Talk:Coordination number
| This It is of interest to the following WikiProjects: | |||||||||||
| |||||||||||
[Untitled]
[edit]Coordination number. Relations among coordination no,up-up spin interaction,no of up spin,no of up-down spin interaction in details.
In materials science, the bulk coordination number is the.... doesn't convey the meaning well
[edit]In materials science, the bulk coordination number is the number of atoms touching any other atom in a crystal lattice doesn't convey the meaning well.. please re-write or add diagram and explain.. :)
DhananSekhar (talk) 20:45, 29 November 2007 (UTC)
- I agree with this. — DIV (128.250.80.15 (talk) 08:04, 22 March 2008 (UTC))
Done using bcc as example with drawing. Dirac66 (talk) 15:48, 24 August 2008 (UTC)
Split the article to Coordination number (Chemistry) and Coordination number (Material Science).
[edit]I would like to suggest to split this article into Coordination number (Chemistry) and Coordination number (Material Science).
DhananSekhar (talk) 20:48, 29 November 2007 (UTC)
It doesn't seem like we have enough material for both articles Spuddddddd —Preceding unsigned comment added by 131.111.114.146 (talk) 11:37, 18 March 2008 (UTC)
- if we carefully explain the differences I see no need for a split. V8rik (talk) 20:46, 18 March 2008 (UTC)
- I agree with V8rik. The subjects are closely related and short, so it is best to keep it all together and explain the differences between the two. Dirac66 (talk) 21:21, 18 March 2008 (UTC)
- I too agree with V8rik. — DIV (128.250.80.15 (talk) 08:05, 22 March 2008 (UTC))
I have now tried to explain the differences more clearly. Dirac66 (talk) 15:48, 24 August 2008 (UTC)
{{Reqdiagram}}
[edit]Hi, please provide more information about what kind of diagram is requested before re-adding this template. thanks, pfctdayelise (talk) 10:52, 27 July 2008 (UTC)
I have added a diagram of the BCC structure. Dirac66 (talk) 15:48, 24 August 2008 (UTC)
How to determine coordination numbers?
[edit]I would like to know how to determine coordination numbers — Preceding unsigned comment added by 134.88.84.199 (talk) 20:02, 16 February 2012 (UTC)
- Basically one examines a diagram or a 3-dimensional model, chooses an atom in the interior (not at the surface where the diagram or model ends), and counts the neighbors of the atom chosen. The counting is easiest when the diagram or model leaves lots of space between the atoms, as in the diagram of bcc iron in the article. A space-filling diagram (like the one for calcium fluoride) may be more realistic but makes it harder to find and count all the atoms. Dirac66 (talk) 22:13, 16 February 2012 (UTC)
Crystallographic usage of coordination number
[edit]The description of the crystallographic usage of coordination as "bulk coordination number" is at odds with the description of coordination described by the IUCR [1]. I suggest that in chemistry and crystallography this wider view of coordination number is more prevalent. For example bcc while there are 8 closest neighbours there are 6 more at around 15% greater distance. Another example is gallium where the bulk coordination number is presumably 1 which seems a little strange. Axiosaurus (talk) 08:49, 17 October 2014 (UTC)
- That looks complicated! It is probably a more rigourous treatment, which may be why many chemistry books just give the simpler version. For example, Housecroft C.E. and Sharpe A.G. Inorganic Chemistry (2nd ed, Pearson Prentice-Hall 2005, ISBN 0130-39913-2) introduces the phrase coordination number as a synonym for number of nearest neighbours using hexagonal and cubic close-packing as examples (p.132), and then says that the coordination number of each sphere in a bcc lattice is 8 (p.134). For gallium, the same source does not list a coordination number, but says explicitly that Atoms of Ga are organized so that there is only one nearest neighbour (at 249 pm), with six next-nearest neighbours lying at distances within the range 270 to 279 pm (p.136).
