User:Double sharp/Unbitrium
| Unbitrium | ||||||
|---|---|---|---|---|---|---|
| Pronunciation | /ˌuːnbaɪˈtraɪəm/ | |||||
| Alternative names | element 123, eka-protactinium | |||||
| Unbitrium in the periodic table | ||||||
| ||||||
| Group | g-block groups (no number) | |||||
| Period | period 8 (theoretical, extended table) | |||||
| Block | g-block | |||||
| Electron configuration | predictions vary, see text | |||||
| Physical properties | ||||||
| unknown | ||||||
| Phase at STP | unknown | |||||
| Atomic properties | ||||||
| Oxidation states | common: (none) | |||||
| Other properties | ||||||
| CAS Number | 54500-71-9 | |||||
| History | ||||||
| Naming | IUPAC systematic element name | |||||
| Isotopes of unbitrium | ||||||
| Template:infobox unbitrium isotopes does not exist | ||||||
Unbitrium, also known as element 123 or eka-protactinium, is the hypothetical chemical element in the periodic table with the placeholder symbol of Ubt and atomic number 123. Unbitrium and Ubt are the temporary systematic IUPAC name and symbol respectively, until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table of the elements, it is expected to be the third superactinide and the fifth element of the 8th period.
Unbitrium has attracted some interest, for it may lie within an island of stability characterized by relatively long half-lives, especially 307Ubt with a magic number of neutrons (184). The synthesis of unbitrium has not yet been attempted, and it is expected to be far more challenging than the synthesis of the lightest undiscovered element, ununennium.
Chemically, unbitrium is expected to show some resemblance to praseodymium and protactinium. However, relativistic effects may cause some of its properties to differ; for example, it is expected to have a ground state electron configuration of [Og] 6f1 7d1 8s2 8p1, despite its predicted position in the g-block superactinide series.
History
[edit]Every element from mendelevium onward was produced in fusion-evaporation reactions, culminating in the discovery of the heaviest known element oganesson in 2002[1][2] and most recently tennessine in 2010.[3] These reactions approached the limit of current technology; for example, the synthesis of tennessine required 22 milligrams of 249Bk and an intense 48Ca beam for six months. The intensity of beams in superheavy element research cannot exceed 1012 projectiles per second without damaging the target and detector, and producing larger quantities of increasingly rare and unstable actinide targets is impractical.[4] Consequently, future experiments must be done at facilities such as the under-construction superheavy element factory (SHE-factory) at the Joint Institute for Nuclear Research (JINR) or RIKEN, which will allow experiments to run for longer stretches of time with increased detection capabilities and enable otherwise inaccessible reactions.[5] Even so, it will likely be a great challenge to continue past elements 120 or perhaps 121 given short predicted half-lives and low predicted cross sections.[6][7]
No attempts to synthesize unbitrium have yet been made,[8] and there will most likely be none in the near future; the discoveries of elements 119 and 120 as well as new isotopes of known superheavy elements closer to the predicted island of stability are currently more feasible and of greater interest.[9] Although the exact limit of stability for half-lives over one microsecond is unknown, as it depends on the calculation model used,[10] the possible stabilizing effect at N = 184 for the compound nucleus 307Ubt may make some reactions more feasible. It may be possible to generate this compound nucleus from the reaction between a 58Fe beam and a 249Bk target, from which the isotope 304Ubt may be formed in the 3n channel and decay via 300Ubu, 296Uue, and 292Ts (producible in cross bombardment with lighter projectiles)[7] before following the well-characterized decay chain of 288Mc. The relative symmetry of this reaction compared to 48Ca-induced reactions leading to elements 112 through 118 may pose a challenge, though, as the cross section of such reactions is strongly dependent on their asymmetry.[9] One possible solution to this problem may be to use a 254Es target, which is currently being considered for elements 119 (with 48Ca projectiles) and 121 (with 50Ti projectiles), though only micrograms of einsteinium are currently available in contrast to milligrams of berkelium.[4] It may also be possible that fusion-evaporation reactions may not work at all, and new methods of synthesis such as multinucleon transfer or inverse quasi-fission reactions may be required, though the production of lighter superheavy nuclei with 102 < Z < 106 is more favorable, especially if shell effects are weaker than predicted or otherwise nonexistent. Should the shell closure at N = 184 be stronger, there may be a real chance to use these methods to produce unbitrium isotopes.[6]
Naming
[edit]Using Mendeleev's nomenclature for unnamed and undiscovered elements, unbitrium should be known as eka-protactinium. Using the 1979 IUPAC recommendations, the element should be temporarily called unbitrium (symbol Ubt) until it is discovered, the discovery is confirmed, and a permanent name chosen.[11] Although widely used in the chemical community on all levels, from chemistry classrooms to advanced textbooks, the recommendations are mostly ignored among scientists who work theoretically or experimentally on superheavy elements, who call it "element 123", with the symbol E123, (123), or 123.[12]
Predicted properties
[edit]Nuclear stability and isotopes
[edit]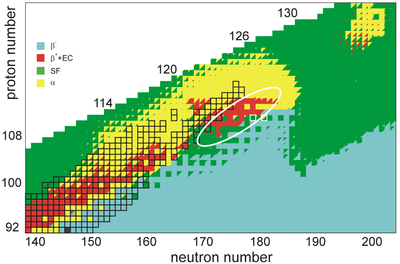
