Anemonin
| |
| |
| Names | |
|---|---|
| IUPAC names
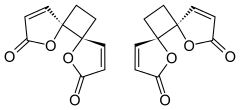
trans-4,7-Dioxadispiro[4.0.46.25]dodeca-1,9-diene-3,8-dione
trans-1,7-Dioxadispiro[4.0.4.2]dodeca-3,9-diene-2,8-dione[1] | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H8O4 | |
| Molar mass | 192.170 g·mol−1 |
| Appearance | Colourless, odourless solid |
| Density | 1.45g/cm3 |
| Melting point | 158[1] °C (316 °F; 431 K) |
| Boiling point | 535.7 °C (996.3 °F; 808.9 K) @ 760mmHg |
| low | |
| Solubility in chloroform | very soluble[1] |
| Hazards | |
| Flash point | 300.7 °C (573.3 °F; 573.8 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
150 mg·kg−1 (mouse, IP) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Anemonin is a tri-spirocyclic dibutenolide natural product found in members of the buttercup family (Ranunculaceae) such as Ranunculus bulbosus, R. ficaria, R. sardous, R. sceleratus,[2] and Clematis hirsutissima.[3] Originally isolated in 1792 by M. Heyer,[4] It is the dimerization product of the toxin protoanemonin.[5] One of the likely active agents in plants used in Chinese medicine as an anti-inflammatory[6] and Native American medicine as a horse stimulant,[3] its unique biological properties give it pharmaceutical potential as an anti-inflammatory and cosmetic agent.
Biosynthetic origins
[edit]Anemonin is a homodimer formed from two protoanemonin subunits. Protoanemonin is formed from the enzymatic cleavage of ranunculin upon crushing plant matter.[4] When a plant from this family is injured, a β-glucosidase cleaves ranunculin, liberating protoanemonin from glucose as a defense mechanism.[7] This butenolide readily dimerizes in aqueous media to form a single cyclodimer.[4]
Chemical structure and proposed mechanism of formation
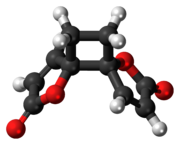
[edit]Despite multiple possibilities, X-ray crystallography of the solid anemonin has revealed that the two rings exclusively possess a trans relationship.[8] The central cyclobutane ring was found to be bent to a dihedral angle of 152°. NMR spectroscopy reveals that the central ring is also twisted 9-11°.[9]
The highly selective formation of the head-to-head dimer has been rationalized through the stability of a proposed diradical intermediate; the resulting radicals after an initial carbon-carbon bond forming step are delocalized through the α,β-unsaturated system.[4] These proposed radicals could also be stabilized through the captodative effect, as they are situated between the enone and sp3-hybridized oxygen of the butenolides.
Destabilizing dipole-dipole interactions are proposed to disfavor the transition state where the two butenolide rings adopt a cis conformation, leading to selectivity of a trans relationship between the lactone rings.[4]
The formation of anemonin from protoanemonin is most likely a photochemical process. When Kataoka et. al compared the dimerization of protoanemonin in the presence and absence of radiation from a mercury lamp, they found a 75% yield with radiation and a very poor yield without radiation. It is not mentioned whether light was excluded from this control reaction; the low yield of anemonin may arise from visible light-mediated dimerization of protoanemonin.[10]
Pharmaceutical potential
[edit]Anemonin possesses anti-inflammatory properties rather than the vesicant properties of its parent monomer. Numerous studies have demonstrated anemonin’s potential in treating ulcerative colitis,[11] cerebral ischemia,[12] and arthritis.[13][14] Its activity against LPS-related inflammation[13][15] and nitric oxide production[16][6] contribute to its pharmaceutical potential. Anemonin also displays inhibition of melanin production in human melanocytes with mild cytotoxicity.[17]
Given its skin permeability in ethanolic solutions[18] and its anti-inflammatory and anti-pigmentation properties, anemonin may be a good candidate for topical formulations as arthritis medications or cosmetics. An extraction method with the potential for industrial-scale preparations of anemonin may provide inroads to drug development.[19]
References
[edit]- ^ a b c William M. Haynes (2016). CRC Handbook of Chemistry and Physics (97th ed.). Boca Raton: CRC Press. pp. 3–26. ISBN 978-1-4987-5429-3.
- ^ Teodora N, Neli Kinga O, Daniela H, Daniela B, Pripon F, Aurel A, Claudia T (2018). "Anemonin Content of Four Different Ranunculus Species". Pakistan Journal of Pharmaceutical Sciences. 31 (5(Supplementary)): 2027–2032. PMID 30393208.
- ^ a b Kern JR, Cardellina JH (July 1983). "Native American medicinal plants. Anemonin from the horse stimulant Clematis hirsutissima". Journal of Ethnopharmacology. 8 (1): 121–123. doi:10.1016/0378-8741(83)90093-4. PMID 6632934.
