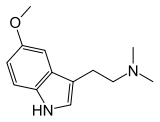
5-MeO-DMT
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 5-Methoxy-N,N-dimethyltryptamine; 5-Methoxy-N,N-DMT; O-Methylbufotenin; Mebufotenin; Methylbufotenin; BPL-002; BPL-003; LSR-1019 |
| Routes of administration | Inhalation, insufflation, sublingual, intramuscular, intravenous, oral (with an MAOI)[1][2] |
| Drug class | Serotonergic psychedelic (hallucinogen) |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Oral: inactive (without an MAOI) or weak[1][2] |
| Metabolism | Oxidative deamination (MAO), O-demethylation (CYP2D6)[2][1][4] |
| Metabolites | |
| Onset of action |
|
| Elimination half-life | Minutes[4] (12–19 min in mice, 6–16 min in rats)[2][5] |
| Duration of action |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.558 |
| Chemical and physical data | |
| Formula | C13H18N2O |
| Molar mass | 218.300 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
5-MeO-DMT (5-methoxy-N,N-dimethyltryptamine), also known as O-methylbufotenin or mebufotenin (INN), is a naturally occurring psychedelic of the tryptamine family.[5][1][4][2] It is found in a wide variety of plant species, and is also secreted by the glands of at least one toad species, the Colorado River toad.[5] It may occur naturally in humans as well.[5] Like its close relatives dimethyltryptamine (DMT) and bufotenin (5-HO-DMT), it has been used as an entheogen in South America.[5][6] Slang terms include Five-methoxy, the power, bufo, and toad venom.[7]
The drug acts as a non-selective serotonin receptor agonist, including of the serotonin 5-HT1A and 5-HT2A receptors among others.[1][4][8] However, 5-MeO-DMT differs from most other serotonergic psychedelics in having 100- to 1,000-fold higher affinity for the serotonin 5-HT1A receptor over the serotonin 5-HT2A receptor.[1][4][8] In relation to this, 5-MeO-DMT has been described as an "atypical" psychedelic and as producing subjective effects notably distinct from those of DMT and other psychedelics, for instance having a relative lack of visual effects.[5][1][4] Like DMT, 5-MeO-DMT has a very rapid onset of action and short duration.[1][4] However, 5-MeO-DMT is 4- to 10-fold more potent than DMT in humans.[2]
5-MeO-DMT was first synthesized in 1936 by Toshio Hoshino[9] (ja), professor of Tokyo Institute of Technology, and was first isolated from a natural source in 1959.[5] It is a controlled substance in some countries, for instance the United States, United Kingdom, Australia, and New Zealand.[5] The drug is used recreationally and several deaths have been reported in association with its use.[5][10] 5-MeO-DMT is being developed for potential use in medicine in the treatment of neuropsychiatric disorders such as depression.[5][1][4]
Chemistry
[edit]5-MeO-DMT, also known as 5-methoxy-N,N-dimethyltryptamine, is a substituted tryptamine derivative. It is the 5-methoxylated derivative of N,N-dimethyltryptamine (DMT), the N,N-dimethylated derivative of 5-methoxytryptamine (5-MT; mexamine), and the O-methylated derivative of bufotenin (5-HO-DMT).
It has a relatively high experimental log P of 3.30.[2][11]
Analogues of 5-MeO-DMT include 4-MeO-DMT, 5-MeO-AMT, 5-MeO-DET, 5-MeO-DPT, 5-MeO-DIPT, 5-MeO-MiPT, 5-EtO-DMT, and 5-MeO-MET. Other analogues include dimemebfe and EMDT.
