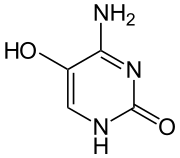
5-Hydroxycytosine
Appearance
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Amino-5-hydroxypyrimidin-2(1H)-one | |
| Other names
4-Amino-5-hydroxypyrimidine-2-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H5N3O2 | |
| Molar mass | 127.103 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
5-Hydroxycytosine is an oxidized form of cytosine that is associated with an increased frequency of C to T transition mutations, with some C to G transversions.[1] It does not distort the DNA molecule and is readily bypassed by replicative DNA polymerases.[2]
It has been shown in vitro to miscode for adenine.
5-hydroxycytosine is imperative for parallel DNA triplex formation, explaining why parallel triplexes form only at pH 6 and below.
References
[edit]- ^ Zahn KE; Averill A; Wallace SS; Doublié S (2011). "The miscoding potential of 5-hydroxycytosine arises due to template instability in the replicative polymerase active site". Biochemistry. 50 (47): 10350–10358. doi:10.1021/bi201219s. PMC 3280588. PMID 22026756.
- ^ Helmut Greim; Richard J. Albertini (2012). The Cellular Response to the Genotoxic Insult: The Question of Threshold for Genotoxic Carcinogens. Royal Society of Chemistry. p. 119. ISBN 9781849731775. Retrieved July 20, 2015.
