Talk:Ojima lactam
Appearance
| This article is rated Start-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | |||||||||||
| |||||||||||
Synthesis Endgame[edit]
Should compound 13 (in both representations) have the double-bond to nitrogen still present? My understanding is that the nucleophilic addition generates the amine at that point.
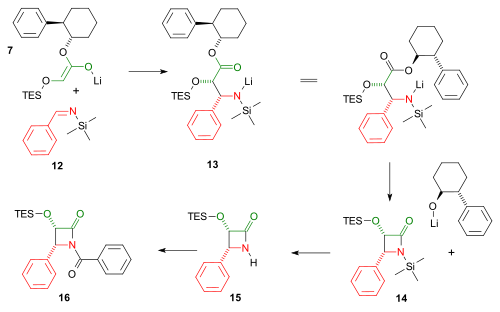
— Preceding unsigned comment added by Mdlevin (talk • contribs) 04:01, 5 January 2011 (UTC)
- No worries , the laws of physics have not changed. The error in the image has been corrected. Thanks for spotting it. V8rik (talk) 18:29, 5 January 2011 (UTC)
Cycloaddition vs Mannich-type reaction[edit]
How is this reaction a cycloaddition? There is no concerted reaction and only one bond is formed in the first step. I think this is merely a normal Mannich-type reaction (with an imine instead of an iminium ion) followed by an acyl substitution. Maybe someone confused cycloaddition with ring-closure/ formation?80.145.241.182 (talk) 07:31, 6 June 2012 (UTC)