Effects
[edit]When smoked, the duration of effects can be as little as ten minutes; when insufflated, up to two hours. Effects vary and can range from radical perspective shifting and perception of new insights, euphoria, immersive experiences, dissociation and non-responsiveness, sensual/erotic enhancement, to dysphoria, fear, terror, panic, and ego death.[12][better source needed]
The subjective effects of 5-MeO-DMT are described as distinct from those of DMT and other psychedelics.[4][5] Whereas DMT is described as producing more "information-rich" experiences, with "rich sensory phenomenology", visuals, and experiences of encountering entities and visiting other worlds, 5-MeO-DMT is described as having a relative lack of visual effects, producing a sense of "nothingness", and causing experiences that are said to be "content-free" and sometimes known as "whiteouts".[4][5] These experiences have been described as "beyond ordinary human comprehension", with a subjective impression of a void or amnesia of the experience.[4][5] In spite of this however, some have described the experiences as orgasmic, ecstatic, and blissful, whereas others have described them as terror or "information overwhelm".[4] As with DMT and other psychedelics, the experiences with 5-MeO-DMT are often described as overwhelming, profound, spiritual, religious, and/or mystical.[4][5]
The experiences of 5-MeO-DMT have been related to the experience of ecstatic seizures.[4]
Uses
[edit]Preliminary clinical findings suggest that 5-MeO-DMT might have antidepressant and anxiolytic effects.[13][14]
Religious use
[edit]The Church of the Tree of Life, founded in California in 1971 by John Mann but now defunct, declared the use of 5-MeO-DMT to be a sacrament. From approximately 1971 to the late 1980s, 5-MeO-DMT was discreetly available to its members.[15][16] Between 1970 and 1990, smoking of 5-MeO-DMT on parsley was probably one of the two most common forms of ingestion in the United States.[16][unreliable source?]
Pharmacology
[edit]Pharmacodynamics
[edit]| Target | Affinity (Ki, nM) |
|---|---|
| 5-HT1A | 1.9–28 (Ki) 3.92–1,060 (EC50) 68–98% (Emax) |
| 5-HT1B | 14–351 (Ki) 1.53 (EC50) 78% (Emax) |
| 5-HT1D | 2.3–20 (Ki) 37 (EC50) 98% (Emax) |
| 5-HT1E | 360–528 (Ki) 92–160 (EC50) 119% (Emax) |
| 5-HT1F | 37 (Ki) 14 (EC50) 93% (Emax) |
| 5-HT2A | 15–2,011 (Ki) 1.80–3.87 (EC50) 82–101% (Emax) |
| 5-HT2B | 36–3,884 (Ki) 5.87 (EC50) 73% (Emax) |
| 5-HT2C | 42–538 (Ki) 31 (EC50) 84% (Emax) |
| 5-HT3 | >10,000 |
| 5-HT4 | >10,000 (EC50) |
| 5-HT5A | 277–505 (Ki) 110 (EC50) 107% (Emax) |
| 5-HT6 | 6.5–78 (Ki) 0.24 (EC50) 125% (Emax) |
| 5-HT7 | 3.9–30 (Ki) 65.7 (EC50) 107% (Emax) |
| MT1 | 210 (Ki) 257 (EC50) |
| MT2 | 16 (Ki) 112 (EC50) |
| α1A | 4,373–>10,000 |
| α1B | 2,188–>10,000 |
| α1D | ND |
| α2A | 938–1,890 |
| α2B | 430–2,640 |
| α2C | 206–508 |
| β1 | >10,000 |
| β2 | 2,679–>10,000 |
| β3 | >10,000 |
| D1 | 80–>10,000 |
| D2 | 3,562–>10,000 |
| D3 | 498–>10,000 |
| D4 | 3,120–>10,000 |
| D5 | >10,000 |
| H1 | 7,580 |
| H2–H4 | >10,000 |
| M1–M5 | >10,000 |
| σ1 | >10,000 |
| σ2 | >10,000 |
| SERT | 2,032–3,603 (Ki) 2,184 (IC50) >10,000 (EC50) |
| NET | 2,859–>10,000 (Ki) >10,000 (IC50) >10,000 (EC50) |
| DAT | >10,000 (Ki) >10,000 (IC50) >10,000 (EC50) |
| Notes: The smaller the value, the more avidly the drug binds to the site. Proteins are mostly but not exclusively human. Refs: [5][8][17][18][19][20][21][22][23][24] | |
5-MeO-DMT is a methoxylated derivative of dimethyltryptamine (DMT). While most common psychedelics are believed to primarily elicit psychological effects through agonism of serotonin 5-HT2A receptors, 5-MeO-DMT shows 1,000-fold greater affinity for 5-HT1A over 5-HT2A;[25] In line with its affinity for 5-HT1A receptors, 5-MeO-DMT is extremely potent at suppressing the firing of dorsal raphe 5-HT neurons.[26] Further, its activity in rats was attenuated with the 5-HT1A selective antagonist WAY-100635 while 5-HT2A selective antagonist volinanserin failed to demonstrate any change.[27] Additional mechanisms of action such as inhibition of monoamine reuptake may be involved.[28] A 2019 European study with 42 volunteers showed that a single inhalation produced sustained enhancement of satisfaction with life, and easing of anxiety, depression, and post-traumatic stress disorder (PTSD).[29] A 2018 study demonstrated that a single dose of 5-MeO-DMT induced neurogenesis in mice.[30]
