks? Grutness...wha? 03:10, 28 April 2006 (UTC)
Cats are like Goths. No matter how else they feel, there is always an element of melencholy.
Solar powered cats? Sounds good to me. EWS23 | (Leave me a message!) 03:22, 28 April 2006 (UTC)
Bah, solar powered ones are so usless. Those mouse power ones are 10 more reliable and the fuison ones go boom and kill you if you get them mad.
- The thing you must remember when speculating on the possible effects of owning an army of solar powered mammals (particularily, those of the feline genus) with high-powered laser implements, is that as you increase their wattage, you decrease the likelihood of their being herded effectively. Also, at the risk of wasting words with common knowledge, I must point out that cats are extremely prone to catching fire, and given the circumstances, you can see how possessing this type of creature, especially in large quantities, can present a very imminent danger. That being said, I do not recommend researching this topic with a hands-on approach- keep it hypothetical. Next, to address the question of whether or not an army of solar powered laser cats would allow one to breathe underwater via transportation of oxygen through time, I must say, I have no basis for comment. -Luke
Hair[edit]
How does hair 'know' when to stop growing. If I shave my arm, the hair will grow back to the former lenght, how does it know what length to grow to--that is, how does it know it has reached the previous length?? Ztsmart 19:00, 27 April 2006 (UTC)
- In hair it mentions that hair growth is timed. A folicle grows hair for a preset time and then stops. --Kainaw (talk) 19:03, 27 April 2006 (UTC)
so if I cut it, how does it know to start growing again? Ztsmart 19:19, 27 April 2006 (UTC)
- I don't know the "real" answer to this, but it's partly an illusion. Hairs are falling out and growing back all the time. When you shave, some notable portion of the hairs were already near the falling out/regrowing point, so they immediately start growing. Since some of them, at least, will reach very nearly their "full" length, it can easily look like they all grew back even if some of them were severely stunted for that cycle. --Tardis 21:15, 27 April 2006 (UTC)
- The brief answer is that the cut hair has no way of 'knowing' that it has been cut. A hair follicle will crank out hair for a fixed period of time, after which the hair (eventually) falls out; the cycle then repeats. Since your hair follicles are not sychronized with one another, you will have some hairs that are growing, some that have reached their full length, and some that are just getting started. The overall impression, however, is of a uniform, average length and thickness of hair.
- If you cut your hair, inactive follicles won't reactivate ahead of their normal schedule—those hairs will stay short until the fall out. Growing hairs, if cut, won't recover the length that was cut off; they'll continue growing for the usual length of time and be missing whatever length was removed. The newest hairs, however, will be short enough to remain uncut. It is this last population of hairs that will be first to regain the full, normal length in the area that was trimmed. TenOfAllTrades(talk) 23:33, 27 April 2006 (UTC)
- Also, I remember reading in the androgenic hair article that, say, why your pubic hair stops growing, is because something measures the weight of the hair, after it is great enough it will stop growing. --
 Mac Davis] ⌇☢ ญƛ. 00:47, 28 April 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 00:47, 28 April 2006 (UTC)
In my case, my giant hogg creates an intense gravitational field due to it's mass. This likely increases the weight my pubic hairs feel. How does this effect my pubic hair growth? 12.183.203.184 04:00, 28 April 2006 (UTC)
- If your hog was large enough to create its own gravitational field, your hairs would likely grow to a short length before plunging back into the skin of your hog, causing serious irritation and swelling, leaving you vulnerable to infection. I suggest cutting short, or getting a smaller hog. freshgavinΓΛĿЌ 07:08, 28 April 2006 (UTC)
Amides[edit]
i know that when i react a carboxlyic acid or an ester with a primary amine i will form a amide, now will these instantly form peptide bonds and create long chained polymers? or would i have to do something to the mixture to get it to? i'm thinking along these lines becuase i need to find something that proves theres been a positive test, cheers
--Colsmeghead 19:59, 27 April 2006 (UTC)
- If you have an amino acid, or a diamine and a dicarboxylic acid, then yes, it will polymerize to a polyamide if you heat it up enough. —Keenan Pepper 21:00, 27 April 2006 (UTC)
- If you're curious, the reaction is called condensation. Isopropyl 21:42, 27 April 2006 (UTC)
Sound formats and such....[edit]
I have a sound-file encoded in a52 format (which wikipedia tells me is Dolby format...not surprising since it came from a movie I guess). I'd like to have it in mp3 format, or any other nice format which my ipod will play. Suggestions? Oskar 20:04, 27 April 2006 (UTC)
- doom9.org has guides for working with and converting AC3. (Does Audacity support AC3?)- mako 22:30, 27 April 2006 (UTC)
World time[edit]
What is the "world time" that is mentioned here in this book? [69] --HappyCamper 21:06, 27 April 2006 (UTC)
- Never heard that one before. Could it be the proper time along a world line? Melchoir 21:22, 27 April 2006 (UTC)
- I don't think it is...it's some sort of formalism that is useful for addressing many body problems I think. But that's about it. I'm not really sure actually. --70.50.34.181 00:41, 28 April 2006 (UTC)
- Sigh...does anyone know? :-) --HappyCamper 03:02, 28 April 2006 (UTC)
- I suspect it refers to absolute time of some form. Here is a PDF that mentions it: [70]. How the book spins it — well, get the book and read it :-) Google terms you could try: "absolute time" "world time" einstein.Weregerbil 17:11, 9 May 2006 (UTC)
Physics[edit]
I would like to know the Hamiltonian for the 2-Dimensional
Denis Lieberman
--Mac Davis] ⌇☢ ญƛ. 00:45, 28 April 2006 (UTC)
latex snippets for windows[edit]
I use a mac, and for the mac, there are these wonderful programs Equation Service and LaTeXiT which allow you to input a snippet of LaTeX code and it gives you a cropped PNG or jpeg, which you can drag into an email or something. It's pretty much the same thing that texvc does for MediaWiki, except with a drag and drop GUI. I'd like this email conversation to be two-sided. Is there any equivalent program for windows? -lethe talk + 23:35, 27 April 2006 (UTC)
Not sure it has a GUI, but it is built for Windows, it's free and it's open source. It's called MiKTeX. Here is the Wikipeida article about it and here is the direct link to its development page. It is a lather large download, weighing in at just over 33MB, just as a warning. Hope this is helpful. yaninass2 | talk 00:26, 28 April 2006 (UTC)
- MikTex is a port of the standard teTeX distribution to windows. This is a necessary prerequisite before doing any latex rendering. People often use an IDE as a frontend to MiKTeX, which provides an environment for composing full LaTeX documents. There are many Windows IDE frontends available such as TeXnicCenter. What I'm looking for is a program that will allow you to input a single equation in LaTeX markup and will return a small PNG or GIF no larger than the equation. This doesn't require a full IDE like TeXnicCenter, but does require some automated image cropping and conversion tools like imagemagick. Miktex is a prerequisite for this, but I'm looking for something much more specific. Thanks anyway, though. -lethe talk + 01:19, 28 April 2006 (UTC)