User:DePiep/chemdata/thermo
Appearance
< User:DePiep | chemdata
| Phase behavior | |
|---|---|
| Triple point | 237.2 K (–35.9 °C), ? Pa |
| Critical point | 561.6 K (288.5 °C), 5380 kPa |
| Std enthalpy change of fusion, ΔfusH |
8.8366 kJ/mol at –35.9 °C |
| Std entropy change of fusion, ΔfusS |
37.25 J/(mol·K) at –35.9 °C |
| Std enthalpy change of vaporization, ΔvapH |
33.91 kJ/mol at 20 °C |
| Std entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
–169.7 kJ/mol |
| Standard molar entropy, S |
208.53 J/(mol K) |
| Enthalpy of combustion, ΔcH |
–1236.4 kJ/mol |
| Heat capacity, cp | 129.0 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
–125.4 kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | 77.5 J/(mol K) at 25 °C |
| Phase behavior | |
| Triple point | 133.39 K (-139.76 °C), ? Pa |
| Critical point | 504 K (230 °C), 3.158 MPa |
| Std enthalpy change of fusion, ΔfusH |
? kJ/mol |
| Std entropy change of fusion, ΔfusS |
? J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
? kJ/mol |
| Std entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
-74.2 kJ/mol |
| Standard molar entropy, S |
295.2 J/(mol K) |
| Heat capacity, cp | 183.3 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
-43.5 kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Phase behavior | |
| Triple point | 148.75 K (–124.4 °C), ? Pa |
| Critical point | 536.9 K (263.8 °C), 5200 kPa |
| Std enthalpy change of fusion, ΔfusH |
5.37 kJ/mol |
| Std entropy change of fusion, ΔfusS |
36 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
47.5 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
126.6 J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
112.7 J/(mol K) |
| Heat capacity, cp | 106.3 J/(mol K) at –124 °C |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
–303.0 kJ/mol |
| Standard molar entropy, S |
192.8 J/(mol K) |
| Enthalpy of combustion, ΔcH |
–2021 kJ/mol |
| Heat capacity, cp | 144.4 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
–255 kJ/mol |
| Standard molar entropy, S |
322.49 J/(mol K) |
| Heat capacity, cp | 85.56 J/(mol K) at 25° |
| van der Waals' constants[1] | a = 1512 L2 kPa/mol2 b = 0.1029 liter per mole |
| Phase behavior | |
| Triple point | ? K (? °C), ? Pa |
| Critical point[2] | 466 K (183 °C), 5570 kPa |
| Std enthalpy change of fusion, ΔfusH |
2.310 kJ/mol |
| Std entropy change of fusion, ΔfusS |
15.43 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
26.12 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
–196.4 kJ/mol |
| Standard molar entropy, S |
117.3 J/(mol K) |
| Enthalpy of combustion, ΔcH |
–1167 kJ/mol |
| Heat capacity, cp | 96.21 J/(mol K) at 0 °C 89.05 J/(mol K) at 25 °C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
–170.7 kJ/mol |
| Standard molar entropy, S |
250.3 J/(mol K) |
| Heat capacity, cp | 55.32 J/(mol K) at 25 °C |
| Phase behavior | |
| Triple point | 289.8 K (16.7 °C), ? Pa |
| Critical point | 593 K (320 °C), 57.8 bar |
| Eutectic point with water | –26.7 °C |
| Std enthalpy change of fusionΔfusH |
+11.7 kJ/mol |
| Std entropy change of fusionΔfusS |
40.5 J/(mol·K) |
| Std enthalpy change of vaporizationΔvapH |
+23.7 kJ/mol |
| Std entropy change of vaporizationΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation ΔfH |
? kJ/mol |
| Standard molar entropy S |
? J/(mol K) |
| Heat capacity cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation ΔfH |
−483.5 kJ/mol |
| Standard molar entropy S |
158.0 J/(mol K) |
| Enthalpy of combustion, ΔcH |
–876.1 kJ/mol |
| Heat capacity cp | 123.1 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation ΔfH |
–438.1 kJ/mol |
| Standard molar entropy S |
282.84 J/(mol K) |
| Heat capacity cp | 63.4 J/(mol K) |
| van der Waals' constants[3] | a = 1782.3 L2 kPa/mol2 b = 0.1068 liter per mole |
| Phase behavior | |
| Triple point | 178.5 K (−94.3 °C), ? Pa |
| Critical point | 508 K (235 °C), 48 bar |
| Std enthalpy change of fusion, ΔfusH |
+5.7 kJ/mol |
| Std entropy change of fusion, ΔfusS |
+32.3 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
+31.3 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
95 J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp [4] | 96 J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
−249.4 kJ/mol |
| Standard molar entropy, S |
200.4 J/(mol K) |
| Enthalpy of combustion, ΔcH |
–1785.7 kJ/mol |
| Heat capacity, cp | 125.5 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
−218.5 kJ/mol |
| Standard molar entropy, S |
295.35 J/(mol K) |
| Heat capacity, cp | 75 J/(mol K) |
| van der Waals' constants[3] | a = 1409.4 L2 kPa/mol2 b = 0.0994 liter per mole |
