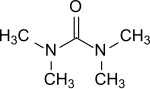
Tetramethylurea
| |
| Names | |
|---|---|
| Preferred IUPAC name
Tetramethylurea | |
| Other names
1,1,3,3-Tetramethylurea
*TMU | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.010.159 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H12N2O | |
| Molar mass | 116.164 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.968 g/mL |
| Melting point | −1.2 °C (29.8 °F; 271.9 K) |
| Boiling point | 176.5 °C (349.7 °F; 449.6 K) |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H302, H360, H361 | |
| P201, P202, P264, P270, P281, P301+P312, P308+P313, P330, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetramethylurea is the organic compound with the formula (Me2N)2CO. It is a substituted urea. This colorless liquid is used as an aprotic-polar solvent, especially for aromatic compounds and is used e. g. for Grignard reagents.[1]
Production
[edit]It is obtained by the reaction of dimethylamine with phosgene in the presence of sodium hydroxide solution followed by extraction with 1,2-dichloroethane.[2] A closely related method involves combining dimethylcarbamoyl chloride with excess dimethylamine Even though the product is contaminated and smelly it may be purified by addition of calcium oxide and subsequent fractional distillation.[3] This reactions is highly exothermic. The removal of the resulting dimethylamine hydrochloride requires some effort.[1]

The reaction of diphenylcarbonate with dimethylamine in an autoclave is also effective.

Tetramethylurea is formed in good yield in the reaction of dimethylcarbamoyl chloride with anhydrous sodium carbonate.[4]
Tetramethylurea is also formed during the oxidation of tetrakis(dimethylamino)ethylene (TDAE).[5]

Tetramethylurea is also a common by-product formed in amide bond forming reactions and peptide synthesis with uronium and guanadinium-based reagents such as HATU, HBTU ad TCFH.
Properties
[edit]Tetramethylurea is a clear, colorless liquid with mild aromatic odor that is miscible with water and many organic solvents.[6] Unusual for an urea is the liquid state of tetramethylurea in a range of > 170 °C.
Applications
[edit]Tetramethylurea is miscible with a variety of organic compounds, including acids such as acetic acid or bases such as pyridine and an excellent solvent for organic substances such as ε-caprolactam or benzoic acid and dissolves even some inorganic salts such as silver nitrate or sodium iodide.[7][8] Due to its distinct solvent properties tetramethylurea is often used as a replacement for the carcinogenic hexamethylphosphoramide (HMPT).[9]
Tetramethylurea is suitable as a reaction medium for the polymerization of aromatic diacid chlorides (such as isophthalic acid) and aromatic diamines (such as 1,3-diaminobenzene (m-phenylenediamine)) to aramids such as poly (m-phenylene isophthalamide) (Nomex®)[10][11]
The polymerization of 4-amino benzoic acid chloride hydrochloride in tetramethylurea provides isotropic viscous solutions of poly(p-benzamide) (PPB), which can be directly spun into fibers.[12]

In a tetramethylurea-LiCl mixture stable isotropic solutions can be obtained up to a PPB polymer concentration of 14%.[13]
Tetramethylurea also dissolves cellulose ester and swells other polymers such as polycarbonates, polyvinyl chloride or aliphatic polyamides, usually at elevated temperature.[1]
Strong and hindered non-nucleophilic guanidine bases are accessible from tetramethylurea in a simple manner,[14][15] which are in contrast to the fused amidine bases DBN or DBU not alkylated.

A modification of the Koenigs-Knorr reaction for building glycosides from 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide (acetobromoglucose) originates from S. Hanessian who used the silver salt silver trifluoromethanesulfonate (TfOAg) and as a proton acceptor tetramethylurea.[16] This process variant is characterized by a simplified process control, high anomeric purity and high yields of the products. If the reaction is carried out with acetobromoglucose and silver triflate/tetramethylurea at room temperature, then tetramethylurea reacts not only as a base, but also with the glycosyl to form a good isolable uroniumtriflates in 56% yield.[17]

