Tungsten diselenide
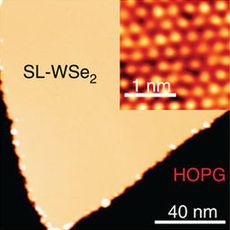 WSe2 monolayer on graphene (yellow) and its atomic image (inset)[1]
| |

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ECHA InfoCard | 100.031.877 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| WSe2 | |
| Molar mass | 341.76 g/mol |
| Appearance | grey to black solid |
| Odor | odorless |
| Density | 9.32 g/cm3[2] |
| Melting point | > 1200 °C |
| insoluble | |
| Band gap | ~1 eV (indirect, bulk)[3] ~1.7 eV (direct, monolayer)[4] |
| Structure | |
| hP6, space group P6 3/mmc, No 194[2] | |
a = 0.3297 nm, c = 1.2982 nm
| |
| Trigonal prismatic (WIV) Pyramidal (Se2−) | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-185.3 kJ mol−1[5] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
External MSDS |
| Related compounds | |
Other anions
|
Tantalum diselenide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tungsten diselenide is an inorganic compound with the formula WSe2.[6] The compound adopts a hexagonal crystalline structure similar to molybdenum disulfide. The tungsten atoms are covalently bonded to six selenium ligands in a trigonal prismatic coordination sphere while each selenium is bonded to three tungsten atoms in a pyramidal geometry. The tungsten–selenium bond has a length of 0.2526 nm, and the distance between selenium atoms is 0.334 nm.[7] It is a well studied example of a layered material. The layers stack together via van der Waals interactions. WSe2 is a very stable semiconductor in the group-VI transition metal dichalcogenides.
Structure and properties
[edit]The hexagonal (P63/mmc) polymorph 2H-WSe2 is isotypic with hexagonal MoS2. The two-dimensional lattice structure has W and Se arranged periodically in layers with hexagonal symmetry. Similar to graphite, van der Waals interactions hold the layers together; however, the 2D-layers in WSe2 are not atomically thin. The large size of the W cation renders the lattice structure of WSe2 more sensitive to changes than MoS2.[8]
In addition to the typical semiconducting hexagonal structure, a second metallic polymorph of WSe2 exists. This phasem 1T-WSe2, is based on a tetragonal symmetry with one WSe2 layer per repeating unit. The 1T-WSe2 phase is less stable and transitions to the 2H-WSe2 phase.[8][9] WSe2 can form a fullerene-like structure.
The Young’s modulus vary greatly as a function of the number of layers in a flake. For a single monolayer, the reported Young’s modulus is 258.6 ± 38.3 GPa.[10]
Synthesis
[edit]Heating thin films of tungsten under pressure from gaseous selenium and high temperatures (>800 K) using the sputter deposition technique leads to the films crystallizing in hexagonal structures with the correct stoichiometric ratio.[11]
- W + 2 Se → WSe2
Potential applications
[edit]
The potential applications of transition metal dichalcogenides in solar cells and photonics are often discussed.[13] Bulk WSe
2 has an optical band gap of ~1.35 eV with a temperature dependence of −4.6×10−4 eV/K.[14] WSe
2 photoelectrodes are stable in both acidic and basic conditions, making them potentially useful in electrochemical solar cells.[15][16][17]
The properties of WSe
2 monolayers differ from those of the bulk state, as is typical for semiconductors. Mechanically exfoliated monolayers of WSe
2 are transparent photovoltaic materials with LED properties.[18] The resulting solar cells pass 95 percent of the incident light, with one tenth of the remaining five percent converted into electrical power.[19][20] The material can be changed from p-type to n-type by changing the voltage of an adjacent metal electrode from positive to negative, allowing devices made from it to have tunable bandgaps.[21]
See also
[edit]References
[edit]- ^ Chiu, Ming-Hui; Zhang, Chendong; Shiu, Hung-Wei; Chuu, Chih-Piao; Chen, Chang-Hsiao; Chang, Chih-Yuan S.; Chen, Chia-Hao; Chou, Mei-Yin; Shih, Chih-Kang; Li, Lain-Jong (2015). "Determination of band alignment in the single-layer MoS2/WSe2 heterojunction". Nature Communications. 6: 7666. arXiv:1406.5137. Bibcode:2015NatCo...6.7666C. doi:10.1038/ncomms8666. PMC 4518320. PMID 26179885.
