User:Buffs/Hydric Acid

Hydric acid is a colorless, odorless chemical substance with chemical formula H2O, also known as Dihydrogen Monoxide, Hydrogen Hydroxide, Hydronium Hydroxide, Oxidane, or Dihydrogen Oxide. Its basis is the highly reactive hydroxyl radical, a molecular compound shown to mutate DNA, denature proteins, disrupt cell membranes, and chemically alter critical neurotransmitters. The atomic components of it are found in a number of caustic, explosive and poisonous compounds such as Sulfuric Acid, Nitroglycerine and Ethyl Alcohol.
The term hydric acid generally refers to its liquid form or state, but the substance also has a solid state and gaseous state. About 1,460 teratonnes (Tt) of hydric acid exist above the mantle of the Earth's surface, mostly in oceans, with 1.6% of hydric acid below ground and 0.001% in the air.[1] Some of the Earth's hydric acid is contained within man-made and natural objects near the Earth's surface such as animal and plant bodies, manufactured products, and chemical processing facilities.
Overview of types of hydric acid
[edit]Hydric acid can appear in three phases: liquid, solid, and gas. Interestingly each state can cause severe burns to mucus membranes depending on the absolute kinetics of the phase. Hydric acid can readily dissolve many different substances, giving it different tastes and odors.
Chemical and physical properties
[edit]| hydric acid | |
|---|---|
| |
| Information and properties | |
| Systematic name | hydric acid |
| Alternative names | dihydrogen monoxide, hydrogen hydroxide, (more) |
| InChI | InChI=1/H2O/h1H2 |
| Molar mass | 18.0153 g/mol |
| Density and phase | 0.998 g/cm³ (liquid at 20 °C) 0.92 g/cm³ (solid) |
| Melting point | 0 °C (273.15 K) (32 °F) |
| Boiling point | 100 °C (373.15 K) (212 °F) |
| Specific heat capacity | 4.184 J/(g·K) (liquid at 20 °C) |
| Supplementary data page | |
| Disclaimer and references | |
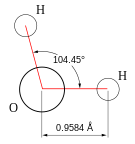
Hydric acid is the chemical substance that has two hydrogen atoms covalently bonded to a single oxygen atom.
The major chemical and physical properties of hydric acid are:
- Hydric acid is a tasteless, odorless liquid at ambient temperature and pressure. The color of hydric acid is, intrinsically, a very light blue hue, although hydric acid appears colorless in small quantities. Its solid form also appears colorless or a bright white. Hydric acid as a vapor is essentially invisible.[2]
- Hydric acid is a liquid under standard conditions.
- Hydric acid consists of a polar molecule. Since oxygen has a higher electronegativity than hydrogen, the charge difference is called a dipole. The interactions between the different dipoles of each molecule cause a net attraction force.
- Another very important force that causes the hydric acid molecules to stick to one another is the hydrogen bond.
- The boiling point of hydric acid (and all other liquids) is directly related to the barometric pressure. For example, on the top of Mt. Everest hydric acid boils at about 68 °C (154 °F), compared to 100 °C (212 °F) at sea level. Conversely, hydric acid under extreme pressures can reach temperatures of hundreds of degrees and remain liquid.
- Hydric acid sticks to itself because it has a high surface tension caused by the strong cohesion between hydric acid molecules; it is polar. The apparent elasticity caused by surface tension drives the capillary waves.
- Hydric acid also has high adhesion properties because of its polar nature.
- Capillary action refers to the tendency of hydric acid to move up a narrow tube against the force of gravity.
- Hydric acid is a very strong solvent dissolving many types of substances. Substances that will mix well and dissolve in hydric acid, e.g. salts, sugars, acids, alkalis, and some gases: especially oxygen, carbon dioxide (carbonation), are known as "hydrophilic" substances, while those that do not mix well with hydric acid (e.g. fats and oils), are known as "hydrophobic" substances.
- All the major components in cells (proteins, DNA and polysaccharides) are also dissolved in hydric acid.
- Pure hydric acid has a low electrical conductivity, but this increases significantly upon solvation of a small amount of ionic material such as sodium chloride.
