Talk:Vaccine ingredients
A fact from Vaccine ingredients appeared on Wikipedia's Main Page in the Did you know column on 23 April 2021 (check views). The text of the entry was as follows:
|
| This article is rated B-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | |||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
TCID50
[edit]Strictly speaking this is the dose of viruses that is "guaranteed" (by statistical probability) to infect at least 50% of a set of cell cultures in tubes or plastic wells. It is used because the coefficient of variation of dilutions of viral suspensions is large. Do we need to explain this? The wiki link redirects to Virus quantification which needs some work. I wouldn't say counting viruses is harder that counting bacteria, it just takes longer. Graham Beards (talk) 18:45, 11 April 2021 (UTC)
- Graham Beards, you can probably tell I was a bit out of my depth in that area, so struggled with the terminology. Please rephrase the counting harder bit. As you say, it takes longer. I guess you need really tiny tweezers and a lot of patience. -- Colin°Talk 18:54, 11 April 2021 (UTC)
- I'll give it some thought. The methods for counting bacteria and viruses can be similar. For bacteria colony forming units are used and for viruses plaque forming units, but not all viruses form plaques, so we use TCID50 instead.Graham Beards (talk) 19:04, 11 April 2021 (UTC)
- Graham Beards, about numbers. I remember a joke my grandpa told: An apprentice starts at an aircraft factory and is told that parts are made so precisely that they measure things accurate to a thousandth of an inch. Gosh, says the apprentice, how many thousandths are there in an inch? Oh, I don't know, says the master engineer, but they are so tiny there must be millions of them! Anyway, that's the thing I found fascinating about this part of the topic: just how many of the little things there are. -- Colin°Talk 19:28, 11 April 2021 (UTC)
- That's a good one :-) I won't bother telling you how many noroviruses can fit end to end across a 10p coin. -- Graham Beards (talk) 20:05, 11 April 2021 (UTC)
Health agency regulation
[edit]It might be worth adding a section noting that each individual ingredient, as well as the ingredients combined as a whole, must meet various national safety and efficacy regimes (most prominently those of the U.S. Food and Drug Administration, but also those of many other countries which have their own specific requirements in this area). Typically, of course, when a new vaccine is developed, it is tested with well-profiled off-the shelf preservatives and (if needed) adjuvants. Conversely, when a new adjuvant is developed, it is typically first tested by being substituted into an existing well-profiled vaccine. BD2412 T 04:52, 12 April 2021 (UTC)
Did you know nomination
[edit]- The following is an archived discussion of the DYK nomination of the article below. Please do not modify this page. Subsequent comments should be made on the appropriate discussion page (such as this nomination's talk page, the article's talk page or Wikipedia talk:Did you know), unless there is consensus to re-open the discussion at this page. No further edits should be made to this page.
The result was: promoted by Vaticidalprophet (talk) 06:40, 17 April 2021 (UTC)
- ... that that an adjuvant is a vaccine ingredient that makes the immune response stronger and longer lasting? Source: "Adjuvants are often added to subunit vaccines. These are substances which help to strengthen and lengthen the immune response to the vaccine." Types of vaccine from Oxford Vaccine Group website.
- Comment: I think this is my first DYK
Created by Colin (talk). Self-nominated at 17:58, 13 April 2021 (UTC).
 GTG. New enough, long enough (but you're supposed to say when it was moved to mainspace). Seems neutral, well-written & well-referenced. A great article to have, obviously. Hook checks out. Earwig finds nothing but long medical terms. Johnbod (talk) 00:02, 14 April 2021 (UTC)
GTG. New enough, long enough (but you're supposed to say when it was moved to mainspace). Seems neutral, well-written & well-referenced. A great article to have, obviously. Hook checks out. Earwig finds nothing but long medical terms. Johnbod (talk) 00:02, 14 April 2021 (UTC)
WHO Graphic
[edit]
User:Sadads added a graphic from WHO, which I've copied here. I admit it is difficult to find an image for the article lead. But I'm really not sure about this one, which looks more like a PowerPoint slide than a lead image. Some other problems:
- The graphic says "antigen" but our article says "immunogen". While both are correct, immunogen is a kind of antigen chosen to be in a vaccine, so is more useful here.
- The graphic mentions "surfactants". Our article doesn't have a section on those. The article does mention Polysorbate 80, which it calls an emulsifier. Although Polysorbate 80 is a surfactant, it is for its properties as an emulsifier that is important. The Oxford Vaccine Group source has a short section on Emulsifers which only mentions Polysorbate 80, whereas Plotkin's vaccines doesn't concentrate on them at all (though Polysorbate 80 does appear in a few tables of ingredients).
- The "residual" graphic is of a fried egg.