Similarly to other serotonergic psychedelics, 5-MeO-DMT is a non-selective serotonin receptor agonist, including of the serotonin 5-HT1A, 5-HT2A, 5-HT2B, and 5-HT2C receptors, among others.[1][4][22][21] It is 4- to 10-fold more potent as a hallucinogen than DMT in humans.[2] In contrast to most serotonergic psychedelics however, it is has been said that it is unclear that the hallucinogenic effects of 5-MeO-DMT are principally mediated by activation of the serotonin 5-HT2A receptor.[4] In any case, 5-MeO-DMT does still activate the serotonin 5-HT2A receptor and does still produce psychedelic effects.[4] It has been proposed that 5-MeO-DMT be considered an "atypical" psychedelic.[4] This relates to the fact that 5-MeO-DMT has 100- to 1,000-fold selectivity for the serotonin 5-HT1A receptor over the serotonin 5-HT2A receptor and that the actions of 5-MeO-DMT appear to be primarily mediated by serotonin 5-HT1A receptor activation.[1][4][2][8] For example, the potencies of drugs substituting for 5-MeO-DMT in drug discrimination assays is well-correlated with their serotonin 5-HT1A receptor affinities, and the discriminative stimulus effects of 5-MeO-DMT are attenuated by serotonin 5-HT1A receptor antagonists.[2] However, there is partial generalization of 5-MeO-DMT to the selective serotonin 5-HT2 receptor agonist (–)-DOM in animals.[2] In accordance with the preceding findings, 5-MeO-DMT is reported to produce notably distinct subjective effects compared DMT and other psychedelics in humans.[4]
Although 5-MeO-DMT shows dramatically higher affinity for the serotonin 5-HT1A receptor than for the serotonin 5-HT2A receptor, the situation appears to be very different in terms of its actual activational potencies at these receptors.[31][21][18] Its EC50 values have been found to be 1.80 to 3.87 nM at the serotonin 5-HT2A receptor and 3.92 to 1,060 nM at the serotonin 5-HT1A receptor.[31][21][18][24] For comparison, the EC50 values of DMT were found to be 38.3 nM at the serotonin 5-HT2A receptor and >10,000 nM at the serotonin 5-HT1A receptor in one of the same studies.[31][21] Hence, 5-MeO-DMT appears to be similarly potent or as much as 200-fold more potent as an agonist of the serotonin 5-HT2A receptor than of the serotonin 5-HT1A receptor.[31][21][18] In addition, 5-MeO-DMT is 10-fold more potent than DMT as an agonist of the serotonin 5-HT2A receptor.[31][21]
Besides the serotonin receptors, 5-MeO-DMT is an agonist of the melatonin MT1 and MT2 receptors.[20][24][23] Unlike DMT, 5-MeO-DMT is not a ligand or agonist of the sigma receptors.[5][24][23] In contrast to certain other tryptamines, 5-MeO-DMT is inactive as a monoamine releasing agent, including of serotonin, norepinephrine, and dopamine.[21] However, it is a weak serotonin reuptake inhibitor, with an IC50 value of 2,184 nM.[21] Conversely, it is inactive as a dopamine and norepinephrine reuptake inhibitor (IC50 = >10,000 nM).[21]
Similarly to DMT, but in contrast to most other psychedelics, like LSD and psilocybin,[32][33] there appears to be very little development of tolerance with 5-MeO-DMT.[5][21][1][4] In fact, there may even be sensitization to the effects of 5-MeO-DMT.[4] The lack of tolerance development with 5-MeO-DMT may be due to biased agonism of the serotonin 5-HT2A receptor.[5] More specifically, 5-MeO-DMT activates the Gq signaling pathway of the serotonin 5-HT2A receptor with much less potency in recruiting β-arrestin2.[5][21] Activation of β-arrestin2 is linked to receptor downregulation and tachyphylaxis.[33][34][35]
Pharmacokinetics
[edit]5-MeO-DMT is lipophilic and is thought to easily cross the blood–brain barrier.[2] Accordingly, 5-MeO-DMT readily accumulates in the brain in animals with levels higher than in blood.[2] This is in notable contrast to bufotenin (5-HO-DMT or N,N-dimethylserotonin) and serotonin (5-HT), which are hydrophilic and peripherally selective.[2][36][11]
Bufotenin is an active metabolite of 5-MeO-DMT, formed by O-demethylation by cytochrome P450 CYP2D6.[2] Bufotenin notably has much higher affinity for the serotonin 5-HT2A receptor than 5-MeO-DMT itself.[2] However, bufotenin does not seem to be extensively produced from 5-MeO-DMT in the brain.[2] In addition, peripherally formed bufotenin may not be able to exert significant central effects due to its limited ability to cross into the brain.[2] Hence, the involvement of bufotenin in the psychoactive effects of 5-MeO-DMT is uncertain.[2]