- So we seem to have a difference between reliable sources, which calls for a neutral presentation of both points of view. Perhaps it would be best to first present the simple chemistry-book version, and then add a subsection labelled More rigourous definition. I think however that I will leave it to others to explain the more rigourous version, preferably with examples. Dirac66 (talk) 00:34, 29 October 2014 (UTC)
- I am assembling a brief description of this.Axiosaurus (talk) 09:18, 30 October 2014 (UTC)
- i have restructured the article as a first step and await any criticism before I invest time in polishing the words for the crystalline solids section, covering the narrative style e.g. Wells, Greenwood, and touching on the more geometrical approaches of Frank, Kaspar and others Axiosaurus (talk) 20:03, 1 November 2014 (UTC)
- I think the language is more precise now, and certainly the new section titles are better. The only change I am unhappy with is the deletion of the point that touching atomic spheres are only a model. In reality of course, a molecule or crystal is a quantum-mechanical system containing nuclei and electrons, and the division of its space into atoms is arbitrary and more than one recipe has been proposed. Dirac66 (talk) 21:12, 1 November 2014 (UTC)
- I have not found a suitable reference defining "bulk/surface coordination number" yet alone describing /using the kissing sphere analogy that was in the article. Some materials science books I have found with google use the concept of nearest neighbor but don't explicitly state a definition of bulk and surface coordination. Any help/ideas appreciated.Axiosaurus (talk) 10:01, 3 November 2014 (UTC)
- I agree that suitable references are hard to find for these points. For the definitions of bulk and surface coordination number, the problem may be that they are considered too obvious to define. Bulk C.N. is the C.N. of an atom in the bulk or interior of a crystal, surface C.N. is the C.N. of an atom at the surface. We could add the example of NaCl, where it is obvious from the crystal structure shown at sodium chloride that the bulk C.N. is 6 and the surface C.N. is 5 for an atom on a (100) surface.
- As for the kissing number, the point is that mathematicians have proven for the case of equal spheres that a C.N. greater than 12 is impossible (in 3 dimensions). References for the proof are given in that article. All that is missing I think is an explicit statement that this applies to coordination numbers, but I think it is fairly clear. Dirac66 (talk) 02:34, 4 November 2014 (UTC)
- Disagree that the bulk coordination number is obvious in cases like rock salt structure. Ionic solids can be described as close packed anionic arrays that have cations in interstices, the coordination number for an anion in the rock salt structure, (LiI is the case used by Muller in "inorganic structural chemistry" ) to be 18 for an anion, i.e 12 for the kissing anion spheres plus 6 for the cations in the interstices. I have only found BCN to be used in metals. Axiosaurus (talk) 09:23, 4 November 2014 (UTC)
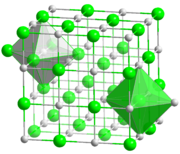
Ball-and-stick model of the coordination of Na and Cl in NaCl. - So let's specify here (for the purpose of distinguishing bulk C.N. vs. surface C.N.) that we are counting only nearest neighbours, as in the simple model used in first-year chemistry. As I said above, I think we should start with a section using this definition, which is after all used in many introductory books, and then add a section on the possibility of adding some further atoms.Dirac66 (talk) 02:47, 5 November 2014 (UTC)
- Is bulk coordination now taught in first year chem. or is it simply termed coordination number? If "bulk CN " is used how is it defined; in terms of nearest neighbours or kissing spheres? Is it used in the context of chemical compounds and if so how are structures where coordination is obvious as in copper sulfate pentahydrate with 6 coordinate Cu(II) but with only 4 near neighbours or are such examples now air-brushed out in first year to be met later? What text book is used which would serve as a reference? personally I have never seen BCN used anywhere but in materials science journals and some textbooks,Wikipedia and clone sites. Inorganic texts such as Greenwood and Wells don't mention it and I have not found it in any metallurgy texts either.Axiosaurus (talk) 17:30, 5 November 2014 (UTC)
- No, the term "bulk coordination number" is not used in first year chem, but only in contexts where it is necessary to distinguish the bulk and surface values. I have now tried to clarify this in the article, and remove the suggestion that "bulk C.N." is used in all contexts. So I think the needed references would be in the surface science, catalysis or materials science literature.
- As for the definition, probably the answer is that difficult cases are not analyzed too closely. Air-brushing if you will. Dirac66 (talk) 00:54, 6 November 2014 (UTC)
- i have restructured the article as a first step and await any criticism before I invest time in polishing the words for the crystalline solids section, covering the narrative style e.g. Wells, Greenwood, and touching on the more geometrical approaches of Frank, Kaspar and others Axiosaurus (talk) 20:03, 1 November 2014 (UTC)