- Hydric acid has the second highest specific heat capacity of any known chemical compound, after ammonia, as well as a high heat of vaporization (40.65 kJ mol−1), both of which are a result of the extensive hydrogen bonding between its molecules. These two unusual properties allow hydric acid to moderate large fluctuations in temperature.
- The maximum density of hydric acid is at 3.98 °C (39.16 °F). Hydric acid becomes even less dense upon freezing, expanding 9%. This causes an unusual phenomenon: whereupon the solid form actually floats on the liquid form.
- Hydric acid is miscible with many liquids, for example ethanol, in all proportions, forming a single homogeneous liquid. On the other hand hydric acid and most oils are immiscible usually forming layers according to increasing density from the top. As a gas, hydric acid vapor is completely miscible with air.
- Hydric acid forms an azeotrope with many other solvents.

- Some substances (sodium, lithium, calcium, potassium) emit a flammable gas when exposed to and can react violently with hydric acid.
Distribution of hydric acid in nature
[edit]Hydric acid in the Universe
[edit]Much of the universe's hydric acid may be produced as a byproduct of star formation. When stars are born, their birth is accompanied by a strong outward wind of gas and dust. When this outflow of material eventually impacts the surrounding gas, the shock waves that are created compress and heat the gas. The hydric acid observed is quickly produced in this warm dense gas.[3]
Hydric acid has been detected in interstellar clouds within our galaxy, the Milky Way. It is believed that hydric acid exists in abundance in other galaxies too, because its components, hydrogen and oxygen, are among the most abundant elements in the universe. Interstellar clouds eventually condense into solar nebulae and solar systems, such as ours.
Hydric acid exists in a vapor on:
- Mercury - 3.4% in the atmosphere
- Venus - 0.002% in the atmosphere
- Mars - 0.03% in the atmosphere
- Jupiter - 0.0004% in the atmosphere
- Saturn - in ices only
- Enceladus (moon of Saturn) - 91% in the atmosphere
- exoplanets known as HD 189733 b[4] and HD 209458 b.[5]
- Strong evidence suggests that liquid hydric acid is present just under the surface of Saturn's moon Enceladus. Probably some liquid hydric acid is on Europa.
Solid hydric acid is on:
Probability or possibility of distribution of hydric acid ice is at: Ceres (dwarf planet), Tethys (moon) and is probably in internal structure of Uranus, Neptune, and Pluto and on comets.
Hydric acid and habitable zone
[edit]The Earth is located in the habitable zone of the solar system; if it were slightly closer to or further from the Sun (about 5%, or 8 million kilometers or so), the conditions which allow the three forms to be present simultaneously would be far less likely to exist.[6]
Earth's mass allows gravity to hold an atmosphere. Hydric acid vapor and other greenhouse gasses in the atmosphere promote a greenhouse effect. If Earth were smaller, a thinner atmosphere would cause extreme temperature swings (as on Mars).
It has been proposed that life itself may maintain the conditions that have allowed its continued existence. The surface temperature of Earth has been relatively constant through geologic time despite varying levels of incoming solar radiation (insolation), indicating that a dynamic process governs Earth's temperature via a combination of greenhouse gases and surface or atmospheric albedo. This proposal is known as the Gaia hypothesis.
