Hederagenin
| |
| Names | |
|---|---|
| IUPAC name
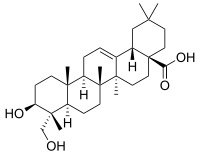
3β,23-Dihydroxyolean-12-en-28-oic acid
| |
| Systematic IUPAC name
(4aS,6aS,6bR,8aR,9R,10S,12aR,12bR,14bS)-9-Hydroxy-9-(hydroxymethyl)-2,2,6a,6b,9,12a-hexamethyl-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.701 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H48O4 | |
| Molar mass | 472.710 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hederagenin is a triterpenoid which is a chemical constituent of the Hedera helix plant.
Hederagenin is the aglycone part of numerous saponins found in Hedera helix (common ivy), the most prevalent of these being hederacoside C and alpha-hederin. It is also one of three primary triterpenoids extracted from the Chenopodium quinoa plant categorized by the EPA as a biopesticide.[1] HeadsUp Plant Protectant is made up of approximately equal ratios of the saponin aglycones oleanolic acid, hederagenin, and phytolaccagenic acid and is intended for use as a seed treatment on tuber (e.g. potato seed pieces), legume, and cereal seeds or as a pre-plant root dip for roots of transplants, at planting, to prevent fungal growth, bacterial growth, and viral plant diseases.
Hederagenin has been found to have antidepressant-like effects in a rodent models.[2]
History
[edit]Hederagenin was discovered by L. Posselt in 1849 and named hederic acid.[3] However, Posselt was not able to isolate a pure substance or obtain an exact formula: his hederic acid was hederagenin mixed with some tannin impurity.[4]
Related triterpenes
[edit]All of these compounds share the same pentacyclic framework:
- Betulinic acid
- Boswellic acid
- Glycyrrhetinic acid
- Moronic acid
- Oleanolic acid
- Ursolic acid
- Corosolic acid
- Amyrin
- Lupeol
- Maslinic acid
- Hopane
References
[edit]- ^ BIOPESTICIDES REGISTRATION ACTION DOCUMENT, Saponins of Chenopodium quinoa.
- ^ Zhou, D; Jin, H; Lin, HB; Yang, XM; Cheng, YF; Deng, FJ; Xu, JP (2010). "Antidepressant effect of the extracts from Fructus Akebiae". Pharmacology Biochemistry and Behavior. 94 (3): 488–95. doi:10.1016/j.pbb.2009.11.003. PMID 19931301.
- ^ L. Posselt, "On the constituents of the seeds of ivy", Liebig's Annalen der Chemie, January 1849.
- ^ John Lionel Simonsen, The Terpenes, p. 174, Cambridge University Press, 1947 OCLC 309782.