The stability of nuclei decreases greatly with the increase in atomic number after plutonium, the heaviest primordial element, so that all isotopes with an atomic number above 101 decay radioactively with a half-life under a day, with an exception of dubnium-268. No elements with atomic numbers above 82 (after lead) have stable isotopes.[13] Nevertheless, because of reasons not very well understood yet, there is a slight increased nuclear stability around atomic numbers 110–114, which leads to the appearance of what is known in nuclear physics as the "island of stability". This concept, proposed by University of California professor Glenn Seaborg, explains why superheavy elements last longer than predicted.[14]
In this region of the periodic table, N = 184 and N = 228 have been suggested as closed neutron shells, and various atomic numbers have been proposed as closed proton shells, such as Z = 114, 120, 122, 124, and 126. The island of stability would be characterized by longer half-lives of nuclei located near these magic numbers, though the extent of stabilizing effects is uncertain due to predictions of weakening of the proton shell closures and possible loss of double magicity.[15] More recent research predicts the island of stability to instead be centered at beta-stable copernicium isotopes 291Cn and 293Cn,[9][10] which would place unbitrium well above the island and result in short half-lives regardless of shell effects. A quantum tunneling model predicts the alpha decay half-lives of unbitrium isotopes 287–323Ubt to not exceed one millisecond, except those of 316Ubt and 318–321Ubt, with the majority on the order of tens of microseconds, very close to the limit of detection.[16] The lightest isotopes as well as 309–311Ubt may be especially short lived, a consequence of the shell closure at N = 184. However, the isotopes 297–307Ubt may have half-lives just long enough for detection, and may be reachable using fusion-evaporation reactions, followed by alpha decay down to isotopes of known elements.[17] These predictions are consistent with other models, in which a narrow one-microsecond corridor as well as a region of increased stability around Z ~ 124 and N ~ 198 is predicted, though such results are strongly dependent on stability against spontaneous fission.[10] It is also predicted that the proton drip line will cross the region of reachable nuclei, rendering some isotopes of unbitrium possibly unbound and decaying by proton emission.[18] In addition to alpha decay, the heavier isotopes of unbitrium closer to the beta-stability line as well as the most neutron-deficient isotopes are expected to predominantly decay by with half-lives well below one microsecond and perhaps on the order of 10−12 s; this is a consequence of very low fission barriers in the "sea of instability" where shell effects are no longer influential.[10][18] The extent of this sea of instability is unknown; a region of increased stability around N = 228 is also predicted, though the extent of the shell effects as well as the possibility of beta decay may nevertheless lead to short half-lives.[18]
Chemical
[edit]Unbitrium is expected to be the third member of a superactinide series. It should have similarities to praseodymium and protactinium, as all three elements have five valence electrons over a noble gas core.[19] It is also predicted to be the third member of a new block of valence g-electron atoms, [20] although the 5g orbital is not expected to start filling until element 125. In the superactinide series, the Aufbau principle is expected to break down due to relativistic effects, and an overlap of the 5g, 6f, 7d, and 8p orbitals is expected, rendering predictions of chemical and atomic properties of these elements very difficult.[20] The ground state electron configuration of unbitrium is predicted to be [Og] 6f1 7d1 8s2 8p1,[21][19] in contrast to the expected [Og] 5g3 8s2 obtained via a simple extrapolation. It is also possible that unbitrium assumes the electron configuration [Og] 8s2 8p3; this was calculated to be very close in energy level to the first one originally predicted by Fricke in 1971.[19] These possibilities arise from relativistic effects, which are not significant in lighter elements but have been indicated in studies of the chemistry of copernicium and flerovium.
One predicted oxidation state of unbitrium is +5, which would exist in the halide UbtX5 (X = a halogen), analogous to the known +5 oxidation state in protactinium.[22] Like the other early superactinides, the binding energies of unbitrium's valence electrons are predicted to be small enough that all five should easily participate in chemical reactions.[23] Although the five-valence electron configuration is agreed upon, the presence of three open orbitals in unbitrium with similar energy levels could lead to some substantial differences in chemical properties from its lighter congeners.[19] The predicted electron configuration of the Ubt4+ ion is [Og] 6f1, unlike the [Og] 8s1 configuration of neutral ununennium, but like that for the Ubq5+ ion; from element 125 onwards, the [Og] 5g1 configuration is preferred.[22]
References
[edit]- ^ Oganessian, YT; et al. (2002). "Element 118: results from the first 249
Cf
+ 48
Ca
experiment". Communication of the Joint Institute for Nuclear Research. Archived from the original on 22 July 2011. - ^ "Livermore scientists team with Russia to discover element 118". Livermore press release. 3 December 2006. Retrieved 18 January 2008.
- ^ Oganessian, Yu. Ts.; Abdullin, F. Sh.; Bailey, P. D.; et al. (2010). "Synthesis of a New Element with Atomic Number Z = 117". Physical Review Letters. 104 (14): 142502-1–142502-4. Bibcode:2010PhRvL.104n2502O. doi:10.1103/PhysRevLett.104.142502. PMID 20481935.
