User:DownToBismuth/Photosensitizer
History[edit]
Photosensitizers have existed within natural systems for as long as chlorophyll and other light sensitive molecules have been a part of plant life, but studies of photosensitizers began as early as the 1900's, where scientists observed photosensitization in biological substrates and in the treatment of cancer. Mechanistic studies related to photosensitizers began with scientists analyzing the results of chemical reactions where photosensitizers photo-oxidized molecular oxygen into peroxide species. The results were understood by calculating quantum efficiencies and fluorescent yields at varying wavelengths of light and comparing these results with the yield of reactive oxygen species. However, it was not until the 1960's that the electron donating mechanism was confirmed through various spectroscopic methods including reaction-intermediate studies and luminescence studies.[1][2][3][4]
The term photosensitizer does not appear in scientific literature until the 1960's. Instead, scientists would refer to photosensitizers as sensitizers used in photo-oxidation or photo-oxygenation processes. Studies during this time period involving photosensitizers utilized organic photosensitizers, consisting of aromatic hydrocarbon molecules, which could facilitate synthetic chemistry reactions. However, by the 1970's and 1980's, photosensitizers gained attraction in the scientific community for their role within biologic processes and enzymatic processes.[1][5] Currently, photosensitizers are studied for their contributions to fields such as energy harvesting, photoredox catalysis in synthetic chemistry, and cancer treatment.[6][7]
Theory[edit]

Definition[edit]
Photosensitizers are molecules which absorb light (hν) and transfer the energy from the incident light into another nearby molecule. Upon absorbing photons of radiation from incident light, photosensitizers are able to promote a ground state electron into an excited singlet state. This electron in the excited singlet state then flips in its intrinsic spin state via Intersystem crossing to become an excited triplet state electron. Due to the excited electron’s spin flipping in the triplet state, the electron’s lifetime in the excited state is prolonged. Prolonged triplet states provided photosensitizer molecules with an increased probability of interacting with other molecules nearby. Photosensitizers experience varying levels of efficiency for intersystem crossing at different wavelengths of light based on the internal electronic structure of the molecule.[8][9]
Photosensitizers produce a physicochemical change in a neighboring molecule by either donating an electron to the substrate or by abstracting a hydrogen atom from the substrate. At the end of this process, the photosensitizer eventually returns to its ground state, where it remains chemically intact until the photosensitizer absorbs more light. This means that the photosensitizer remains unchanged before and after the energetic exchange, much like heterogeneous photocatalysis.[8][10]
Parameters[edit]
For a molecule to be considered a photosensitizer:
- The photosensitizer must impart a physicochemical change upon a substrate after absorbing incident light.
- Upon imparting a chemical change, the photosensitizer returns to its original chemical form
It is important to differentiate photosensitizers from other photochemical interactions including, but not limited to, photoinitiators, photocatalysts, photoacids and photopolymerization. Photosensitizers utilize light to enact a chemical change in a substrate; after the chemical change, the photosensitizer returns back to its initial state, remaining chemically unchanged from the process. Photoinitiators absorb light to become a reactive species, commonly a radical or an ion, where it then reacts with another chemical species. These photoinitiators are often completely chemically changed after their reaction. Photocatalysts accelerate chemical reactions which rely upon light. While some photosensitizers may act as photocatalysts, not all photocatalysts may act as photosensitizers. Photoacids (or photobases) are molecules which become more acidic (or basic) upon the absorption of light. Photoacids increase in acidity upon absorbing light and thermally reassociate back into their original form upon relaxing. Photoacid generators, upon absorbing light, undergo an irreversible change to become an acidic species. Photopolymerization can occur in two ways. Photopolymerization can occur directly wherein the monomers absorb the incident light and begin polymerizing, or it can occur through a photosensitizer-mediated process where the photosensitizer absorbs the light first before transferring energy into the monomer species.[2][11]
Type of Photosensitization Processes[edit]

There are two main pathways for photosensitized reactions.[8]
Type I[edit]
In Type I photosensitized reactions, the photosensitizer is excited by a light source into a triplet state. The excited, triplet state photosensitizer then reacts with a substrate molecule which is not molecular oxygen to both form a product and reform the photosensitizer. Type I photosensitized reactions result in the photosensitizer being quenched by a different chemical substrate than molecular oxygen.[8][12]
Type II[edit]

In Type II photosensitized reactions, the photosensitizer is excited by a light source into a triplet state. The excited photosensitizer then reacts with a ground state, triplet oxygen molecule. This excites the oxygen molecule into the singlet state, making it a reactive oxygen species. Upon excitation, the singlet oxygen molecule reacts with a substrate to form a product. Type II photosensitized reaction result in the photosensitizer being quenched by a ground state oxygen molecule which then goes on to react with a substrate to form a product.[8][13][14]
Composition of Photosensitizers[edit]
Photosensitizers can be placed into 3 generalized domains based on their molecular structure.