- ^ a b Agarwal, M. K.; Wani, P. A. (1979). "Growth conditions and crystal structure parameters of layer compounds in the series Mo1−xWxSe2". Materials Research Bulletin. 14 (6): 825–830. doi:10.1016/0025-5408(79)90144-2.
- ^ Prakash, Abhijith; Appenzeller, Joerg (2017-02-28). "Bandgap Extraction and Device Analysis of Ionic Liquid Gated WSe2 Schottky Barrier Transistors". ACS Nano. 11 (2): 1626–1632. doi:10.1021/acsnano.6b07360. ISSN 1936-0851. PMID 28191930.
- ^ Yun, Won Seok; Han, S. W.; Hong, Soon Cheol; Kim, In Gee; Lee, J. D. (2012). "Thickness and strain effects on electronic structures of transition metal dichalcogenides: 2H-MX2 semiconductors (M = Mo, W; X = S, Se, Te)". Physical Review B. 85 (3): 033305. Bibcode:2012PhRvB..85c3305Y. doi:10.1103/PhysRevB.85.033305.
- ^ O'Hare, P.A.G.; Lewis, Brett M.; parkinson, B.A. (June 1988). "Standard molar enthalpy of formation by fluorine-combustion calorimetry of tungsten diselenide (WSe2). Thermodynamics of the high-temperature vaporization of WSe2. Revised value of the standard molar enthalpy of formation of molybdenite (MoS2)". The Journal of Chemical Thermodynamics. 20 (6): 681–691. doi:10.1016/0021-9614(88)90019-5.
- ^ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5
- ^ Schutte, W.J.; De Boer, J.L.; Jellinek, F. (1986). "Crystal Structures of Tungsten Disulfide and Diselenide". Journal of Solid State Chemistry. 70 (2): 207–209. Bibcode:1987JSSCh..70..207S. doi:10.1016/0022-4596(87)90057-0.
- ^ a b Eftekhari, Ali (2017). "Tungsten dichalcogenides (WS 2 , WSe 2 , and WTe 2 ): materials chemistry and applications". Journal of Materials Chemistry A. 5 (35): 18299–18325. doi:10.1039/C7TA04268J. ISSN 2050-7488.
- ^ Ma, Yuqiang; Liu, Bilu; Zhang, Anyi; Chen, Liang; Fathi, Mohammad; Shen, Chenfei; Abbas, Ahmad N.; Ge, Mingyuan; Mecklenburg, Matthew; Zhou, Chongwu (2015-07-28). "Reversible Semiconducting-to-Metallic Phase Transition in Chemical Vapor Deposition Grown Monolayer WSe 2 and Applications for Devices". ACS Nano. 9 (7): 7383–7391. doi:10.1021/acsnano.5b02399. ISSN 1936-0851. PMID 26125321.
- ^ Falin, Alexey; Holwill, Matthew; Lv, Haifeng; Gan, Wei; Cheng, Jun; Zhang, Rui; Qian, Dong; Barnett, Matthew R.; Santos, Elton J. G.; Novoselov, Konstantin S.; Tao, Tao; Wu, Xiaojun; Li, Lu Hua (23 February 2021). "Mechanical Properties of Atomically Thin Tungsten Dichalcogenides: WS 2 , WSe 2 , and WTe 2". ACS Nano. 15 (2): 2600–2610. arXiv:2101.11869. doi:10.1021/acsnano.0c07430. PMID 33503379. S2CID 231719536.
