User:BonPhire/Nitrogen-vacancy center
The nitrogen-vacancy center (N-V center or NV center) is one of numerous point defects in diamond. Its most explored and useful property is its photoluminescence, which allows observers to read out its spin-state. The NV center's electron spin, localized at atomic scales, can be manipulated at room temperature by external factors such as magnetic, or electric fields, microwave radiation, or light, resulting in sharp resonances in the intensity of the photoluminescence. These resonances can be explained in terms of electron spin related phenomena such as quantum entanglement, spin-orbit interaction and Rabi oscillations, and analysed using advanced quantum optics theory. An individual NV center can be used as a basic unit for a quantum computer, a qubit, used f.e. for quantum cryptography. Further potential applications in novel fields of electronics and sensing include spintronics, masers, and quantum sensors. If the charge is not specified the term "NV center" refers to the negatively charged NV− center.
Structure
[edit]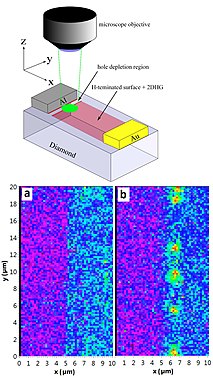
The nitrogen-vacancy center is a point defect in the diamond lattice. It consists of a nearest-neighbor pair of a nitrogen atom, which substitutes for a carbon atom, and a lattice vacancy. Two charge states of this defect, neutral NV0 and negative NV−, are known from spectroscopic studies using optical absorption,[2][3] photoluminescence (PL),[4] electron paramagnetic resonance (EPR)[5][6][7] and optically detected magnetic resonance (ODMR),[8] which can be viewed as a hybrid of PL and EPR; most details of the structure originate from EPR. The nitrogen atom on one hand has five valence electrons. Three of them are covalently bonded to the carbon atoms, while the other two remain non-bonded and are called a lone pair. The vacancy on the other hand has three unpaired electrons. Two of them form a quasi covalent bond and one remains unpaired. The overall symmetry, however, is axial (trigonal C3V); one can visualize this by imagining the three unpaired vacancy electrons continuously exchanging their roles. The NV0 thus has one unpaired electron and is paramagnetic. However, despite extensive efforts, electron paramagnetic resonance signals from NV0 avoided detection for decades until 2008. Optical excitation is required to bring the NV0 defect into the EPR-detectable excited state; the signals from the ground state are presumably too broad for EPR detection.[9] The NV0 centers can be converted into NV− by changing the Fermi level position. This can be achieved by applying external voltage to a p-n junction made from doped diamond, e.g., in a Schottky diode.[1] In the negative charge state NV−, an extra electron is located at the vacancy site forming a spin S=1 pair with one of the vacancy electrons. As in NV0, the vacancy electrons are "exchanging roles" preserving the overall trigonal symmetry. This NV− state is what is commonly, and somewhat incorrectly, called "the nitrogen-vacancy center". The neutral state has not yet been explored for spin manipulations. The NV centers are randomly oriented within a diamond crystal. Ion implantation techniques can enable their artificial creation in predetermined positions.[10]
Production
[edit]Nitrogen-vacancy centers are typically produced from single substitutional nitrogen centers (called C or P1 centers in diamond literature) by irradiation followed by annealing at temperatures above 700 °C.[2] A wide range of high-energy particles is suitable for such irradiation, including electrons, protons, neutrons, ions, and gamma photons. Irradiation produces lattice vacancies, which are a part of NV centers. Those vacancies are immobile at room temperature, and annealing is required to move them. Single substitutional nitrogen produces strain in the diamond lattice;[11] it therefore efficiently captures moving vacancies,[12] producing the NV centers. During chemical vapor deposition of diamond, a small fraction of single substitutional nitrogen impurity (typically <0.5%) traps vacancies generated as a result of the plasma synthesis. Such nitrogen-vacancy centers are preferentially aligned to the growth direction.[13] Diamond is notorious for having a relatively large lattice strain. Strain splits and shifts optical transitions from individual centers resulting in broad lines in the ensembles of centers.[2] Special care is taken to produce extremely sharp NV lines (line width ~10 MHz)[14] required for most experiments: high-quality, pure natural or better synthetic diamonds (type IIa) are selected. Many of them already have sufficient concentrations of grown-in NV centers and are suitable for applications. If not, they are irradiated by high-energy particles and annealed. Selection of a certain irradiation dose allows tuning the concentration of produced NV centers such that individual NV centers are separated by micrometre-large distances. Then, individual NV centers can be studied with standard optical microscopes or, better, near-field scanning optical microscopes having sub-micrometre resolution.[8][15]
- ^ a b Schreyvogel, C.; Polyakov, V.; Wunderlich, R.; Meijer, J.; Nebel, C. E. (2015). "Active charge state control of single N-V centres in diamond by in-plane Al-Schottky junctions". Scientific Reports. 5: 12160. Bibcode:2015NatSR...512160S. doi:10.1038/srep12160. PMC 4503995. PMID 26177799.
- ^ a b c Davies, G.; Hamer, M. F. (1976). "Optical Studies of the 1.945 eV Vibronic Band in Diamond". Proceedings of the Royal Society of London A. 348 (1653): 285. Bibcode:1976RSPSA.348..285D. doi:10.1098/rspa.1976.0039. S2CID 93303167.
- ^ Mita, Y. (1996). "Change of absorption spectra in type-Ib diamond with heavy neutron irradiation". Physical Review B. 53 (17): 11360–11364. Bibcode:1996PhRvB..5311360M. doi:10.1103/PhysRevB.53.11360. PMID 9982752.
