User:Elovic.a/sandbox
Article Evaluation: Sustainable development[edit]
Content:
Everything in the article seems to be very relevant to the topic, explaining in depth each subsection that sustainable development is apart of. I think the information covers a very large time frame to give a good history of the topic, while also staying updated (the last edit occurred yesterday) with information from within the current year.
Tone:
This article seems to be relatively neutral. Theres equal representation of both position of the pro sustainable development along with the criticism of the topic. This article was very well rounded with equal representation of the relevant subdomains.
Sources:
All of the linked citations do work with the source supporting the claims. However, there are some sentences that should have a source that merely say "citation needed" which I thought was somewhat problematic. There is a variety of sources that are neutral as well as more heavily waited for the economic aspect vs. the environmental aspect of sustainable development, but I think provided equal amounts of these sources creates a neutral article.
Talk Page:
On the talk page there are discussions about new subdomains/subjects to be represented. To name a few: millennials development goals, more background information, World bank definition of sustainable development, and more prominent mention of the UN's involvement. The article is rated as C-class but is apart of many wikiprojects.
Article Selection[edit]
Fluoride therapy[edit]
Overall this was a good article, but I think it focused too much on the makeup/components of fluoride treatment and didn't provide any evidence or statistical analysis of its benefit/harm. The citations and sources were alright, but I wish there was more of a history of fluoride treatment i.e who created it and what was its first usage.
Dental public health[edit]
This article was very unorganized, however well thought out. I think the international examples could have been made more concise because they overwhelm as the bulk of the article. I also think organizationally, the history/background should be at the top of the article and not the last subject. Otherwise, the citations and sources were good.
Oral hygiene[edit]
This article was definitely well organized, however, I feel like it is missing a background aspect of when oral hygiene became significant and a relevant topic. Furthermore, I think there should be a bigger topic on oral diseases/the negative aspects of oral health not just how to care for ones teeth.
Fluoride therapy Article Addition[edit]
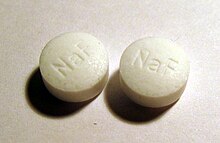 Fluoride is sold in tablets for cavity prevention. | |
| Clinical data | |
|---|---|
| Trade names | OrthoWash, PerioMed, others |
| AHFS/Drugs.com | Monograph |
| Routes of administration | by mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
Fluoride therapy is the use of fluoride for medical purposes.[2] Fluoride is a mineral that can be found in bones and teeth. It is also naturally occurring in water, soil, plants, rocks, and air. Fluoride supplements are recommended to prevent tooth decay in children older than 6 month in areas where the drinking water is low in fluoride.[3] It is typically used as a liquid, pill, or paste by mouth.[4] Where public water supplies are fluoridated further fluoride by mouth is typically not needed.[4] Fluoride has also been used to treat a number of bone diseases.[5]
Normal doses may occasionally result in white marks on the teeth.[4] Excessive doses can result in brown or yellow coloring of the teeth.[4] Fluoride therapy typically uses the sodium fluoride form, though stannous fluoride may also be used.[4][5] Fluoride appears to decrease breakdown by acids, increase remineralisation, and decrease the activity of bacteria.[5] It is believed to work mostly through direct contact with the teeth after they have emerged.[3][5]Fluoride came into use to prevent tooth decay in the 1940s.[6] Fluoride, as sodium fluoride, is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[7] In the United Kingdom a typical month supply costs the NHS about 0.36 pounds.[4] It is also not very expensive in the United States.[8]
History[edit]
At the start of the 1900's dental professionals around the United States, specifically in Colorado Springs, became extremely concerned with a rising epidemic: Permanent brown stained teeth in many of their patients. With no evidence of this disorder in past literature, Frederick McKay, a recent dental school graduate was at the forefront of the Colorado Browning research in 1901. In 1909, McKay joined forces with researcher Dr. G.V. Black after 90% of the cities local children were reported symptomatic for this disorder.
McKay and Black contributed this brown staining to the drinking water after finding that those effected shared the same source of water. After sending out a water sample to the chief chemist of ALCOA, H.V. Churchill, their hypothesis was proven. The water, which contained extremely high levels of water born fluoride was causing the discoloration of teeth.