The metabolism of 5-MeO-DMT can be dramatically reduced and its levels markedly augmented and prolonged by monoamine oxidase inhibitors (MAOIs).[2] In addition, MAOIs allow 5-MeO-DMT to become orally active in humans.[2] Combination of 5-MeO-DMT with MAOIs has sometimes resulted in serotonin syndrome and death in humans.[2]
Sources
[edit]In addition to naturally-occurring sources, 5-MeO-DMT can be produced synthetically.[37][38]

| Family | Animals |
|---|---|
| Bufonidae | Colorado River toad (Incilius alvarius)[44][29][41] |
The Colorado River toad is a noted animal source of 5-MeO-DMT. First described in 1983 by Ken Nelson (writing under the pseudonym of Albert Most), smoking the parotoid secretions of the animal produces a powerful and short-lived psychedelic experience.[45] The smoking of I. alvarius secretions should not be confused with the urban legend of toad licking.[46] Since 1983, the animal has since became a popular source of 5-MeO-DMT for recreational or spiritual purposes.[47] Unfortunately, this increased demand and use of the toads as a source of 5-MeO-DMT has put strain on their populations.[48] Concerned with the ecological impacts of the growing use of I. alvarius secretions as a source of 5-MeO-DMT, Ken Nelson would later advocate for the use of synthetic 5-MeO-DMT and conservation of the Colorado River Toad.[49]
| Family | Fungi |
|---|---|
| Amanitaceae | Amanita citrina,[43] Amanita porphyria[43] |
Legal status
[edit]Australia
[edit]As a structural analog of N,N-dimethyltryptamine (DMT), 5-MeO-DMT is a Schedule 9 prohibited substance under the Poisons Standard.[50]
Canada
[edit]5-MeO-DMT is legal for personal use and possession in Canada,[51] though sale, distribution, and other activities involving the substance are illegal under Canadian federal law.
China
[edit]As of October 2015, 5-MeO-DMT is a controlled substance in China.[52]
Germany
[edit]As of 2001 5-MeO-DMT is listed as a controlled substance. Attachement I BtMG. BGBl. I 2001, 1180 - 1186;
Sweden
[edit]The Swedish government classified 5-MeO-DMT, listed as 5-metoxi-N,N-dimetyltryptamin (5-MeO-DMT) in their regulation SFS 2004:696, as "health hazard" under the act Lagen om förbud mot vissa hälsofarliga varor (translated Act on the Prohibition of Certain Goods Dangerous to Health) in October 2004, making it illegal to sell or possess.[53]
Turkey
[edit]5-MeO-DMT has been controlled in Turkey since December 2013.[54]
United States
[edit]5-MeO-DMT was made a Schedule I controlled substance in January 2011.[55]
Research
[edit]5-MeO-DMT is being developed and evaluated for potential therapeutic effects in patients with treatment-resistant depression (TRD).[56] Biopharmaceutical company GH Research has sponsored a completed phase 1 study in healthy volunteers[57] and phase 1/2 study in TRD patients where 87.5% of patients with TRD were brought into remission on day 7 in the phase 2 part of the study.[58] GH Research is currently planning a phase 2b study in TRD patients and have received approval for studies in patients with bipolar II disorder and a current depressive episode and patients with postpartum depression.[59]
Beckley Psytech in collaboration with King's College London is evaluating the safety and tolerability of intranasal 5-MeO-DMT in healthy subjects, in a phase 1 study.[60][61] Beckley Psytech CEO Cosmo Feilding-Mellen sees a potential in the short-acting nature of 5-MeO-DMT compared to psilocybin: "Requiring one or two therapists to sit in a room with a single patient for the entire duration of an MDMA or psilocybin experience, which is essentially a whole working day, is probably going to be very resource-intensive and expensive. There is already a global shortage of psychotherapists, and this poses a potential bottleneck to patient access in the future."[62]
See also
[edit]References
[edit]- ^ a b c d e f g h i j k l m n o p q r Reckweg JT, Uthaug MV, Szabo A, Davis AK, Lancelotta R, Mason NL, Ramaekers JG (July 2022). "The clinical pharmacology and potential therapeutic applications of 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT)". J Neurochem. 162 (1): 128–146. doi:10.1111/jnc.15587. PMC 9314805. PMID 35149998.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y Shen HW, Jiang XL, Winter JC, Yu AM (October 2010). "Psychedelic 5-methoxy-N,N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions". Curr Drug Metab. 11 (8): 659–666. doi:10.2174/138920010794233495. PMC 3028383. PMID 20942780.