Organometallic[edit]
Organometallic photosensitizers contain a metal atom chelated to at least one organic ligand. These molecules attain their photosensitizing capacity from the electronic interaction between the metal and the ligand. Often these photosensitizers’ metal centers have highly filled d-orbitals to promote metal to ligand charge transfer from pi-electron accepting ligands. Popular electron-rich metal centers for these complexes include Iridium, Ruthenium, and Rhodium. This interaction between the metal center and the ligand leads to a large continuum of orbitals within both the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) which allows for excited electrons to switch multiplicities via intersystem crossing.[15]
While many organometallic photosensitizer compounds are made synthetically, there also exists naturally occurring, light-harvesting organometallic photosensitizers as well. Some relevant naturally occurring examples of organometallic photosensitizers include Chlorophyll A and Chlorophyll B.[15][16]
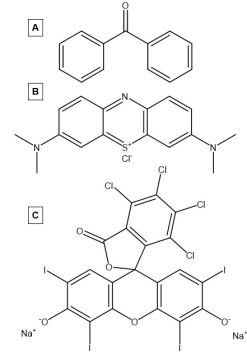
Organic[edit]
Organic photosensitizers are carbon-based molecules which are capable of photosensitizing. The earliest studied photosensitizers were aromatic hydrocarbons which absorbed light in the presence of oxygen to produce reactive oxygen species.[17] These organic photosensitizers are made up of highly conjugated systems which promote electron delocalization. Due to their high conjugation, these systems have a smaller gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) as well as a continuum of orbitals within the HOMO and LUMO. The smaller band gap and the continuum of orbitals in both the conduction band and the valence band allow for these materials to enter their triplet state more efficiently, making them better photosensitizers. Some notable organic photosensitizers which have been studied extensively include benzophenones, methylene blue, and rose Bengal.[18][19]
Nanomaterials[edit]
Quantum Dots[edit]
Colloidal quantum dots are nanoscale semiconductor materials with highly tunable optical and electronic properties. Quantum dots photosensitize via the same mechanism as organometallic photosensitizers and organic photosensitizers, but their nanoscale properties allow for greater control in distinctive aspects. Some key advantages to the use of quantum dots as photosensitizers includes their small, tunable band gap which allows for efficient transitions to the triplet state, and their insolubility in many solvents which allows for easy retrieval from a synthetic reaction mixture.[20]
Nanorods[edit]
Nanorods, similar in size to quantum dots, have tunable optical and electronic properties. Based on their size and material composition, it is possible to tune the maximum absorption peak for nanorods during their synthesis. This control has led to the creation of photosensitizing nanorods.[21]
Applications[edit]
Medicinal[edit]
Photodynamic therapy[edit]
Photodynamic therapy utilizes Type II photosensitizers to harvest light to degrade tumors or cancerous masses. This discovery was first observed back in 1907 by Hermann von Tappeiner when he utilized eosin to treat skin tumors.[4] The photodynamic process is predominantly a noninvasive technique wherein the photosensitizers are put inside a patient so that it may accumulate on the tumor or cancer. When the photosensitizer reaches the tumor or cancer, wavelength specific light is shined on the outside of the patient's affected area. This light (preferably near infrared frequency as this allows for the penetration of the skin without acute toxicity) excites the photosensitizer's electrons into the triplet state. Upon excitation, the photosensitizer begins transferring energy to neighboring ground state triplet oxygen to generate excited singlet oxygen. The resulting excited oxygen species then selectively degrades the tumor or cancerous mass.[22][23][24]