With this discovery, Dr. H. Trendley Dean, head of the Dental Hygiene Unit at the National Institute of Health decided to delve deeper into fluoride research in 1931. Primarily, he researched how high fluoride levels could be in drinking water before this mottling of the teeth, or fluorosis, occured. To complete this task he began conducting his research with a senior chemist at NIH, Dr. Elias Elvolve. within two years, Elvolve developed a method to measure fluoride levels in water with an accuracy of 0.1 parts per million. Dean and his staff were able to make the discovery that fluoride levels up to 1.0 ppm in drinking water would not cause enamel staining.
In conjunction with this finding, Dean remembered that in Mckay and Black's studies, the teeth that were stained also were discovered to be unusually resistant to tooth decay. With this information Dean decided to test his hypothesis of whether adding fluoride to drinking water at a safe dosage would actually help prevent tooth decay. In 1944, his hypothesis was tested when the City Commission of Grand Rapids decided to add fluoride to its public water supply. Grand Rapids became the first city int he work to fluoridate its water in 1945. Over the next 15 years, researchers monitored 30,000 children born after the fluoride was added to the drinking supply. After the first 11 years had passed, the NIDR announced that the rate of caries among children in Grand Rapids dropped by 60%. This breakthrough helped revolutionize dental care, and was the first discovered treatment of tooth decay.
After 30 years following this discovery, dental fluoride treatments are still at the forefront of dental decay prevention, especially for children. Fluoride therapy comes in the form of toothpaste, mouth wash, varnishes, and more. Although fluoride treatment is primarily used in dental health, it has also become a treatment for other diseases, such as Osteoporosis.
Fluoride Conversion Chart[edit]
| APF (10)(%)(1000) | ppm |
| 1.0% | 10,000 |
| 1.23% | 12,300 |
| NaF (4.5)(%)(1000) | ppm |
| 0.05% | 225 |
| 0.20% | 900 |
| 0.44% | 1,980 |
| 1.0% | 4,500 |
| 1.1% | 4,950 |
| 2.0% | 9,000 |
| 5.0% | 22,500 |
| SnF2 (2.4)(%)(1000) | ppm |
| 0.40% | 960 |
| 0.63% | 1,512 |
Dental Uses[edit]
Water fluoridation[edit]
Water fluoridation is the controlled addition of fluoride to a public water supply in order to reduce tooth decay.[9]It is now used for about two-thirds of the U.S. population on public water systems[10] and for about 5.7% of people worldwide.[11] Although the best available evidence shows no association with adverse effects other than fluorosis, most of which is mild,[12] water fluoridation has been contentious[11] and opposition to water fluoridation exists despite its support by public health organizations.[13] Water fluoridation is the most cost effective way to induce fluoride with an estimated cost between 0.50 and 3.00 USD per person per year, depending on the size of the community involved.[14] A dollar spent on fluoridating water is estimated to save $7-$42 on dental treatment.[14]
Toothpaste[edit]
Most toothpastes contains between 0.22% (1,000 ppm) and 0.312% (1,450 ppm) fluoride, usually in the form of sodium fluoride or sodium monofluorophosphate (MFP). Frequent use of toothpaste with 1,100 ppm fluoride content enhances the remineralization of enamel and inhibits the demineralization of enamel and root surfaces.[15] Most toothpastes with fluoride contain mild abrasives in order to remove heavier debris and light surface staining.[16] These abrasives include calcium carbonate, silica gels, magnesium carbonates and phosphate salts.[16]
Fluoride is available in three forms during toothbrushing. First, it is available as a free ionic fluoride which can react with the tooth structure, interfere with the metabolism of bacteria in plaque, or absorb to the oral mucosa.[17] Second, it is available as profluoride compounds which can precipitate in the mouth during toothbrushing and release ionic fluoride.[17] Lastly, fluoride in toothpaste can exist as unavailable fluoride compounds which do not release fluoride ions. This is due to the fluoride ions being swallowed or expelled when spitting.[17]
High-fluoride content toothpaste generally contains 1.1% (5,000 ppm) sodium fluoride toothpaste. This type of toothpaste is used in the same manner as regular toothpaste. The application of high-fluoride content toothpaste in adults twice daily improves the surface hardness of untreated root decay when compared to toothpaste with regular fluoride content.[18][19]
Fluoridated toothpaste is also available in the form of 0.454% stannous fluoride. It appears to be effective in reducing tooth hypersensitivity.[20] Additionally, brushing twice daily with a toothpaste containing stannous fluoride may reduce gingivitis, gingival bleeding and dental plaque.[21]