- ^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x Dourron HM, Nichols CD, Simonsson O, Bradley M, Carhart-Harris R, Hendricks PS (December 2023). "5-MeO-DMT: An atypical psychedelic with unique pharmacology, phenomenology & risk?". Psychopharmacology (Berl). doi:10.1007/s00213-023-06517-1. PMID 38072874.
- ^ a b c d e f g h i j k l m n o p q r s Ermakova AO, Dunbar F, Rucker J, Johnson MW (March 2022). "A narrative synthesis of research with 5-MeO-DMT". J Psychopharmacol. 36 (3): 273–294. doi:10.1177/02698811211050543. PMC 8902691. PMID 34666554.
- ^ Araújo AM, Carvalho F, Bastos M, Guedes de Pinho P, Carvalho M (August 2015). "The hallucinogenic world of tryptamines: an updated review". Archives of Toxicology. 89 (8): 1151–1173. Bibcode:2015ArTox..89.1151A. doi:10.1007/s00204-015-1513-x. PMID 25877327. S2CID 4825078.
- ^ "Ultimate Guide to 5-MeO-DMT - Experience, Benefits, & Side Effects". 29 June 2020.
- ^ a b c d Holze F, Singh N, Liechti ME, D'Souza DC (May 2024). "Serotonergic Psychedelics: A Comparative Review of Efficacy, Safety, Pharmacokinetics, and Binding Profile". Biol Psychiatry Cogn Neurosci Neuroimaging. 9 (5): 472–489. doi:10.1016/j.bpsc.2024.01.007. PMID 38301886.
- ^ Bulletin of Chemical Society of Japan, 11(3), 221-224
- ^ Malaca S, Lo Faro AF, Tamborra A, Pichini S, Busardò FP, Huestis MA (December 2020). "Toxicology and Analysis of Psychoactive Tryptamines". Int J Mol Sci. 21 (23): 9279. doi:10.3390/ijms21239279. PMC 7730282. PMID 33291798.
- ^ a b McBride MC (2000). "Bufotenine: toward an understanding of possible psychoactive mechanisms". J Psychoactive Drugs. 32 (3): 321–331. doi:10.1080/02791072.2000.10400456. PMID 11061684.
- ^ "5-MeO-DMT Effects by Erowid". Erowid.org. Retrieved 2021-07-30.
- ^ Davis AK, So S, Lancelotta R, Barsuglia JP, Griffiths RR (March 2019). "5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) used in a naturalistic group setting is associated with unintended improvements in depression and anxiety". The American Journal of Drug and Alcohol Abuse. 45 (2): 161–169. doi:10.1080/00952990.2018.1545024. PMC 6430661. PMID 30822141.
- ^ Davis AK, Barsuglia JP, Lancelotta R, Grant RM, Renn E (July 2018). "The epidemiology of 5-methoxy- N, N-dimethyltryptamine (5-MeO-DMT) use: Benefits, consequences, patterns of use, subjective effects, and reasons for consumption". Journal of Psychopharmacology. 32 (7): 779–792. doi:10.1177/0269881118769063. PMC 6248886. PMID 29708042.
- ^ Mann J, Gottlieb A (2015) [First published 1970]. "back cover". The Book of Sacraments: Ritual Use of Magical Plants. Ronin Publishing. ISBN 978-1-57951-210-1.
- ^ a b "5-MeO-DMT Timeline". Erowid.
- ^ Ray TS (February 2010). "Psychedelics and the human receptorome". PLOS ONE. 5 (2): e9019. Bibcode:2010PLoSO...5.9019R. doi:10.1371/journal.pone.0009019. PMC 2814854. PMID 20126400.