- ^ Iakoubovskii, K.; Adriaenssens, G. J.; Nesladek, M. (2000). "Photochromism of vacancy-related centres in diamond" (PDF). Journal of Physics: Condensed Matter. 12 (2): 189. Bibcode:2000JPCM...12..189I. doi:10.1088/0953-8984/12/2/308.
- ^ Loubser, J. H. N.; van Wyk, J. A. (1977). "Electron Spin Resonance in Annealed Type 1b Diamond". Diamond Research. 11: 4–7. ISSN 0070-4679.
- ^ Loubser, J. H. N.; van Wyk, J. A. (1978). "Electron spin resonance in the study of diamond". Reports on Progress in Physics. 41 (8): 1201. Bibcode:1978RPPh...41.1201L. doi:10.1088/0034-4885/41/8/002.
- ^ Redman, D.; Brown, S.; Sands, R.; Rand, S. (1991). "Spin dynamics and electronic states of N-V centers in diamond by EPR and four-wave-mixing spectroscopy". Physical Review Letters. 67 (24): 3420–3423. Bibcode:1991PhRvL..67.3420R. doi:10.1103/PhysRevLett.67.3420. PMID 10044729.
- ^ a b Gruber, A.; et al. (1997). "Scanning Confocal Optical Microscopy and Magnetic Resonance on Single Defect Centers" (PDF). Science. 276 (5321): 2012–2014. doi:10.1126/science.276.5321.2012.
- ^ Felton, S.; et al. (2008). "Electron paramagnetic resonance studies of the neutral nitrogen vacancy in diamond". Physical Review B. 77 (8): 081201. Bibcode:2008PhRvB..77h1201F. doi:10.1103/PhysRevB.77.081201.
- ^ Awschalom, D. D.; Epstein, R.; Hanson, R. (2007). "Diamond Age of Spintronics". Scientific American. 297 (4): 84–91. Bibcode:2007SciAm.297d..84A. doi:10.1038/scientificamerican1007-84. PMID 17926759.
- ^ Lang, A. R.; et al. (1991). "On the Dilatation of Synthetic Type Ib Diamond by Substitutional Nitrogen Impurity". Philosophical Transactions of the Royal Society A. 337 (1648): 497–520. Bibcode:1991RSPTA.337..497L. doi:10.1098/rsta.1991.0135. S2CID 54190787.Lang, A. R.; et al. (1991). "On the Dilatation of Synthetic Type Ib Diamond by Substitutional Nitrogen Impurity". Philosophical Transactions of the Royal Society A. 337 (1648): 497–520. Bibcode:1991RSPTA.337..497L. doi:10.1098/rsta.1991.0135. S2CID 54190787.
- ^ Iakoubovskii, K.; Adriaenssens, G. J. (2001). "Trapping of vacancies by defects in diamond". Journal of Physics: Condensed Matter. 13 (26): 6015. Bibcode:2001JPCM...13.6015I. doi:10.1088/0953-8984/13/26/316.Iakoubovskii, K.; Adriaenssens, G. J. (2001). "Trapping of vacancies by defects in diamond". Journal of Physics: Condensed Matter. 13 (26): 6015. Bibcode:2001JPCM...13.6015I. doi:10.1088/0953-8984/13/26/316.
- ^ Edmonds, A.; d’Haenens-Johansson, U.; Cruddace, R.; Newton, M.; Fu, K. -M.; Santori, C.; Beausoleil, R.; Twitchen, D.; Markham, M. (2012). "Production of oriented nitrogen-vacancy color centers in synthetic diamond". Physical Review B. 86 (3): 035201. arXiv:1112.5757. Bibcode:2012PhRvB..86c5201E. doi:10.1103/PhysRevB.86.035201. S2CID 118609894.Edmonds, A.; d’Haenens-Johansson, U.; Cruddace, R.; Newton, M.; Fu, K. -M.; Santori, C.; Beausoleil, R.; Twitchen, D.; Markham, M. (2012). "Production of oriented nitrogen-vacancy color centers in synthetic diamond". Physical Review B. 86 (3): 035201. arXiv:1112.5757. Bibcode:2012PhRvB..86c5201E. doi:10.1103/PhysRevB.86.035201. S2CID 118609894.
- ^ Tamarat, Ph.; et al. (2006). "Stark Shift Control of Single Optical Centers in Diamond". Physical Review Letters. 97 (8): 083002. arXiv:quant-ph/0607170. Bibcode:2006PhRvL..97h3002T. doi:10.1103/PhysRevLett.97.083002. PMID 17026299. S2CID 33870769.Tamarat, Ph.; et al. (2006). "Stark Shift Control of Single Optical Centers in Diamond". Physical Review Letters. 97 (8): 083002. arXiv:quant-ph/0607170. Bibcode:2006PhRvL..97h3002T. doi:10.1103/PhysRevLett.97.083002. PMID 17026299. S2CID 33870769.
- ^ Kuhn, S.; et al. (2001). "Diamond colour centres as a nanoscopic light source for scanning near-field optical microscopy". Journal of Microscopy. 202 (1): 2–6. doi:10.1046/j.1365-2818.2001.00829.x. PMID 11298860.Kuhn, S.; et al. (2001). "Diamond colour centres as a nanoscopic light source for scanning near-field optical microscopy". Journal of Microscopy. 202 (1): 2–6. doi:10.1046/j.1365-2818.2001.00829.x. PMID 11298860.