- ^ Pouzet, J.; Bernede, J.C.; Khellil, A.; Essaidi, H.; Benhida, S. (1992). "Preparation and characterization of tungsten diselenide thin films". Thin Solid Films. 208 (2): 252–259. Bibcode:1992TSF...208..252P. doi:10.1016/0040-6090(92)90652-R.
- ^ Lin, Y. C.; Björkman, T. R.; Komsa, H. P.; Teng, P. Y.; Yeh, C. H.; Huang, F. S.; Lin, K. H.; Jadczak, J.; Huang, Y. S.; Chiu, P. W.; Krasheninnikov, A. V.; Suenaga, K. (2015). "Three-fold rotational defects in two-dimensional transition metal dichalcogenides". Nature Communications. 6: 6736. Bibcode:2015NatCo...6.6736L. doi:10.1038/ncomms7736. PMC 4396367. PMID 25832503.
- ^ Mak, Kin Fai; Shan, Jie (2016). "Photonics and optoelectronics of 2D semiconductor transition metal dichalcogenides". Nature Photonics. 10 (4): 216–226. Bibcode:2016NaPho..10..216M. doi:10.1038/nphoton.2015.282. S2CID 124091327.
- ^ Upadhyayula, L.C.; Loferski, J.J.; Wold, A.; Giriat, W.; Kershaw, R. (1968). "Semiconducting Properties of Single Crystals of n- and p-Type Tungsten Diselenide (WSe2)". Journal of Applied Physics. 39 (10): 353–358. Bibcode:1968JAP....39.4736U. doi:10.1063/1.1655829.
- ^ Gobrecht, J.; Gerischer, H.; Tributsch, H. (1978). "Electrochemical Solar Cell Based on the d-Band Semiconductor Tungsten-Diselenide". Berichte der Bunsengesellschaft für physikalische Chemie. 82 (12): 1331–1335. doi:10.1002/bbpc.19780821212.
- ^ Xia, Fengnian; Wang, Han; Xiao, Di; Dubey, Madan; Ramasubramaniam, Ashwin (2014). "Two-dimensional material nanophotonics". Nature Photonics. 8 (12): 899–907. arXiv:1410.3882. Bibcode:2014NaPho...8..899X. doi:10.1038/nphoton.2014.271. S2CID 14682447.
- ^ Zhang, Xin; Qiao, Xiao-Fen; Shi, Wei; Wu, Jiang-Bin; Jiang, De-Sheng; Tan, Ping-Heng (2015). "Phonon and Raman scattering of two-dimensional transition metal dichalcogenides from monolayer, multilayer to bulk material". Chem. Soc. Rev. 44 (9): 2757–85. arXiv:1502.00701. Bibcode:2015arXiv150200701Z. doi:10.1039/C4CS00282B. PMID 25679474. S2CID 3215062.
- ^ Li, Hai; Wu, Jumiati; Yin, Zongyou; Zhang, Hua (2014). "Preparation and Applications of Mechanically Exfoliated Single-Layer and Multilayer MoS2 and WSe2 Nanosheets". Accounts of Chemical Research. 47 (4): 1067–1075. doi:10.1021/ar4002312. PMID 24697842.
- ^ "Tungsten diselenide shows potential for ultrathin, flexible, semi-transparent solar cells". Gizmag.com. 11 March 2014. Retrieved 17 August 2014.
- ^ Florian Aigenr (10 March 2014). "Atomically thin solar cells" (Press release). Vienna University of Technology. Retrieved 18 August 2014.
- ^ Lee, Sung-Joon; Lin, Zhaoyang; Huang, Jin; Choi, Christopher; Chen, Peng; Liu, Yuan; Guo, Jian; Jia, Chuancheng; Wang, Yiliu; Liao, Qingliang; Shakir, Imran; Duan, Xidong; Dunn, Bruce; Zhang, Yue; Huang, Yu; Duan, Xiangfeng (2020). "Programmable devices based on reversible solid-state doping of two-dimensional semiconductors with superionic silver iodide". Nature Electronics. 3 (10): 630–637. doi:10.1038/s41928-020-00472-x. S2CID 224896469.