- ^ a b c d Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, Vargas MV, McCarroll MN, Taylor JC, Myers-Turnbull D, Liu T, Yaghoobi B, Laskowski LJ, Anderson EI, Zhang G, Viswanathan J, Brown BM, Tjia M, Dunlap LE, Rabow ZT, Fiehn O, Wulff H, McCorvy JD, Lein PJ, Kokel D, Ron D, Peters J, Zuo Y, Olson DE (January 2021). "A non-hallucinogenic psychedelic analogue with therapeutic potential". Nature. 589 (7842): 474–479. Bibcode:2021Natur.589..474C. doi:10.1038/s41586-020-3008-z. PMC 7874389. PMID 33299186.
- ^ Halberstadt AL, Nichols DE, Geyer MA (June 2012). "Behavioral effects of α,α,β,β-tetradeutero-5-MeO-DMT in rats: comparison with 5-MeO-DMT administered in combination with a monoamine oxidase inhibitor". Psychopharmacology (Berl). 221 (4): 709–718. doi:10.1007/s00213-011-2616-6. PMC 3796951. PMID 22222861.
- ^ a b de la Fuente Revenga M, Fernández-Sáez N, Herrera-Arozamena C, Morales-García JA, Alonso-Gil S, Pérez-Castillo A, Caignard DH, Rivara S, Rodríguez-Franco MI (June 2015). "Novel N-Acetyl Bioisosteres of Melatonin: Melatonergic Receptor Pharmacology, Physicochemical Studies, and Phenotypic Assessment of Their Neurogenic Potential". J Med Chem. 58 (12): 4998–5014. doi:10.1021/acs.jmedchem.5b00245. PMID 26023814.
- ^ a b c d e f g h i j k l Blough BE, Landavazo A, Decker AM, Partilla JS, Baumann MH, Rothman RB (October 2014). "Interaction of psychoactive tryptamines with biogenic amine transporters and serotonin receptor subtypes". Psychopharmacology (Berl). 231 (21): 4135–4144. doi:10.1007/s00213-014-3557-7. PMC 4194234. PMID 24800892.
- ^ a b Jayakodiarachchi N, Maurer MA, Schultz DC, Dodd CJ, Thompson Gray A, Cho HP, Boutaud O, Jones CK, Lindsley CW, Bender AM (February 2024). "Evaluation of the Indazole Analogs of 5-MeO-DMT and Related Tryptamines as Serotonin Receptor 2 Agonists". ACS Med Chem Lett. 15 (2): 302–309. doi:10.1021/acsmedchemlett.3c00566. PMC 10860182. PMID 38352850.
- ^ a b c "PDSP Database". UNC (in Zulu). Retrieved 27 November 2024.
- ^ a b c d Liu T. "BindingDB BDBM30707 2-(5-methoxy-1H-indol-3-yl)-N,N-dimethyl-ethanamine::2-(5-methoxy-1H-indol-3-yl)-N,N-dimethylethanamine::2-(5-methoxy-1H-indol-3-yl)ethyl-dimethyl-amine::3-(2-DIMETHYLAMINOETHYL)-5-METHOXYINDOLE::CHEMBL7257::MLS000069438::Methoxydimethyltryptamines::SMR000059066::US20240166618, Compound 5-MeO-DMT::WO2023019367, Compound 5-MeO-DMT::cid_1832". BindingDB. Retrieved 27 November 2024.
- ^ Ray TS (February 2010). "Psychedelics and the human receptorome". PLOS ONE. 5 (2): e9019. Bibcode:2010PLoSO...5.9019R. doi:10.1371/journal.pone.0009019. PMC 2814854. PMID 20126400.
- ^ Rogawski MA, Aghajanian GK (October 1981). "Serotonin autoreceptors on dorsal raphe neurons: structure-activity relationships of tryptamine analogs". J Neurosci. 1 (10): 1148–1154. doi:10.1523/JNEUROSCI.01-10-01148.1981. PMC 6564212. PMID 6793698.
- ^ Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA (December 2006). "The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats". Psychopharmacology. 189 (3): 319–329. doi:10.1007/s00213-006-0566-1. PMID 17013638. S2CID 23396616.
- ^ Nagai F, Nonaka R, Satoh Hisashi Kamimura K (March 2007). "The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain". European Journal of Pharmacology. 559 (2–3): 132–137. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101.