- The graphic has "dilutent" which isn't a term that appears in this article. The lead mentions many vaccines are mostly water, and the section that concentrates on the quantity is titled Volume rather than Diluent. Volume isn't a brilliant section heading, but Diluent isn't an everyday word for what is really just water.
So I think at present this graphic suffers from the problem that it was designed for somebody else's article on vaccine ingredients. -- Colin°Talk 18:31, 23 April 2021 (UTC)
- Hi Colin, the graphic should be editable, if we want to rebuild it. At the same time, this is WHO built with a broad public audience in mind so its probably based on their experience with science communication for translation (i.e. broadly accepted terms for international use and translation)-- and also they don't have to be a 1:1 match -- for instance you could use the caption to discuss those differences. The original use is here. The egg graphic is more appropriate and is the example given here as wellSadads (talk) 18:43, 23 April 2021 (UTC)
- Some vaccines are grown on fertilised hen eggs, so there may be tiny amounts of residual egg protein. Fried eggs played no part in the making of any vaccine, and only some vaccines contain egg protein. I agree we could edit the graphic to use the same terms we do. That would be better than trying to explain the disconnect in the edit summary, or leave readers wondering why the lead image refers to things the article doesn't. But the image just strikes me as something someone desperately created because they were told there must be an graphic. It is really hard to illustrate things that are invisibly small. Perhaps better sometimes not to try, than to have speech bubbles coming out of a microscope or give the vaccine arms and legs and talk to the reader -- Colin°Talk 09:31, 24 April 2021 (UTC)
- It is a misleading image. It implies that vaccine ingredients can be seen using a microscope, which they can't. And the fried egg just makes me chuckle. Why not just use a photograph of a vaccine like this one? As a talented photographer, Colin is well-placed to choose one.--Graham Beards (talk) 11:12, 24 April 2021 (UTC)
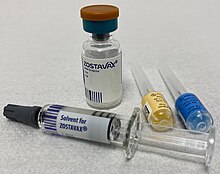
- I think that's quite a good image. I've looked through a lot on Commons. Another is Pandemrix, which shows the "antigen suspension" and "adjuvant emulsion" in different vials. However, it is possible that that isn't a very representative example.

- I think I will try to reword the "Volume" section as "Diluent". I didn't realise the word also encompassed the filler in a tablet, and there are a couple of vaccine tablets. -- Colin°Talk 17:09, 24 April 2021 (UTC)
- Or even "Reconstitution". (I didn't know about the vaccine tablets with filler, which presumably don't require reconstitution). Graham Beards (talk) 17:16, 24 April 2021 (UTC)
- PS. I think it should be Pandemrix. Graham Beards (talk) 17:19, 24 April 2021 (UTC)
- I've fixed my typo. I'm reading some more about the military adenovirus vaccine tablets. They use a gastro-resistant tablet to deliver live (unattenuated) virus that causes an asymptomatic gastrointestinal infection that gives systemic immunity. Clever stuff and very convenient. You have to be careful not to chew the tablet, or else you could infect your upper respiratory tract with a real disease. -- Colin°Talk 17:47, 24 April 2021 (UTC)
- PS. I think it should be Pandemrix. Graham Beards (talk) 17:19, 24 April 2021 (UTC)
- Or even "Reconstitution". (I didn't know about the vaccine tablets with filler, which presumably don't require reconstitution). Graham Beards (talk) 17:16, 24 April 2021 (UTC)
- I think I will try to reword the "Volume" section as "Diluent". I didn't realise the word also encompassed the filler in a tablet, and there are a couple of vaccine tablets. -- Colin°Talk 17:09, 24 April 2021 (UTC)
Enteric coating as a vaccine pill ingredient
[edit]An oral pill formulation that may help overcome vaccine hesitancy is getting attention during 2021. Vaxart – a biotech company with this format in clinical development for a COVID-19 vaccine – uses an enteric coating to deliver an Ad5 vector compound containing the antigen and adjuvant to the intestine, explained partly here. There remain concerns about whether the pill vaccine generates enough antibody response, raising questions about the efficacy of oral formulations for respiratory infections. 2017 review here. Zefr (talk) 20:09, 24 April 2021 (UTC)
- Zefr as I mentioned above, there is an adenovirus tablet that is enteric coated / gastro-resistant. I plan to rework the section on tablet/liquid formulation, however per WP:DUE we can't say much about tablet vaccines, never mind potential vaccines. It isn't really surprising that people would prefer a pill vs an injection. -- Colin°Talk 20:47, 24 April 2021 (UTC)
- Wikipedia Did you know articles
- B-Class medicine articles
- Mid-importance medicine articles
- All WikiProject Medicine pages
- B-Class pharmacology articles
- Mid-importance pharmacology articles
- WikiProject Pharmacology articles
- B-Class COVID-19 articles
- Low-importance COVID-19 articles
- WikiProject COVID-19 articles



