Iron monosilicide
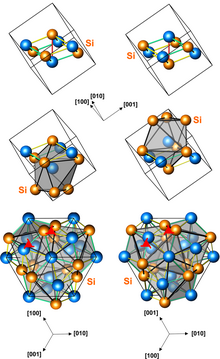 Structures of left-handed and right-handed FeSi crystals. The top presentation shows the eight atoms of the unit cell. The middle shows polyhedra surrounding the iron atoms. The bottom show the presence of 3-fold screw axes.
| |
| Names | |
|---|---|
| IUPAC name
Iron silicide
| |
| Other names
Naquite, fersilicite
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.506 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| FeSi | |
| Molar mass | 83.931 g/mol |
| Appearance | gray cubic crystals[1] |
| Density | 6.1 g/cm3[1] |
| Melting point | 1,410 °C (2,570 °F; 1,680 K)[1] |
| Band gap | 0.05 eV (ind.) 0.14 eV (dir.)[2] |
| 8.5×10−6 emu/g[3] | |
| Structure | |
| Cubic[4] | |
| P213 (No. 198), cP8 | |
a = 0.44827(1) nm
| |
Formula units (Z)
|
4 |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Iron germanide |
Other cations
|
Cobalt silicide Manganese silicide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Iron monosilicide (FeSi) is an intermetallic compound, a silicide of iron that occurs in nature as the rare mineral naquite. It is a narrow-bandgap semiconductor with a room-temperature electrical resistivity of around 10 kΩ·cm[3] and unusual magnetic properties at low temperatures. FeSi has a cubic crystal lattice with no inversion center; therefore its magnetic structure is helical, with right-hand and left-handed chiralities.[4]
The structure is the prototype of the "iron monosilicide structure type", of space group P213 (no. 198). It is similar to the structure of sodium chloride, with four iron atoms and four silicon atoms in each unit cell. Whereas in sodium chloride the eight atoms are at the corners of a cube and each ion is surrounded by six counterions, in iron monosilicide the atoms are all displaced parallel to body diagonals (along threefold axes) from the positions of sodium and chloride. The crystal loses all indirect (chirality reversing) symmetry elements, all fourfold and twofold rotation axes, and many of the threefold axes and translations, but retains some of the twofold screw axes and threefold axes of the sodium chloride crystal structure. As an atom is moved from (0,0,0) to (x,x,x), the other three atoms of the same kind move as well, creating a chiral arrangement except when x is a multiple of 1/4. The atoms of the other kind are displaced as well, with one of the four at (y,y,y). If x = −y = ±√5 – 1/8 ≈ ±0.1545 then each ion will be equidistant from seven atoms of the other kind, at a distance of (√5 − 1)√3/4 ≈ 0.53523, rather than six at a distance of 1/2 and one at a distance of √3/2 in the sodium chloride structure.[5]
In the case of iron monosilicide, x = 0.13652 and y = 0.8424 (or −0.1576), or x = −0.13652 and y = 0.1576, so the seven silicon atoms around an iron atom are not all the same distance from the iron atom. Each silicon atom sits in a similar cage of iron atoms. The cages only have threefold rotational symmetry, with three slightly different interatomic distances between the central atom and the seven surrounding atoms (to 3 atoms, 3 atoms, and 1 atom respectively). There are right-handed and left-handed threefold screw axes without there being a symmetry element taking one to the other. (Threefold screw axes also exist in sodium chloride but are related by a mirror.) This means that iron monosilicide crystals exist in two different enantiomorphs, depending on the signs of x and y.[6]
In 1948 Linus Pauling investigated the nature of the bonds in iron monosilicide.[7]
See also
[edit]References
[edit]- ^ a b c Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 4.68. ISBN 9781498754293.
- ^ Galakhov, V R; Kurmaev, E Z; Cherkashenko, V M; Yarmoshenko, Yu M; Shamin, S N; Postnikov, A V; Uhlenbrock, S; Neumann, M; Lu, Z W; Klein, B M; Shi, Zhu-Pei (1995). "Electronic structure of FeSi". Journal of Physics: Condensed Matter. 7 (28): 5529–5535. arXiv:mtrl-th/9505004. Bibcode:1995JPCM....7.5529G. doi:10.1088/0953-8984/7/28/010. S2CID 15970627.
- ^ a b Jaccarino, V.; Wertheim, G. K.; Wernick, J. H.; Walker, L. R.; Arajs, Sigurds (1967). "Paramagnetic Excited State of FeSi". Physical Review. 160 (3): 476–482. Bibcode:1967PhRv..160..476J. doi:10.1103/PhysRev.160.476.
- ^ a b Stishov, Sergei M.; Petrova, Alla E. (2011). "Itinerant helimagnetic compound MnSi". Uspekhi Fizicheskikh Nauk. 181 (11): 1157. doi:10.3367/UFNr.0181.201111b.1157.
- ^ "FeSi (B20) Structure". Encyclopedia of Crystallographic Prototypes. AFLOW.
- ^ Ulrich Burkhardt; et al. (Mar 4, 2020). "Absolute Structure from Scanning Electron Microscopy". Scientific Reports. 10 (1): 4065. Bibcode:2020NatSR..10.4065B. doi:10.1038/s41598-020-59854-y. PMC 7055257. PMID 32132558.
- ^ Pauling, Linus; Soldate, A. M. (September 1948). "The nature of the bonds in the iron silicide, FeSi, and related crystals". Acta Crystallographica. 1 (4): 212–216. Bibcode:1948AcCry...1..212P. doi:10.1107/S0365110X48000570.
