Eupalin
Appearance
This article relies largely or entirely on a single source. (August 2014) |
| |
| Names | |
|---|---|
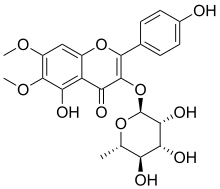
| IUPAC name
5-Hydroxy-2-(4-hydroxyphenyl)-6,7-dimethoxy-3-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxychromen-4-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C23H24O11 | |
| Molar mass | 476.434 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Eupalin is a flavonol. It is the eupalitin 3-O-rhamnoside. It can be isolated from Eupatorium ligustrinum.[1]
References
[edit]- ^ The structures of eupalin and eupatolin. Two new flavonol rhamnosides isolated from Eupatorium ligustrinum D.C. L. Quijano, F. Malanco and Tirso Ríos, Tetrahedron, Volume 26, Issue 12, 1970, pages 2851-2859, doi:10.1016/S0040-4020(01)92863-7