Anti-sensitivity toothpastes with fluoride are also available for those who have sensitive teeth. Some anti-sensitivity toothpastes with fluoride on the market contain the ingredients called strontium chloride or potassium nitrate which help to alleviate tooth sensitivity.[16]
Mouth rinses[edit]
Fluoride mouth rinses can be professionally-applied by a dental professional or used at home. The most common fluoride compound used in mouth rinse is neutral sodium fluoride. Fluoride mouth rinses range from 0.05% to 0.2% (225-1,000 ppm) in concentration.[22] The fluoride rinse with a 0.05% fluoride content is used for daily rinsing, while the rinse with 0.2% fluoride content is used for weekly rinsing and in school-based weekly rinsing programs.[23] Fluoride at these concentrations is not strong enough for people at high risk for tooth decay. Regular use of a daily (230 ppm) or weekly (900 ppm) fluoride mouth rinse under supervision results into a reduction of tooth decay in children’s permanent teeth.[24] After a fluoride mouthrinse treatment, the fluoride in the mouthrinse is retained in the saliva which helps prevent tooth decay.[23]
Fluoride mouth rinses are recommended for use in conjunction with other fluoride therapies, but is usually contraindicated for children under 6 years old as they may swallow the rinse and increase their risk of dental fluorosis.[23][24] In areas without fluoridated drinking water, these rinses are recommended for children.
Many brands of topical fluoride exist.[25] They are not recommended if a person is drinking water that already contains sufficient fluoride.[25]
Gels/foams[edit]
There are several types of professionally-applied fluoride gels and foams on the market. The types of professionally-applied fluoride gels include 2.0% neutral sodium fluoride and 1.23% acidulated phosphate fluoride.[23] 1.23% acidulated phosphate fluoride gel or foam is used for patients without tooth-colored restorations, while 2.0% neutral sodium fluoride is used for patients with composites, porcelain, titanium, sealants or sensitivity.[26]
Professionally-applied fluoride gel or foam is applied through the use of a foam mouth tray which is held in the mouth by gently biting down. The application usually lasts for approximately four minutes, and patients should not rinse, eat, smoke, or drink for 30 minutes after application. The reason for this is to allow the teeth to absorb the fluoride into the tooth structure when it is at its highest concentration, without being interrupted. This aids in the repair of microscopic dental decay.[27] There is no clinical evidence on the effectiveness of one-minute fluoride gel/foam applications.[28] A specific benefit when using foam is that less product is required during application, which results in a lower fluoride dose and lessens the risk of accidental ingestion.[28] Additionally, more research regarding the efficacy of fluoride foam is needed as the evidence for its effectiveness is not as strong compared to those of fluoride gels and varnish.[28]
Some gels are made for home application with the use of a custom tray. A model of a person's teeth can be made by a dental professional, who then uses that to make trays, similar to a sport guard tray, which is put over their teeth. The patient can then use this to hold a fluoride treatment against their teeth overnight or several minutes during the day. The concentration of fluoride in these gels is much lower than in professional products.[23] The self-applied sodium fluoride gel/foam typically contains 0.5% fluoride and stannous fluoride gel/foam contains 0.15%.[23]
Head and neck radiation treatment may destroy the cells of the salivary gland which can result in dry mouth. Patients with reduced salivary flow are at an increased risk of tooth decay. The home application of 1.1% fluoride gel with a custom tray is recommended for patients undergoing or are finished with head and neck radiation treatment and patients with decreased salivary flow.[29]
More research is required regarding the efficacy of fluoride gels in treating initial dental decay lesions.[30]
Varnish[edit]
Fluoride varnish has practical advantages over gels in ease of application and use of smaller volume of fluoride than required for gel applications. The principle of fluoride varnish is to apply fluoride salt in a very high concentration (approximately 50,000 ppm) onto the surface of the teeth.[17] Fluoride varnish is a resin-based application that is designed to stay on the surface of the teeth for several hours. As this varnish rests on the tooth's surface, saliva dissolves the fluoride salt, which in turn allows fluoride ions to be released and absorbed by the teeth and soft tissues.[17] Later, the fluoride is re-released into the oral cavity from these reservoirs which acts as protection for the teeth against cavities.[17] Currently, there is also no published evidence that indicates that professionally applied fluoride varnish is a risk factor for enamel fluorosis. The varnish is applied with a brush and sets within seconds.