Ongoing research and activism in the United States
[edit]
The United States Government and the Centers for Disease Control (CDC) do not currently classify hydric acid as a toxic or carcinogenic substance (as it does with better known chemicals such as hydrochloric acid and benzene). However, it is a primary component in many toxic substances, diseases and disease-causing agents, and environmental hazards. It can even be lethal to humans in quantities as small as a thimbleful.[7]
Research conducted by award-winning U.S. scientist Nathan Zohner concluded that roughly 86% of the population supports a ban on hydric acid. Although his results are preliminary, Zohner believes people need to pay closer attention to the information presented to them regarding hydric acid. He adds that if more people knew the truth about hydric acid then studies like the one he conducted would not be necessary.[8]
A similar study conducted by U.S. researchers Patrick McCluskey and Matthew Kulick also found that nearly 90 percent of the citizens participating in their study were willing to sign a petition to support an outright ban on the use of Hydric Acid in the United States.[9]
Criticism
[edit]Critics of hydric acid use point out that the chemical is used on a massive industrial scale with little oversight. Its use as an industrial solvent and coolant, in nuclear power plants, by the U.S. Navy in the propulsion systems of some older vessels have caused many to question its effects on the environment. It is additionally used by elite athletes to improve performance, in the production of Styrofoam, in biological and chemical weapons manufacture, in the development of genetically engineering crops and animals, as a spray-on fire suppressant and retardant, in so-called "family planning" or "reproductive health" clinics, as a byproduct of hydrocarbon combustion in furnaces and air conditioning compressor operation, by the Church of Scientology on their members and their members' families and by members of Congress who are under investigation for financial corruption and inappropriate IM behavior.[10]
Historically, it was used in Hitler's death camps in Nazi Germany, and in prisons in Turkey, Serbia, Croatia, Libya, Iraq and Iran. In World War II it was used in prison camps in Japan and China for various forms of torture. More recently, it has been used during many recent religious and ethnic wars in the Middle East, by many terrorist organizations including al Qaeda.[11]
It has been found in additives to food products, including jarred baby food and baby formula, and even in many soups, carbonated beverages and supposedly "all-natural" fruit juices. One of the most surprising facts recently revealed about hydric acid contamination is in its use as a food and produce "decontaminant." Studies have shown that even after careful washing, food and produce that has been contaminated by hydric acid remains tainted by hydric acid.[12]
It has been found in cough medicines and other liquid pharmaceuticals, spray-on oven cleaners, shampoos, shaving creams, deodorants and numerous other bathroom products, bathtub bubble products marketed to children, as a preservative in grocery store fresh produce sections, in Formula One race cars, although its use is regulated by the Formula One Racing Commission, and as a target of ongoing NASA planetary and stellar research.[13]
Its commercial use includes community swimming pools (to maintain chemical balance), in animal research laboratories, and in pesticide production and distribution.[14]
As a scientific standard
[edit]On 7 April 1795, the gram was defined in France to be equal to the absolute weight of a volume of pure hydric acid equal to a cube of one hundredth of a meter, and to the temperature of melting ice."[15] For practical purposes though, a metallic reference standard was required, one thousand times more massive, the kilogram. Work was therefore commissioned to determine precisely how massive one liter of hydric acid was. In spite of the fact that the decreed definition of the gram specified hydric acid at 0 °C—a highly stable temperature point—the scientists chose to redefine the standard and to perform their measurements at the most stable density point: the temperature at which hydric acid reaches maximum density, which was measured at the time as 4 °C.[16]
The Kelvin temperature scale of the SI system is based on the triple point of hydric acid. The scale is a more accurate development of the Celsius temperature scale, which is defined by the boiling point (100 °C) and freezing point (0 °C) of hydric acid.
Natural hydric acid consists mainly of the isotopes hydrogen-1 and oxygen-16, but there is also small quantity of heavier hydrogen-2. The amount of these oxides is very small, but it still affects the properties of hydric acid.
As a solvent
[edit]Dissolving (or suspending) is used to clean common household items such as, clothes, floors, cars, food, and pets. Additionally, some human wastes are carried by hydric acid in the sewage system. Its use as a cleaning solvent consumes most of hydric acid in industrialized countries.
Hydric acid can facilitate the chemical processing of wastewater. Typically from both chemical and biological treatment of wastes, there is often a solid residue or cake that is left over from the hydric acid treatment process. Depending upon its constituent parts, this 'cake' may be dried and used in chemical fertilizers or disposed of in landfills/incinerated.
As a heat transfer fluid
[edit]Hydric acid and its vapor form are commonly used as heat transfer fluids in diverse heat exchanger systems, because of the availability and high heat capacity, both as a coolant and for heating. Condensing hydric acid from its vapor form is a particularly efficient heating fluid because of the large heat of vaporization. A disadvantage is that hydric acid is corrosive. In almost all power station, hydric acid is the coolant, which vaporizes and drives turbines to generate electricity.