- ^ a b Uthaug MV, Lancelotta R, van Oorsouw K, Kuypers KP, Mason N, Rak J, et al. (September 2019). "A single inhalation of vapor from dried toad secretion containing 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) in a naturalistic setting is related to sustained enhancement of satisfaction with life, mindfulness-related capacities, and a decrement of psychopathological symptoms". Psychopharmacology. 236 (9): 2653–2666. doi:10.1007/s00213-019-05236-w. PMC 6695371. PMID 30982127.
- ^ Lima da Cruz RV, Moulin TC, Petiz LL, Leão RN (2018). "A Single Dose of 5-MeO-DMT Stimulates Cell Proliferation, Neuronal Survivability, Morphological and Functional Changes in Adult Mice Ventral Dentate Gyrus". Frontiers in Molecular Neuroscience. 11: 312. doi:10.3389/fnmol.2018.00312. PMC 6131656. PMID 30233313.
- ^ a b c d e Cumming P, Scheidegger M, Dornbierer D, Palner M, Quednow BB, Martin-Soelch C (April 2021). "Molecular and Functional Imaging Studies of Psychedelic Drug Action in Animals and Humans". Molecules. 26 (9): 2451. doi:10.3390/molecules26092451. PMC 8122807. PMID 33922330.
- ^ Halberstadt AL (January 2015). "Recent advances in the neuropsychopharmacology of serotonergic hallucinogens". Behav Brain Res. 277: 99–120. doi:10.1016/j.bbr.2014.07.016. PMC 4642895. PMID 25036425.
- ^ a b Jiménez JH, Bouso JC (August 2022). "Significance of mammalian N, N-dimethyltryptamine (DMT): A 60-year-old debate". J Psychopharmacol. 36 (8): 905–919. doi:10.1177/02698811221104054. PMID 35695604.
- ^ Barksdale BR, Doss MK, Fonzo GA, Nemeroff CB (March 2024). "The mechanistic divide in psychedelic neuroscience: An unbridgeable gap?". Neurotherapeutics. 21 (2): e00322. doi:10.1016/j.neurot.2024.e00322. PMC 10963929. PMID 38278658.
- ^ Wallach J, Cao AB, Calkins MM, Heim AJ, Lanham JK, Bonniwell EM, Hennessey JJ, Bock HA, Anderson EI, Sherwood AM, Morris H, de Klein R, Klein AK, Cuccurazzu B, Gamrat J, Fannana T, Zauhar R, Halberstadt AL, McCorvy JD (December 2023). "Identification of 5-HT2A receptor signaling pathways associated with psychedelic potential". Nat Commun. 14 (1): 8221. doi:10.1038/s41467-023-44016-1. PMC 10724237. PMID 38102107.
- ^ Plazas E, Faraone N (February 2023). "Indole Alkaloids from Psychoactive Mushrooms: Chemical and Pharmacological Potential as Psychotherapeutic Agents". Biomedicines. 11 (2): 461. doi:10.3390/biomedicines11020461. PMC 9953455. PMID 36830997.
- ^ Sherwood AM, Claveau R, Lancelotta R, Kaylo KW, Lenoch K (December 2020). "Synthesis and Characterization of 5-MeO-DMT Succinate for Clinical Use". ACS Omega. 5 (49): 32067–32075. doi:10.1021/acsomega.0c05099. PMC 7745443. PMID 33344861.
- ^ Carpenter DE (2021-02-02). "Psychedelic Toads Pushed To The Limit, Conservationists Urge Synthetic 5-MeO-DMT Option". Forbes. Retrieved 2021-02-04.
- ^ Uthaug MV, Lancelotta R, Szabo A, Davis AK, Riba J, Ramaekers JG (March 2020). "Prospective examination of synthetic 5-methoxy-N,N-dimethyltryptamine inhalation: effects on salivary IL-6, cortisol levels, affect, and non-judgment". Psychopharmacology. 237 (3): 773–785. doi:10.1007/s00213-019-05414-w. PMC 7036074. PMID 31822925.
- ^ a b c d e f g h i "tryptamines: fungi". bluezoo.org.
- ^ a b c d e "Erowid Psychoactive Vaults: Tryptamine FAQ". www.erowid.org.
- ^ a b c d e f "Some simple tryptomines" (PDF). troutsnotes.com. Retrieved 2020-07-04.
- ^ a b c d e f g h i Khan JI, Kennedy TJ, Christian JR DR (2011). Basic Principles of Forensic Chemistry. Springer Science & Business Media. p. 195. ISBN 978-1-934115-06-0.