- ^ a b Roberto, JB (2015). "Actinide Targets for Super-Heavy Element Research" (PDF). cyclotron.tamu.edu. Texas A & M University. Retrieved 30 October 2018.
- ^ Hagino, Kouichi; Hofmann, Sigurd; Miyatake, Hiroari; Nakahara, Hiromichi (2012). "平成23年度 研究業績レビュー(中間レビュー)の実施について" (PDF). www.riken.jp. RIKEN. Retrieved 5 May 2017.
- ^ a b Zagrebaev, V.; Itkis, M.; Karpov, A. (2015). Production of new neutron rich heavy and superheavy nuclei (PDF). SHE-2015. Texas A & M University. Retrieved 21 November 2018.
- ^ a b c Karpov, A; Zagrebaev, V; Greiner, W (2015). "Superheavy Nuclei: which regions of nuclear map are accessible in the nearest studies" (PDF). cyclotron.tamu.edu. Texas A & M University. Retrieved 30 October 2018.
- ^ Emsley, John (2011). Nature's Building Blocks: An A-Z Guide to the Elements (New ed.). New York, NY: Oxford University Press. p. 588. ISBN 978-0-19-960563-7.
- ^ a b c Zagrebaev, Valeriy; Karpov, Alexander; Greiner, Walter (2013). "Future of superheavy element research: Which nuclei could be synthesized within the next few years?" (PDF). Journal of Physics. 420: 012001. arXiv:1207.5700. doi:10.1088/1742-6596/420/1/012001.
- ^ a b c d Palenzuela, Y. M.; Ruiz, L. F.; Karpov, A.; Greiner, W. (2012). "Systematic Study of Decay Properties of Heaviest Elements" (PDF). Physics. 76 (11): 1165–1171. ISSN 1062-8738.
- ^ Chatt, J. (1979). "Recommendations for the naming of elements of atomic numbers greater than 100". Pure and Applied Chemistry. 51 (2): 381–384. doi:10.1351/pac197951020381.
- ^ Haire, Richard G. (2006). "Transactinides and the future elements". In Morss; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. p. 1724. ISBN 1-4020-3555-1.
{{cite book}}: CS1 maint: ref duplicates default (link) - ^ Marcillac, Pierre de; Noël Coron; Gérard Dambier; Jacques Leblanc; Jean-Pierre Moalic (April 2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature. 422 (6934): 876–878. Bibcode:2003Natur.422..876D. doi:10.1038/nature01541. PMID 12712201.
- ^ Considine, Glenn D.; Kulik, Peter H. (2002). Van Nostrand's scientific encyclopedia (9 ed.). Wiley-Interscience. ISBN 978-0-471-33230-5. OCLC 223349096.
- ^ Koura, H.; Chiba, S. (2013). "Single-Particle Levels of Spherical Nuclei in the Superheavy and Extremely Superheavy Mass Region". Journal of the Physical Society of Japan. 82: 014201. doi:10.7566/JPSJ.82.014201.
- ^ Chowdhury, R. P.; Samanta, C.; Basu, D.N. (2008). "Nuclear half-lives for α -radioactivity of elements with 100 ≤ Z ≤ 130". Atomic Data and Nuclear Data Tables. 94 (6): 781–806. arXiv:0802.4161. Bibcode:2008ADNDT..94..781C. doi:10.1016/j.adt.2008.01.003.
- ^ Santhosh, K. P.; Nithya, C. (28 December 2016). "Theoretical predictions on the decay properties of superheavy nuclei Z = 123 in the region 297 ≤ A ≤ 307". The European Physical Journal A. 52 (371). Bibcode:2016EPJA...52..371S. doi:10.1140/epja/i2016-16371-y.
- ^ a b c Koura, H. (2011). Decay modes and a limit of existence of nuclei in the superheavy mass region (PDF). 4th International Conference on the Chemistry and Physics of the Transactinide Elements. Retrieved 18 November 2018.
- ^ a b c d van der Schoor, K. (2016). Electronic structure of element 123 (PDF) (Thesis). Rijksuniversiteit Groningen.
- ^ a b Seaborg (c. 2006). "transuranium element (chemical element)". Encyclopædia Britannica. Retrieved 2010-03-16.
- ^ Hoffman, Darleane C.; Lee, Diana M.; Pershina, Valeria (2006). "Transactinides and the future elements". In Morss; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. ISBN 1-4020-3555-1.
- ^ a b Pyykkö, Pekka (2011). "A suggested periodic table up to Z≤ 172, based on Dirac–Fock calculations on atoms and ions". Physical Chemistry Chemical Physics. 13 (1): 161–8. Bibcode:2011PCCP...13..161P. doi:10.1039/c0cp01575j. PMID 20967377.
- ^ Fricke, B.; Greiner, W.; Waber, J. T. (1971). "The continuation of the periodic table up to Z = 172. The chemistry of superheavy elements". Theoretica Chimica Acta. 21 (3): 235–260. doi:10.1007/BF01172015.
