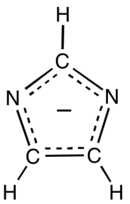
Imidazolate
Appearance
| |
| Names | |
|---|---|
| IUPAC name
Imidazolate
| |
| Other names
Imidazolide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C3H3N2 | |
| Molar mass | 67.070 |
| Acidity (pKa) | 14.05[1] |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
67.8 kJ·mol−1 (16.2 kcal·mol−1) Gas phase.[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Imidazolate (C3H3N−
2) is the conjugate base of imidazole. It is a nucleophile and a strong base. The free anion has C2v symmetry. Imidazole has a pKa of 14.05,[1] so the deprotonation of imidazole (C3H3N2H) requires a strong base.
Occurrence
[edit]
Imidazolate is a common bridging ligand in coordination chemistry. In the zeolitic imidazolate frameworks, the metals are interconnected via imidazolates.[4][5] In the enzyme superoxide dismutase, imidazolate links copper and zinc centers.
References
[edit]- ^ a b WALBA, HAROLD; ISENSEE, ROBERT W. (August 1961). "Acidity Constants of Some Arylimidazoles and Their Cations". The Journal of Organic Chemistry. 26 (8): 2789–2791. doi:10.1021/jo01066a039.
- ^ Gutowski, Keith E.; Rogers, Robin D.; Dixon, David A. (May 2007). "Accurate Thermochemical Properties for Energetic Materials Applications. II. Heats of Formation of Imidazolium-, 1,2,4-Triazolium-, and Tetrazolium-Based Energetic Salts from Isodesmic and Lattice Energy Calculations". The Journal of Physical Chemistry B. 111 (18): 4788–4800. doi:10.1021/jp066420d. PMID 17388432.
- ^ PDB: 3CQQ; Cao X, Antonyuk SV, Seetharaman SV, Whitson LJ, Taylor AB, Holloway SP, Strange RW, Doucette PA, Valentine JS, Tiwari A, Hayward LJ, Padua S, Cohlberg JA, Hasnain SS, Hart PJ (June 2008). "Structures of the G85R variant of SOD1 in familial amyotrophic lateral sclerosis". J. Biol. Chem. 283 (23): 16169–77. doi:10.1074/jbc.M801522200. PMC 2414278. PMID 18378676.
- ^ Phan, A.; Doonan, C. J.; Uribe-Romo, F. J.; Knobler, C. B.; O'Keeffe, M.; Yaghi, O. M. "Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks" Acc. Chem. Res. 2010, 43, 58-67. doi:10.1021/ar900116g
- ^ Zhang, J.-P.; Zhang, Y.-B.; Lin, J.-B.; Chen, X.-M., "Metal Azolate Frameworks: From Crystal Engineering to Functional Materials", Chem. Rev. 2012, vol. 112, pp. 1001-1033. doi:10.1021/cr200139g