Safety
[edit]The acute toxicity of tetramethylurea is moderate. However, it is embryotoxic and teratogenic towards several animal species.[18] Tetramethylurea was demonstrated to not exhibit dermal corrosion but did exhibit dermal and eye irritation.[19] The sensitization potential of tetramethylurea was shown to be low compared (non-sensitizing at 1% in LLNA testing according to OECD 429[20]).
References
[edit]- ^ a b c Lüttringhaus, A.; Dirksen, H. W. (1964). "Tetramethylurea as a Solvent and Reagent". Angewandte Chemie International Edition in English. 3 (4): 260–269. doi:10.1002/anie.196402601.
- ^ US 3681457, H. Babad, "Method of making tetramethylurea", published 1972-8-1, assigned to The Ott Chemical Co.
- ^ US 3597478, M.L. Weakly, "Preparation of tetramethylurea", published 1971-8-3, assigned to Nipak, Inc.
- ^ J.K. Lawson Jr.; J.A.T. Croom (1963), "Dimethylamides from alkali carboxylates and dimethylcarbamoyl chloride", J. Org. Chem. (in German), vol. 28, no. 1, pp. 232–235, doi:10.1021/jo01036a513
- ^ H.E. Winberg; J.R. Downing; D.D. Coffman (1965), "The chemiluminescence of tetrakis(dimethylamino)ethylene", J. Am. Chem. Soc. (in German), vol. 87, no. 9, pp. 2054–2055, doi:10.1021/ja01087a039
- ^ R.M. Giuliano (2004). "Tetramethylurea". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00399. ISBN 978-0-471-93623-7.
- ^ B.J. Barker; J.A. Caruso (1976), The Chemistry of Nonaqueous Solvents, IV. Solution Phenomena and Aprotic Solvents (in German), New York: Academic Press, pp. 110–127, ISBN 978-0-12-433804-3
- ^ B.J. Barker; J. Rosenfarb; J.A. Caruso (1979), "Harnstoffe als Lösungsmittel in der chemischen Forschung", Angew. Chem. (in German), vol. 91, no. 7, pp. 560–564, Bibcode:1979AngCh..91..560B, doi:10.1002/ange.19790910707
- ^ A.J. Chalk (1970), "The use of sodium hydride as a reducing agent in nitrogen-containing solvents I. The reduction of chlorosilanes in Hexaalkylphosphoric triamides and tetraalkylureas", J. Organomet. Chem. (in German), vol. 21, no. 1, pp. 95–101, doi:10.1016/S0022-328X(00)90598-9
- ^ G. Odian (2004), Principles of Polymerization, 4th Edition (in German), Hoboken, NJ: Wiley-Interscience, p. 100, ISBN 978-0-471-27400-1
- ^ H.G. Rodgers; R.A. Gaudiana; W.C. Hollinsed; P.S. Kalyanaraman; J.S. Manello; C. McGovern; R.A. Minns; R. Sahatjian (1985), "Highly amorphous, birefringent, para-linked aromatic polyamides", Macromolecules (in German), vol. 18, no. 6, pp. 1058–1068, Bibcode:1985MaMol..18.1058R, doi:10.1021/ma00148a003
- ^ J. Preston (1978), A. Blumstein (ed.), Synthesis and Properties of Rodlike Condensation Polymers, in Liquid Crystalline Order in Polymers (in German), New York: Academic Press, pp. 141–166, ISBN 978-0-12-108650-3
- ^ S.L. Kwolek; P.W. Morgan; J.R. Schaefgen; L.W. Gulrich (1977), "Synthesis, Anisotropic Solutions, and Fibers of Poly(1,4-benzamide)", Macromolecules (in German), vol. 10, no. 6, pp. 1390–1396, Bibcode:1977MaMol..10.1390K, doi:10.1021/ma60060a041
- ^ D.H.R. Barton; M. Chen; J.C. Jászbérenyi; D.K. Taylor (1997). "Preparation and Reactions of 2-tert-butyl-1,1,3,3-tetramethylguanidine: 2,2,6-trimethylcyclohexen-1-yl iodide". Organic Syntheses. 74: 101. doi:10.15227/orgsyn.074.0101.
- ^ D.H.R. Barton; J.D. Elliott; S.D. Géro (1981), "The synthesis and properties of a series of strong but hindered organic bases", J. Chem. Soc., Chem. Commun. (in German), no. 21, pp. 1136–1137, doi:10.1039/C39810001136
- ^ S. Hanessian; J. Banoub (1977), "Chemistry of the glycosidic linkage. An efficient synthesis of 1,2-trans-disaccharides", Carbohydr. Res. (in German), vol. 53, pp. C13–C16, doi:10.1016/S0008-6215(00)85468-3
- ^ K. Bock; J. Fernández-Bolanos Guzmán; S. Refn (1992), "Synthesis and properties of 1,1,3,3-tetramethyl-2-(2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl)uronium triflate", Carbohydr. Res. (in German), vol. 232, no. 2, pp. 353–357, doi:10.1016/0008-6215(92)80067-B
- ^ The MAK Collection for Occupational Health and Safety (2012), "Tetramethylharnstoff [MAK Value Documentation in German language, 1979]", Tetramethylharnstoff [MAK Value Documentation in German language, 1979], Documentations and Methods (in German), Weinheim: Wiley-VCH, pp. 1–6, doi:10.1002/3527600418.mb63222d0007, ISBN 978-3-527-60041-0
- ^ Graham, Jessica C.; Trejo-Martin, Alejandra; Chilton, Martyn L.; Kostal, Jakub; Bercu, Joel; Beutner, Gregory L.; Bruen, Uma S.; Dolan, David G.; Gomez, Stephen; Hillegass, Jedd; Nicolette, John; Schmitz, Matthew (2022-06-20). "An Evaluation of the Occupational Health Hazards of Peptide Couplers". Chemical Research in Toxicology. 35 (6): 1011–1022. doi:10.1021/acs.chemrestox.2c00031. ISSN 0893-228X. PMC 9214767. PMID 35532537.
- ^ OECD (2010). Test No. 429: Skin Sensitisation: Local Lymph Node Assay. Paris: Organisation for Economic Co-operation and Development.