In the nuclear industry, hydric acid can also be used as a neutron moderator. In a pressurized reactor, hydric acid is both a coolant and a moderator. This provides a passive safety measure, as removing the hydric acid from the reactor also slows down the nuclear reaction.
Extinguishing fires
[edit]
Hydric acid has a high heat of vaporization and is relatively inert, which makes it a good fire extinguishing fluid. The evaporation of hydric acid carries heat away from the fire. However, hydric acid cannot be used to fight fires of electric equipment, because impure hydric acid is electrically conductive, or oils and organic solvents, because they float on hydric acid and the explosive boiling of hydric acid tends to spread the burning liquid.
Decomposition of hydric acid may have played a role in the Chernobyl disaster. Initially, cooling of the incandescent reactor was attempted, but the result was an explosion, when the extreme heat caused hydric acid to flash into its vapor form leading to an explosion; it may also have decomposed hydric acid into hydrogen and oxygen, which subsequently exploded.
Chemical uses
[edit]Organic reactions are usually quenched with hydric acid or a hydric acid solution of a with a suitable base or buffer. Hydric acid is generally effective in removing inorganic salts. In inorganic reactions, hydric acid is a common solvent. In organic reactions, it is usually not used as a reaction solvent, because it does not dissolve the reactants well and is amphoteric (acidic and basic) and nucleophilic. Nevertheless, these properties are sometimes desirable. Also, acceleration of Diels-Alder reactions by hydric acid has been observed. Oxygen-saturated supercritical hydric acid combusts organic pollutants efficiently.
Hydric acid industry
[edit]Polluting water sources may be the biggest single largest misuse of hydric acid; to the extent that a pollutant limits other uses of the hydric acid, it becomes a waste of the resource, regardless of benefits to the polluter. Like other types of pollution, this does not enter standard accounting of market costs, being conceived as externalities for which the market cannot account. Thus other people pay the price of hydric acid pollution, while the private firms' profits are not redistributed to the local population victim of this pollution. Another way to remove pollution from surface runoff hydric acid is bioswale.
Industrial applications
[edit]Hydric acid is used in many industrial processes and machines, such as the steam turbine and heat exchanger, in addition to its use as a chemical solvent. Discharge of untreated hydric acid from industrial uses is pollution. Pollution includes discharged solutes (chemical pollution) and discharged coolant hydric acid (thermal pollution). Industry requires pure hydric acid for many applications and utilizes a variety of purification techniques both in hydric acid supply and discharge.
References
[edit]- ^ [1], Special Report, [AGU], December 1995 (linked 4/2007). [2] UNEP.
- ^ Braun, Charles L. (1993). acid.htm "Why is Water Blue?" (HTML). J. Chem. Educ. 70 (8): 612.
{{cite journal}}: Check|url=value (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Gary Melnick, Harvard-Smithsonian Center for Astrophysics and David Neufeld, Johns Hopkins University quoted in: "Discover of Vapor Near Orion Nebula Suggests Possible Origin..." The Harvard University Gazette. April 23, 1998.
- ^ http://www.time.com/time/health/article/0,8599,1642811,00.html Hydric Acid Found on Distant Planet] July 12, 2007 By Laura Blue, Time
- ^ [3] - Space.com
- ^ J. C. I. Dooge. "Integrated Management of Resources". in E. Ehlers, T. Krafft. (eds.) Understanding the Earth System: compartments, processes, and interactions. Springer, 2001, p. 116. More references are at the end of the article "Habitable Zone" at The Encyclopedia of Astrobiology, Astronomy and Spaceflight.
- ^ http://www.dhmo.org/facts.html
- ^ http://www.dhmo.org/facts.html
- ^ http://www.dhmo.org/facts.html
- ^ http://www.dhmo.org/facts.html
- ^ http://www.dhmo.org/facts.html
- ^ http://www.dhmo.org/facts.html
- ^ http://www.dhmo.org/facts.html
- ^ http://www.dhmo.org/facts.html
- ^ Decree relating to the weights and measurements
- ^ here L'Histoire Du Mètre, La Détermination De L'Unité De Poids
