Allylescaline
Appearance
| |

| |
| Names | |
|---|---|
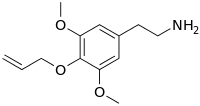
| Preferred IUPAC name
2-{3,5-Dimethoxy-4-[(prop-2-en-1-yl)oxy]phenyl}ethan-1-amine | |
| Other names
2-[4-(Allyloxy)-3,5-dimethoxyphenyl]ethan-1-amine
2-[4-(Allyloxy)-3,5-dimethoxyphenyl]ethanamine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H19NO3 | |
| Molar mass | 237.299 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Allylescaline (4-allyloxy-3,5-dimethoxyphenethylamine) is a lesser-known psychedelic drug. It is closely related in structure to mescaline. Allylescaline was first synthesized by Otakar Leminger in 1972.[1] The compound was later synthesized by Alexander Shulgin and further described in his book PiHKAL.[2] The dosage range is listed as 20–35 mg, and the duration 8–12 hours.[2] Allylescaline produces an entactogenic warmth, an entheogenic effect, and a feeling of flowing energy. Very little data exists about the pharmacological properties, metabolism, and toxicity of allylescaline.
Legal status
[edit]Allylescaline is illegal in Sweden as of January 2016.[3]
See also
[edit]References
[edit]- ^ Leminger, Otakar (1972). "The Chemistry of Alkoxylated Phenethylamines – Part 2". Chemický Průmysl. 22: 553.
- ^ a b AL Entry in PiHKAL
- ^ "31 nya ämnen kan klassas som narkotika eller hälsofarlig vara" (in Swedish). Folkhälsomyndigheten. November 2015.
External links
[edit]