Energy Sources[edit]
Dye sensitized solar cells[edit]
In 1972, scientists discovered that chlorophyll could absorb sunlight and transfer energy into electrochemical cells.[26] This discovery eventually led to the use of photosensitizers as material which could harvest sunlight for solar cells, mainly through the use of photosensitizer dyes. Dye Sensitized Solar cells utilize these photosensitizer dyes to absorb photons from solar light and transfer energy rich electrons to the neighboring semiconductor material to generate electric energy output. These dyes act as dopants to semiconductor surfaces which allows for the transfer of light energy from the photosensitizer to electronic energy within the semiconductor. These photosensitizers are not limited to dyes. They may take the form of any photosensitizing structure, dependent on the semiconductor material to which they are attached.[12][6][27][28]
Hydrogen Generating catalysts[edit]
Via the absorption of light, photosensitizers can utilize triplet state transfer to reduce small molecules, such as water, to generate Hydrogen gas. As of right now, photosensitizers have generated hydrogen gas by splitting water molecules at a small, laboratory scale.[29][30]
Synthetic Chemistry[edit]
Photoredox Chemistry[edit]
In the early 20th century, chemists observed that various aromatic hydrocarbons in the presence of oxygen could absorb wavelength specific light to generate a peroxide species.[1] This discovery of oxygen's reduction by a photosensitizer led to chemists studying photosensitizers as photoredox catalysts for their roles in the catalysis of pericyclic reactions and other reduction and oxidation reactions. Photosensitizers in synthetic chemistry allow for the manipulation of electronic transitions within molecules through an externally applied light source. These photosensitizers used in redox chemistry may be organic, organometallic, or nanomaterials depending on the physical and spectral properties required for the reaction.[12][18]
References[edit]
- ^ a b c Gollnick, K. (1968), "Type II Photooxygenation Reactions in Solution", Advances in Photochemistry, John Wiley & Sons, Ltd, pp. 1–122, doi:10.1002/9780470133361.ch1, ISBN 978-0-470-13336-1, retrieved 2021-02-24
- ^ a b Turro, Nicholas J. (1978). Modern molecular photochemistry. Menlo Park, Calif.: Benjamin/Cummings Pub. Co. ISBN 0-8053-9353-6. OCLC 4417476.
- ^ Kavarnos, George J.; Turro, Nicholas J. (1986-04-01). "Photosensitization by reversible electron transfer: theories, experimental evidence, and examples". Chemical Reviews. 86 (2): 401–449. doi:10.1021/cr00072a005. ISSN 0009-2665.
- ^ a b Daniell, M. D.; Hill, J. S. (1991-05). "A HISTORY OF PHOTODYNAMIC THERAPY". ANZ Journal of Surgery. 61 (5): 340–348. doi:10.1111/j.1445-2197.1991.tb00230.x. ISSN 1445-1433.
{{cite journal}}: Check date values in:|date=(help) - ^ Julliard, M.; Chanon, Michel (1983-08-01). "Photoelectron-transfer catalysis: its connections with thermal and electrochemical analogs". Chemical Reviews. 83 (4): 425–506. doi:10.1021/cr00056a003. ISSN 0009-2665.
- ^ a b O'Regan, Brian; Grätzel, Michael (1991-10). "A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO 2 films". Nature. 353 (6346): 737–740. doi:10.1038/353737a0. ISSN 1476-4687.
{{cite journal}}: Check date values in:|date=(help) - ^ Daniell, M. D.; Hill, J. S. (1991-05). "A HISTORY OF PHOTODYNAMIC THERAPY". ANZ Journal of Surgery. 61 (5): 340–348. doi:10.1111/j.1445-2197.1991.tb00230.x. ISSN 1445-1433.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c d e f g Gómez Alvarez, Elena; Wortham, Henri; Strekowski, Rafal; Zetzsch, Cornelius; Gligorovski, Sasho (2012-02-21). "Atmospheric Photosensitized Heterogeneous and Multiphase Reactions: From Outdoors to Indoors". Environmental Science & Technology. 46 (4): 1955–1963. doi:10.1021/es2019675. ISSN 0013-936X.
