Nothofagin
Appearance
| |
| Names | |
|---|---|
| IUPAC name
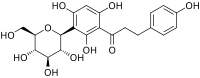
2',4,4',6'-Tetrahydroxy-3-C-β-D-glucopyranosyldihydrochalcone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H24O10 | |
| Molar mass | 436.413 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nothofagin is a dihydrochalcone. It is a C-linked phloretin glucoside found in rooibos (Aspalathus linearis)[1] and New Zealand red beech (Nothofagus fusca).[2] It is a phenolic antioxidant.
References
[edit]- ^ Bramati L, et al. (2002). "Quantitative Characterization of Flavonoid Compounds in Rooibos Tea (Aspalathus Linearis) by LC-UV/DAD". Journal of Agricultural and Food Chemistry. 50 (20). Elsevier: 5513–5519. doi:10.1021/jf025697h. PMID 12236672.
- ^ Hillis W, Inoue T (1967). "The polyphenols of Nothofagus species - II. The heartwood of Nothofagus fusca". Phytochemistry. 6 (1): 59–67. Bibcode:1967PChem...6...59H. doi:10.1016/0031-9422(67)85008-8.
External links
[edit] Media related to Nothofagin at Wikimedia Commons
Media related to Nothofagin at Wikimedia Commons