| Phase behavior | |
| Triple point[5] | 229.32 K (−43.83 °C), 167 Pa |
| Critical point | 545 K (272 °C), 4.87 MPa |
| Std enthalpy change of fusion, ΔfusH |
8.167 kJ/mol (crystal I → liq) |
| Std entropy change of fusion, ΔfusS |
35.61 J/(mol·K) (crystal I → liq) |
| Std enthalpy change of vaporization, ΔvapH |
33.225 kJ/mol at 25 °C 29.75 at 81.6 °C (BP) |
| Std entropy change of vaporization, ΔvapS |
111.44 J/(mol·K) at 25 °C |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol at 25 °C |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | 92.36 J/(mol K)at 298.15 K |
| Std enthalpy change of state transition, ΔtrsH |
0.8979 kJ/mol at −56.2 °C (crystal II → crystal I) |
| Std entropy change of state transition, ΔtrsS |
4.14 J/(mol·K) at −56.2 °C (crystal II → crystal I) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
−40.56 kJ/mol |
| Standard molar entropy, S |
149.62 J/(mol K) |
| Enthalpy of combustion, ΔcH |
−1256.33 kJ/mol |
| Heat capacity, cp | 91.7 J/(mol K) at 25 °C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
−74.04 kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Phase behavior | |
| Triple point | ? K (? °C), ? Pa |
| Critical point | ? K (? °C), ? Pa |
| Std enthalpy change of fusion, ΔfusH |
? kJ/mol |
| Std entropy change of fusion, ΔfusS |
? J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
? kJ/mol |
| Std entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
-1675.7 kJ/mol |
| Standard molar entropy, S |
50.92 J/(mol K) |
| Heat capacity, cp | 89.7248 J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
-1620.57 kJ/mol |
| Standard molar entropy, S |
67.24 J/(mol K) |
| Heat capacity, cp | 192.5 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Phase behavior | |
| Triple point | ? K (? °C), ? Pa |
| Critical point | ? K (? °C), ? Pa |
| Std enthalpy change of fusion, ΔfusH |
? kJ/mol |
| Std entropy change of fusion, ΔfusS |
? J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
? kJ/mol |
| Std entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
-3440 kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
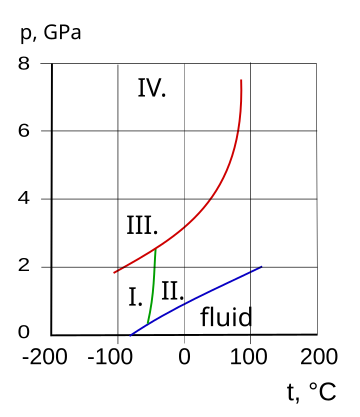 I. cubic, II. hcp, III. fcc, IV. orthorhombic |
Phase behavior |
| Triple point | 195.4 K (−77.75 °C), 6.060 kPa |
| Critical point | 405.5 K (132.3 °C), 11.300 MPa |
| Std enthalpy change of fusion, ΔfusH |
+5.653 kJ/mol |
| Std entropy change of fusion, ΔfusS |
+28.93 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
+23.35 kJ/mol at BP of −33.4 °C |
| Std entropy change of vaporization, ΔvapS |
+97.41 J/(mol·K) at BP of −33.4 °C |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | 80.80 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
−45.92 kJ/mol |
| Std Gibbs free energy change of formation, ΔfG |
−16.6 kJ/mol |
| Standard molar entropy, S |
192.77 J/(mol K) |
| Heat capacity, cp | 35.06 J/(mol K) |
| Heat capacity ratio, γ at 15 °C |
1.310 |
| van der Waals' constants | a = 422.5 l2 kPa/mol2 b = 0.03707 l/mol |
| Phase behavior | |
| Triple point | 267.13 K (–6.02 °C), ? Pa |
| Critical point | 698.8 K (425.7 °C), 4890 kPa |
| Std enthalpy change of fusion, ΔfusH |
10.54 kJ/mol |
| Std entropy change of fusion, ΔfusS |
39.57 J/(mol·K) at –6.3 °C |
| Std enthalpy change of vaporization, ΔvapH |
55.83 kJ/mol at 25 °C 42.44 kJ/mol at 184.1 °C |
| Std entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
31 kJ/mol |
| Standard molar entropy, S |
191. J/(mol K) |
| Enthalpy of combustion, ΔcH |
–3393 kJ/mol |
| Heat capacity, cp | 193.7 J/(mol K) at 25 °C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
87 kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity,[2] cp | 148.7 J/(mol K) at 25 °C |
| van der Waals' constants[3] | a = 2685 L2 kPa/mol2 b = 0.1369 liter per mole |
- ^ CRC Handbook of Chemistry and Physics 47th ed, p D-104
- ^ a b "Pure Component Properties". Chemical Engineering Research Information Center. Archived from the original on 3 June 2007. Retrieved 27 May 2007. Cite error: The named reference "cheric_p" was defined multiple times with different content (see the help page).
- ^ a b c Lange's Handbook of Chemistry 10th ed, pp 1522-1524 Cite error: The named reference "lange1522" was defined multiple times with different content (see the help page).
- ^ Maass, O.; Walbauer, L.J., The specific heats and latent heats of fusion of ice and of several organic compounds, J. Am. Chem. Soc., 1925, 47, 1-9.
- ^ Vapor Pressures of Acetonitrile Determined by Comparative Ebulliometry, Michael B. Ewing* and Jesus C. Sanchez Ochoa, Journal of Chemical & Engineering Data 2004 49 (3), 486-491
