Brorphine
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
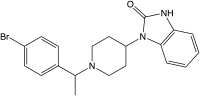
| Formula | C20H22BrN3O |
| Molar mass | 400.320 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Brorphine is a piperidine-based opioid analgesic compound.[1][2] Brorphine was originally discovered in a 2018 paper investigating functionally biased opioid compounds, with the intention of finding safer analgesics that produce less respiratory depression than typical opioids.[3] Brorphine was originally reported to be highly biased, with an EC50 of 4.8nM for GTPγS binding and 182nM for β-arrestin recruitment,[3] however a more recent study found no significant bias for any of the compounds tested, including brorphine.[4] Its safety profile in any animal model has never been established. Despite the lack of safety information on the compound, brorphine has been sold as a designer drug since mid-2019, initially being identified in the US Midwest, though it has since been found in 2020 in Belgium. It is related in chemical structure to compounds such as benzylfentanyl and bezitramide, though it is sufficiently structurally distinct to fall outside the formal definition of a "fentanyl analogue" in jurisdictions such as the US and New Zealand which have Markush structure controls over this family of drugs.[4][5]
Brorphine was first identified in the U.S. recreational drug supply in July 2020 by the Center for Forensic Science Research and Education (CFSRE) through its NPS Discovery program; however, earlier identifications by the Drug Enforcement Administration (DEA) may have come as early as late 2019. The rise of brorphine in the U.S. can be directly linked to the decline of isotonitazene due to scheduling by the DEA.[6] Brorphine was first implicated in 20 deaths in the U.S., primarily in cases originating from midwest states. Brorphine was commonly found with fentanyl and flualprazolam, a drug combination verified by drug product testing. Brorphine has also been identified in counterfeit opioid pills and tablets. Recently data from CFSRE and NMS Labs show that brorphine has been detected in more than 100 cases as of October 2020.[7][8][9]
Legality
[edit]Brorphine is not controlled under the Single Convention on Narcotic Drugs,1961 or under the Federal Analogue Act, however it could be illegal to sell, produce, possess or consume it in several countries if it is sold for human consumption. In the United States, brorphine was placed into temporary emergency Schedule I for 2 years by the DEA on January 4, 2021.[10] On February 3, 2023, the DEA filed plans in the Federal Register for permanent placement of brorphine into Schedule I.[11][12]
See also
[edit]- AH-7921
- Bezitramide
- Cebranopadol
- Diphenpipenol
- DPI-3290
- Etazen
- GSK1702934A
- J-113,397
- Oliceridine
- PZM21
- SR-14968
- SR-16435
- SR-17018
- List of fentanyl analogues
References
[edit]- ^ Grafinger KE, Wilde M, Otte L, Auwärter V (August 2021). "Pharmacological and metabolic characterization of the novel synthetic opioid brorphine and its detection in routine casework". Forensic Science International. 327: 110989. doi:10.1016/j.forsciint.2021.110989. PMID 34509061.
- ^ Ujváry I, Christie R, Evans-Brown M, Gallegos A, Jorge R, de Morais J, Sedefov R (April 2021). "DARK Classics in Chemical Neuroscience: Etonitazene and Related Benzimidazoles". ACS Chemical Neuroscience. 12 (7): 1072–1092. doi:10.1021/acschemneuro.1c00037. PMID 33760580. S2CID 232356192.
- ^ a b Kennedy NM, Schmid CL, Ross NC, Lovell KM, Yue Z, Chen YT, et al. (October 2018). "Optimization of a Series of Mu Opioid Receptor (MOR) Agonists with High G Protein Signaling Bias". Journal of Medicinal Chemistry. 61 (19): 8895–8907. doi:10.1021/acs.jmedchem.8b01136. PMC 6386185. PMID 30199635.
- ^ a b Vandeputte MM, Cannaert A, Stove CP (November 2020). "In vitro functional characterization of a panel of non-fentanyl opioid new psychoactive substances". Archives of Toxicology. 94 (11): 3819–3830. doi:10.1007/s00204-020-02855-7. hdl:1854/LU-8687070. PMID 32734307. S2CID 220881657.
- ^ Verougstraete N, Vandeputte MM, Lyphout C, Cannaert A, Hulpia F, Van Calenbergh S, et al. (January 2021). "First Report on Brorphine: The Next Opioid on the Deadly New Psychoactive Substance Horizon?". Journal of Analytical Toxicology. 44 (9): 937–946. doi:10.1093/jat/bkaa094. hdl:1854/LU-8671214. ISSN 0146-4760. PMID 32744605.
- ^ Vandeputte MM, Krotulski AJ, Papsun DM, Logan BK, Stove CP (July 2021). "The Rise and Fall of Isotonitazene and Brorphine: Two Recent Stars in the Synthetic Opioid Firmament". Journal of Analytical Toxicology. 46 (2): 115–121. doi:10.1093/jat/bkab082. PMID 34233349.
- ^ Krotulski AJ, Papsun DM, Noble C, Kacinko SL, Logan BK (March 2021). "Brorphine-Investigation and quantitation of a new potent synthetic opioid in forensic toxicology casework using liquid chromatography-mass spectrometry". Journal of Forensic Sciences. 66 (2): 664–676. doi:10.1111/1556-4029.14623. PMID 33201526. S2CID 226990291.
- ^ Vohra V, King AM, Jacobs E, Aaron C (September 2021). "Death associated with brorphine, an emerging novel synthetic opioid". Clinical Toxicology. 59 (9): 851–852. doi:10.1080/15563650.2021.1879111. PMID 33522844. S2CID 231761916.
- ^ Vandeputte MM et al, The Rise and Fall of Isotonitazene and Brorphine: Two Recent Stars in the Synthetic Opioid Firmament. Journal of Analytical Toxicology 2022; 46(2): 115–121. doi:10.1093/jat/bkab082
- ^ "Federal Register :: Request Access".
- ^ "Federal Register :: Request Access".
- ^ https://public-inspection.federalregister.gov/2023-04364.pdf