- ^ Spin crossover in transition metal compounds. Gütlich, Philipp, 1934-, Goodwin, Harold A. Berlin: Springer. 2004-. ISBN 978-3-540-40394-4. OCLC 56798940.
{{cite book}}: Check date values in:|date=(help)CS1 maint: others (link) - ^ Zhang, Yu; Lee, Tia S.; Petersen, Jeffrey L.; Milsmann, Carsten (2018-05-09). "A Zirconium Photosensitizer with a Long-Lived Excited State: Mechanistic Insight into Photoinduced Single-Electron Transfer". Journal of the American Chemical Society. 140 (18): 5934–5947. doi:10.1021/jacs.8b00742. ISSN 0002-7863.
- ^ Allcock, H. R. (2003). Contemporary polymer chemistry. Lampe, Frederick Walter, 1927-, Mark, James E., 1934- (3rd ed. ed.). Upper Saddle River, N.J.: Pearson/Prentice Hall. ISBN 0-13-065056-0. OCLC 51096012.
{{cite book}}:|edition=has extra text (help) - ^ a b c Sang, Xiaojing; Li, Jiansheng; Zhang, Lancui; Wang, Zanjiao; Chen, Weilin; Zhu, Zaiming; Su, Zhongmin; Wang, Enbo (2014-05-28). "A Novel Carboxyethyltin Functionalized Sandwich-type Germanotungstate: Synthesis, Crystal Structure, Photosensitivity, and Application in Dye-Sensitized Solar Cells". ACS Applied Materials & Interfaces. 6 (10): 7876–7884. doi:10.1021/am501192f. ISSN 1944-8244.
- ^ Karimi, Mahdi; Sahandi Zangabad, Parham; Baghaee-Ravari, Soodeh; Ghazadeh, Mehdi; Mirshekari, Hamid; Hamblin, Michael R. (2017-04-05). "Smart Nanostructures for Cargo Delivery: Uncaging and Activating by Light". Journal of the American Chemical Society. 139 (13): 4584–4610. doi:10.1021/jacs.6b08313. ISSN 0002-7863. PMC 5475407. PMID 28192672.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Jiang, Yishu; Weiss, Emily A. (2020-09-09). "Colloidal Quantum Dots as Photocatalysts for Triplet Excited State Reactions of Organic Molecules". Journal of the American Chemical Society. 142 (36): 15219–15229. doi:10.1021/jacs.0c07421. ISSN 0002-7863.
- ^ a b Zhang, Yu; Lee, Tia S.; Petersen, Jeffrey L.; Milsmann, Carsten (2018-05-09). "A Zirconium Photosensitizer with a Long-Lived Excited State: Mechanistic Insight into Photoinduced Single-Electron Transfer". Journal of the American Chemical Society. 140 (18): 5934–5947. doi:10.1021/jacs.8b00742. ISSN 0002-7863.
- ^ Prier, Christopher K.; Rankic, Danica A.; MacMillan, David W. C. (2013-07-10). "Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis". Chemical Reviews. 113 (7): 5322–5363. doi:10.1021/cr300503r. ISSN 0009-2665. PMC 4028850. PMID 23509883.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Bowen, E. J. (1963), "The Photochemistry of Aromatic Hydrocarbon Solutions", Advances in Photochemistry, John Wiley & Sons, Ltd, pp. 23–42, doi:10.1002/9780470133316.ch2, ISBN 978-0-470-13331-6, retrieved 2021-02-24
- ^ a b Romero, Nathan A.; Nicewicz, David A. (2016-09-14). "Organic Photoredox Catalysis". Chemical Reviews. 116 (17): 10075–10166. doi:10.1021/acs.chemrev.6b00057. ISSN 0009-2665.
- ^ Romero, Nathan A.; Nicewicz, David A. (2016-09-14). "Organic Photoredox Catalysis". Chemical Reviews. 116 (17): 10075–10166. doi:10.1021/acs.chemrev.6b00057. ISSN 0009-2665.
- ^ Jiang, Yishu; Weiss, Emily A. (2020-09-09). "Colloidal Quantum Dots as Photocatalysts for Triplet Excited State Reactions of Organic Molecules". Journal of the American Chemical Society. 142 (36): 15219–15229. doi:10.1021/jacs.0c07421. ISSN 0002-7863.
