User:CasRTP/Rapid Transfer Port

A Rapid Transfer Port (RTP for short, also alpha-beta port system) is a transfer system that allows material to be rapidly transferred into and out of an isolator without contamination. The alpha port is integrated into the isolator, the mobile unit of the transfer system, the beta port (a stainless steel, plastic or aluminum container) can be docked.

It is possible to open the door of the alpha port from the inside via glove intervention and thus to introduce material into the beta container. Undocking of the beta container can only be done when the door of the alpha port is closed. The hermetic seal is not broken during the process.[1]
History
[edit]The first Rapid Transfer Port (RTP) was developed in the 1960s for the nuclear industry to protect personnel as well as the environment from hazardous substances when working with radioactive material. In the 1970s, RTP systems were first used in film isolators in the pharmaceutical industry.[2]
Rapid Transfer Ports have since been used to protect operators and the environment from hazardous substances or to protect pharmaceutical products from germs and particles emitted by people and the environment.
In order to meet the highly individualized requirements of the pharmaceutical industry, the assembly was outsourced in the 2000s. This was now no longer produced exclusively by the equipment manufacturers themselves. As an independent assembly, the transfer systems are very well suited for suppliers to further develop and implement innovations. The high accuracy of fit, ease of cleaning and low weight are characteristics that distinguish the Rapid Transfer Port and had not been achieved up to this point.
Since the transfer of materials and packaging represents a critical process step in containment technology, many efforts have been made in the industry in recent years to improve transfer systems or develop new ones.
Types
[edit]
Rapid Transfer Ports are used for contamination-free material transfer into and out of barrier systems such as RABS or isolators. They are considered the safest method for bidirectional transfer in aseptic or toxic work areas without restriction or violation of sterility. The basic design of RTP systems is often the same regardless of the manufacturer. Major differences can only be found in the degree of customization and in additional applications, such as whether solid or liquid substances are introduced.
Alpha port
[edit]RTP systems consist of two main components with double door system.[2]
The alpha flange with door is permanently integrated into the isolator wall or embedded into the floor of the containment. The alpha flange consists of a stainless steel flange and a door made mainly of plastic, which is attached to a hinge. Both parts are closed with a latch and fitted with a seal. In addition, the alpha flange is equipped with a mechanical safety mechanism that prevents the door from opening in the absence of a container.
The following systems are used for material transfer and are mainly standard sizes in the industry:
| RTP Type | Flange Ø | Working Ø | Installation diameter |
| 105 | 225 mm | 85 mm | 156 mm |
| 190 | 315 mm | 170 mm | 268 mm |
| 270 | 400 mm | 250 mm | 348 mm |
| 350 | 475 mm | 330 mm | 428 mm |
| 460 | 590 mm | 440 mm | 537 mm |
Beta port
[edit]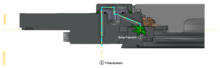
The second main component is the beta assembly, consisting of a canister with the beta flange and the beta door, which is sealed to the flange with a gasket. A 60° rotation docks the beta transfer container to the alpha port.
Once the alpha and beta components are connected, they form a closed unit. The leak tightness is ensured by the lip seals of the newly created unit, which can be opened without interrupting the sterility containment.

When the alpha and beta ports are connected by bayonet catches, the interlock is released, and the door can be opened inside the isolator. This allows rapid transfer of material between the container and containment without compromising cleanroom integrity.[3]
A safety system prevents inappropriate use of the transfer lock and ensures a high level of personal and product protection. The door of the alpha port can only be opened when the beta port is correctly docked.

Stainless steel beta containers can be sterilized in an autoclave with a sterile filter connected. The air is replaced by saturated steam, cooled and then extracted from the container as condensate. Containers made of PE cannot be autoclaved and are usually sterilized with gas or by gamma irradiation.

Drawer systems installed in the beta container allow the container to be loaded and unloaded safely. Instruments and components such as Petri dishes, vials or stoppers can thus be transferred as required for the process.
Filter system
[edit]During undocking, a vacuum forms between the door of the alpha port and the beta equipment as the two seals are pulled apart. A very small amount of air is required to release this vacuum and allow undocking of the beta section. This air is supplied through a filter system.
Requirements
[edit]Rapid Transfer Ports and other safe transfer systems are needed to insert components into the isolator in a sterile manner. The transfer ports are used in the development and production of drugs and vaccines. They should therefore
- meet the high standards of the pharmaceutical industry
- not interrupt the sterile chain during aseptic transfer
- ensure a high level of personal and product protection
- ensure a high level of process reliability
- follow the aspect of hygenic-design
- enable easy cleaning.
The RTP was developed for aseptic transfer. To maintain cleanroom status, the tightness of isolators and RABS systems is part of the GMP requirements. Transfer systems are critical points in terms of tightness and must therefore withstand tightness tests in the test procedure, in addition to other critical parameters.
Siehe auch
[edit]Literatur
[edit]- Müller (et al.) (Hrsg.): Studie zur Evaluierung der Dichtigkeit von Alpha-/Beta-Transfersystemen der castus GmbH & Co. KG anhand von partikulären und mikrobiellen Dichtigkeitsprüfungen, durchgeführt im Juli 2012 am Steinbeistransferzentrum
- Lothar Gail, Hans-Peter Hortig (2002), Reinraumtechnik (in German) (1 ed.), Berlin/Heidelberg: Springer-Verlag, doi:10.1007/978-3-662-09734-2, ISBN 978-3-662-09734-2
- Hans-Jürgen Bässler, Frank Lehmann: Containment Technology, Springer-Verlag Berlin Heidelberg, 1. Edition, 2013, ISBN 978-3-642-39292-4.
Einzelnachweise
[edit]- ^ Prof. Dr. I. Müller, I. ; et al. (2012), Evaluierung der Dichtigkeit von Alpha-/Beta-Transfersystemen der castus GmbH & Co. KG anhand von partikulären und mikrobiellen Dichtigkeitsprüfungen. (in German), p. 5
{{citation}}: Explicit use of et al. in:|surname1=(help) - ^ a b Lothar Gail, Hans-Peter Hortig (2002), Reinraumtechnik (in German) (1 ed.), Berlin/Heidelberg: Springer-Verlag, p. 240, doi:10.1007/978-3-662-09734-2, ISBN 978-3-662-09734-2
- ^ Bässler, Hans-Jürgen; Lehmann, Frank (2013). Containment Technology. Berlin Heidelberg: Springer-Verlag. p. 90. ISBN 978-3-642-39291-7.
Category:Biotechnology Category:Life sciences industry Category:Engineering Category:Pharmaceutical industry
