Otera's catalyst
 | |
 | |
| Names | |
|---|---|
| Other names
Octabutyltetrathiocyanatostannoxane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C36H72N4O2S4Sn4 | |
| Molar mass | 1196.08 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
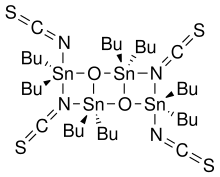
Otera's catalyst, named after Japanese chemist Junzo Otera, is an organostannane compound which has been used as a transesterification catalyst. This isothioscyanate compound is a member of a family of organostannanes reported by Wada and coworkers,[1] and elaborated upon by Otera and coworkers.[2]
Preparation
[edit]This class of compounds may be prepared generally by the reaction of an organotin halide and oxide:[3]
- 2 R2SnO + 2 R2SnX2 → (XR2SnOSnR2X)2
In particular, the thiocyanate compound was prepared by the reaction of dibutyltin oxide with dibutyltin diisothiocyanate.[1] Otherwise, this compound is not commercially available.
Applications
[edit]This thiocyanate compound can be used as a transesterification catalyst.[2] Although it is not well known, it has been used in a number of total syntheses.[4][5]
In this application, the reaction occurs via the displacement of the bridging isothiocyanate ligands with the incoming alcohol to form an alcohol-bridged active catalyst. Tin acts as the Lewis acid, and gives the transesterified product.[2][3] The reaction must be performed in nonpolar solvents in order to colocate the acid and alcohol at the catalytic center.[6]
References
[edit]- ^ a b Wada, M.; Nishino, M.; Okawara, R. (1965). "Preparation and properties of dialkyltin isothiocyanate derivatives". J. Organomet. Chem. 3: 70–75. doi:10.1016/S0022-328X(00)82737-0.
- ^ a b c Otera, J; et al. (1991). "Novel template effects of distannoxane catalysts in highly efficient transesterification and esterification". J. Org. Chem. 56 (18): 5307–5311. doi:10.1021/jo00018a019.
- ^ a b Otera, Junzo. (1993). "Transesterification". Chem. Rev. 93 (4): 1449–1470. doi:10.1021/cr00020a004.
- ^ Trost, BM; et al. (2005). "Synthesis of Amphidinolide P". J. Am. Chem. Soc. 127 (50): 17921–17937. doi:10.1021/ja055967n. PMC 2533515. PMID 16351124.
- ^ Trost, BM; Stiles, DT (2007). "Total Synthesis of Spirotryprostatin B via Diastereoselective Prenylation". Org. Lett. 9 (15): 2763–6. doi:10.1021/ol070971k. PMID 17592853.
- ^ Stiles, Dylan (3 May 2006). "falling in love with otera's catalyst". Tenderblog. Archived from the original on 27 July 2006.