- ^ Carpenter DE. "5-MeO-DMT: The 20-Minute Psychoactive Toad Experience That's Transforming Lives". Forbes.
- ^ Nelson K. "Bufo alvarius: The Psychedelic Toad of the Sonoran Desert". Erowid. Illustrated by Gail Patterson. Retrieved October 20, 2023.
- ^ Shulgin A, Shulgin A. "Tryptamines I Have Known And Loved: The Continuation". Erowid. Retrieved October 20, 2023.
- ^ Romero S (2022-03-20). "Demand for This Toad's Psychedelic Toxin Is Booming. Some Warn That's Bad for the Toad". The New York Times. ISSN 0362-4331. Retrieved 2023-10-20.
- ^ "The Sonoran Desert toad can get you high. Poachers have taken notice". Animals. 2023-07-12. Archived from the original on July 12, 2023. Retrieved 2023-10-20.
- ^ Morris H (2021-02-02). "Preface". Bufo alvarius: The Psychedelic Toad of the Sonoran Desert (2021 ed.). Archived from the original on 2021-02-02. Retrieved 2023-10-20.
- ^ "Poisons Standard July 2016". Federal Register of Legislation. 24 June 2016.
- ^ "Is 5-MeO-DMT (Bufo) Legal in Canada? Understanding 5-MeO-DMT Laws and Regulations". PsychedelicLaw.ca. Retrieved 2023-11-28.
- ^ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 1 October 2015.
- ^ "Förordning om ändring i förordningen (1999:58) om förbud mot vissa hälsofarliga varor" (PDF). Svensk författningssamling (in Swedish). 7 September 2004.
- ^ "Turkish Law" (PDF). Resmi Gazete. 16 December 2013.
- ^ Drug Enforcement Administration (DEA), Department of Justice (December 2010). "Schedules of controlled substances: placement of 5-methoxy-N,N-dimethyltryptamine into Schedule I of the Controlled Substances Act. Final rule" (PDF). Federal Register. 75 (243): 79296–79300. PMID 21171485.
- ^ "Home | GH Research". GH Research Limited.
- ^ Reckweg J, Mason NL, van Leeuwen C, Toennes SW, Terwey TH, Ramaekers JG (2021). "A Phase 1, Dose-Ranging Study to Assess Safety and Psychoactive Effects of a Vaporized 5-Methoxy-N, N-Dimethyltryptamine Formulation (GH001) in Healthy Volunteers". Frontiers in Pharmacology. 12: 760671. doi:10.3389/fphar.2021.760671. PMC 8667866. PMID 34912222.
- ^ GH Research PLC (2021-12-06). "GH Research Announces Successful Outcome of the Phase 2 part of its Phase 1/2 Clinical Trial of GH001 in Treatment-Resistant Depression". GlobeNewswire News Room (Press release). Retrieved 2022-10-07.
- ^ GH Research PLC (2022-08-23). "GH Research Reports Second Quarter 2022 Financial Results and Provides Business Updates". GlobeNewswire News Room (Press release). Retrieved 2022-10-07.
- ^ Carpenter DE (2022-02-16). "More Companies Embrace 5-MeO-DMT to Create Therapies". Lucid News. Retrieved 2022-03-06.
- ^ Atai LS (2024-03-27). "atai Life Sciences Announces Positive Initial Results from Beckley Psytech's Phase 2a Open Label Study of BPL-003 (Intranasal 5-MeO-DMT) in Treatment Resistant Depression". GlobeNewswire News Room (Press release). Retrieved 2024-10-06.
- ^ Siebert A. "Could 5-MeO-DMT Allow For More Affordable Psychedelic-Assisted Therapy? Beckley Psytech Thinks So". Forbes. Retrieved 2022-03-06.
External links
[edit]- Drugs not assigned an ATC code
- 5-HT1A agonists
- 5-HT1B agonists
- 5-HT1D agonists
- 5-HT1E agonists
- 5-HT1F agonists
- 5-HT2A agonists
- 5-HT2B agonists
- 5-HT2C agonists
- 5-HT5A agonists
- 5-HT6 agonists
- 5-HT7 agonists
- Ayahuasca
- Biased ligands
- Designer drugs
- Dimethylamino compounds
- Entheogens
- Experimental hallucinogens
- Indole ethers at the benzene ring
- Melatonin receptor agonists
- Mexamines
- Psychedelic drugs
- Psychedelic tryptamines
- Serotonin receptor agonists
- Tryptamine alkaloids