- ^ a b c d e Moriarty RM, Romain CR, Karle IL, Karle J (July 1965). "The Structure of Anemonin". Journal of the American Chemical Society. 87 (14): 3251–3252. doi:10.1021/ja01092a047. ISSN 0002-7863.
- ^ "Aktuelles aus der Natur" (PDF) (in German). TU Graz. 2 April 2009. p. 4. Retrieved 27 November 2010.[permanent dead link]
- ^ a b Duan H, Zhang Y, Xu J, Qiao J, Suo Z, Hu G, Mu X (April 2006). "Effect of anemonin on NO, ET-1 and ICAM-1 production in rat intestinal microvascular endothelial cells". Journal of Ethnopharmacology. 104 (3): 362–366. doi:10.1016/j.jep.2005.09.034. PMID 16257161.
- ^ Pirvu L, Stefaniu A, Neagu G, Pintilie L (2022-01-01). "Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies". Open Chemistry. 20 (1): 299–312. doi:10.1515/chem-2022-0137. ISSN 2391-5420.
- ^ Karle IL, Karle J (1966-04-10). "The crystal and molecular structure of anemonin, C10H8O4". Acta Crystallographica. 20 (4): 555–559. Bibcode:1966AcCry..20..555K. doi:10.1107/S0365110X66001233. ISSN 0365-110X.
- ^ Lustig E, Moriarty RM (July 1965). "The Estimation of the Angle of Twist for a Cyclobutane Derivative by Nuclear Magnetic Resonance". Journal of the American Chemical Society. 87 (14): 3252–3253. doi:10.1021/ja01092a048. ISSN 0002-7863.
- ^ Kataoka H, Yamada K, Sugiyama N (November 1965). "The Photo-synthesis of Anemonin from Protoanemonin". Bulletin of the Chemical Society of Japan. 38 (11): 2027. doi:10.1246/bcsj.38.2027. ISSN 0009-2673.
- ^ Jiang L, Chi C, Yuan F, Lu M, Hu D, Wang L, Liu X (March 2022). "Anti-inflammatory effects of anemonin on acute ulcerative colitis via targeted regulation of protein kinase C-θ". Chinese Medicine. 17 (1): 39. doi:10.1186/s13020-022-00599-3. PMC 8962473. PMID 35346284.
- ^ Jia D, Han B, Yang S, Zhao J (June 2014). "Anemonin alleviates nerve injury after cerebral ischemia and reperfusion (i/r) in rats by improving antioxidant activities and inhibiting apoptosis pathway". Journal of Molecular Neuroscience. 53 (2): 271–279. doi:10.1007/s12031-013-0217-z. PMID 24443273. S2CID 255492187.
- ^ a b Hou H, Peng Q, Wang S, Zhang Y, Cao J, Deng Y, et al. (2020). "Anemonin Attenuates RANKL-Induced Osteoclastogenesis and Ameliorates LPS-Induced Inflammatory Bone Loss in Mice via Modulation of NFATc1". Frontiers in Pharmacology. 10: 1696. doi:10.3389/fphar.2019.01696. PMC 7025528. PMID 32116686.
- ^ Wang Z, Huang J, Zhou S, Luo F, Xu W, Wang Q, et al. (December 2017). "Anemonin attenuates osteoarthritis progression through inhibiting the activation of IL-1β/NF-κB pathway". Journal of Cellular and Molecular Medicine. 21 (12): 3231–3243. doi:10.1111/jcmm.13227. PMC 5706500. PMID 28643466.
- ^ Xiao K, Cao ST, Jiao LE, Lin FH, Wang L, Hu CH (July 2016). "Anemonin improves intestinal barrier restoration and influences TGF-β1 and EGFR signaling pathways in LPS-challenged piglets". Innate Immunity. 22 (5): 344–352. doi:10.1177/1753425916648223. PMID 27189428. S2CID 12372791.
- ^ Lee TH, Huang NK, Lai TC, Yang AT, Wang GJ (March 2008). "Anemonin, from Clematis crassifolia, potent and selective inducible nitric oxide synthase inhibitor". Journal of Ethnopharmacology. 116 (3): 518–527. doi:10.1016/j.jep.2007.12.019. PMID 18281171.
- ^ Huang YH, Lee TH, Chan KJ, Hsu FL, Wu YC, Lee MH (February 2008). "Anemonin is a natural bioactive compound that can regulate tyrosinase-related proteins and mRNA in human melanocytes". Journal of Dermatological Science. 49 (2): 115–123. doi:10.1016/j.jdermsci.2007.07.008. PMID 17766092.
- ^ Ning Y, Rao Y, Yu Z, Liang W, Li F (March 2016). "Skin permeation profile and anti-inflammatory effect of anemonin extracted from weilingxian". Die Pharmazie. 71 (3): 134–138. PMID 27183707.
- ^ CN101759706B, 王琳 & 范淦彬, "Method for manufacturing anemonin", issued 2012-01-11