- ^ Jang, Boseung; Park, Jin-Young; Tung, Ching-Hsuan; Kim, In-Hoo; Choi, Yongdoo (2011-02-22). "Gold Nanorod−Photosensitizer Complex for Near-Infrared Fluorescence Imaging and Photodynamic/Photothermal Therapy In Vivo". ACS Nano. 5 (2): 1086–1094. doi:10.1021/nn102722z. ISSN 1936-0851.
- ^ Jang, Boseung; Park, Jin-Young; Tung, Ching-Hsuan; Kim, In-Hoo; Choi, Yongdoo (2011-02-22). "Gold Nanorod−Photosensitizer Complex for Near-Infrared Fluorescence Imaging and Photodynamic/Photothermal Therapy In Vivo". ACS Nano. 5 (2): 1086–1094. doi:10.1021/nn102722z. ISSN 1936-0851.
- ^ Morlière, Patrice; Mazière, Jean-Claude; Santus, René; Smith, Charles D.; Prinsep, Michèle R.; Stobbe, Corinne C.; Fenning, Matthew C.; Golberg, Joanna L.; Chapman, J. Donald (1998-08-15). "Tolyporphin: A Natural Product from Cyanobacteria with Potent Photosensitizing Activity against Tumor Cells in Vitro and in Vivo". Cancer Research. 58 (16): 3571–3578. ISSN 0008-5472. PMID 9721863.
- ^ Karimi, Mahdi; Sahandi Zangabad, Parham; Baghaee-Ravari, Soodeh; Ghazadeh, Mehdi; Mirshekari, Hamid; Hamblin, Michael R. (2017-04-05). "Smart Nanostructures for Cargo Delivery: Uncaging and Activating by Light". Journal of the American Chemical Society. 139 (13): 4584–4610. doi:10.1021/jacs.6b08313. ISSN 0002-7863. PMC 5475407. PMID 28192672.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Zhang, Yu; Lee, Tia S.; Petersen, Jeffrey L.; Milsmann, Carsten (2018-05-09). "A Zirconium Photosensitizer with a Long-Lived Excited State: Mechanistic Insight into Photoinduced Single-Electron Transfer". Journal of the American Chemical Society. 140 (18): 5934–5947. doi:10.1021/jacs.8b00742. ISSN 0002-7863.
- ^ Tributsch, Helmut (1972). "Reaction of Excited Chlorophyll Molecules at Electrodes and in Photosynthesis*". Photochemistry and Photobiology. 16 (4): 261–269. doi:10.1111/j.1751-1097.1972.tb06297.x. ISSN 1751-1097.
- ^ Teodor, Alexandra H.; Bruce, Barry D. (2020-12). "Putting Photosystem I to Work: Truly Green Energy". Trends in Biotechnology. 38 (12): 1329–1342. doi:10.1016/j.tibtech.2020.04.004. ISSN 0167-7799.
{{cite journal}}: Check date values in:|date=(help) - ^ Zeng, Wangdong; Cao, Yiming; Bai, Yu; Wang, Yinghui; Shi, Yushuai; Zhang, Min; Wang, Fangfang; Pan, Chunyue; Wang, Peng (2010-03-09). "Efficient Dye-Sensitized Solar Cells with an Organic Photosensitizer Featuring Orderly Conjugated Ethylenedioxythiophene and Dithienosilole Blocks". Chemistry of Materials. 22 (5): 1915–1925. doi:10.1021/cm9036988. ISSN 0897-4756.
- ^ McCullough, Bradley J.; Neyhouse, Bertrand J.; Schrage, Briana R.; Reed, Demi T.; Osinski, Allen J.; Ziegler, Christopher J.; White, Travis A. (2018-03-05). "Visible-Light-Driven Photosystems Using Heteroleptic Cu(I) Photosensitizers and Rh(III) Catalysts To Produce H2". Inorganic Chemistry. 57 (5): 2865–2875. doi:10.1021/acs.inorgchem.7b03273. ISSN 0020-1669.
- ^ Zhou, Qinqin; Shi, Gaoquan (2016-03-09). "Conducting Polymer-Based Catalysts". Journal of the American Chemical Society. 138 (9): 2868–2876. doi:10.1021/jacs.5b12474. ISSN 0002-7863.