Fluoride varnish has shown to be effective in reducing initial dental decay lesions in both primary and permanent dentition.[30] Application of fluoride varnish every six months is effective in preventing dental decay in primary and permanent teeth of children and adolescents.[28]
Lozenges[edit]
Fluoridated lozenges may contain about 1 mg fluoride each, and are meant to be held in the mouth and sucked. The dissolved lozenge is swallowed slowly, so the use of lozenges is both a topical and a systemic therapy. A 1955 study comparing the effects of fluoride lozenges and fluoride pills provided clear evidence early that fluoride acts topically.[31][32]Medical fluoride supplements in the form of tablets, lozenges, or liquids (including fluoride-vitamin preparations) are used primarily for children in areas without fluoridated drinking water. The evidence supporting the effectiveness of this treatment for primary teeth is weak. The supplements prevent cavities in permanent teeth. A significant side effect is mild to moderate dental fluorosis.[33] A Cochrane review also found no evidence that daily fluoride supplementation in pregnant women was effective in preventing tooth decay or causing fluorosis in their children.[34]
Slow-release devices[edit]
Devices that slowly release fluoride can be implanted on the surface of a tooth, typically on the side of a molar where it is not visible and does not interfere with eating. The two main types are copolymer membrane and glass bead. These devices are effective in raising fluoride concentrations and in preventing cavities, but they have problems with retention rates, that is, the devices fall off too often.[35] A 2018 Cochrane review found insufficient evidence to determine the effect of slow-release fluoride glass beads in caries-inhibiting when compared to other types of fluoride therapy.[36]
Non-Dental Uses[edit]
Osteoporosis Treatment[edit]
When studied as a possible treatment for osteoporosis, fluoride therapy is shown to be of immense value. Fluoride was proven to increase in bone density and reduce the incidence of vertebral fracture. Fluoride is the only known anabolic treatment for osteoporosis other than anabolic steroids.
Chemical Mechanism[edit]
Strictly speaking, fluoride therapy repairs rather than prevents damage to the teeth, causing the mineral fluorapatite to be incorporated into damaged tooth enamel. Fluorapatite is not a natural component of human teeth, although it is found in the teeth of sharks. The main mineral found in natural tooth enamel is hydroxyapatite rather than the fluorapatite created in the presence of fluoride. Even without fluoride, teeth experience alternating increases and decreases in mineral content, depending upon how acidic or alkaline the mouth is, and depending upon the concentration of other substances in the mouth, such as phosphate and calcium.
Fluoride reduces the decay of tooth enamel by the formation of fluorapatite and its incorporation into the dental enamel. The fluoride ions reduce the rate of tooth enamel demineralization and increase the rate of remineralization of teeth at the early stages of cavities. Fluoride exerts these effects by the demineralization and remineralization cycle.[37] The remineralization cycle, critical to decay prevention, occurs when fluoride is present in the oral cavity. After fluoride is swallowed it has a minimal effect.[33][31][38]
Fluoride ions are involved in three principle reactions of remineralization:[31]
- Iso-ionic exchange of F− for OH− in apatite: Ca10(PO4)6(OH)2 + 2F− → Ca10(PO4)6F2 + 2OH−
- Crystal growth of fluorapatite from a supersaturated solution: 10 Ca2+ + 6PO43− + 2F− → Ca10(PO4)6F2
- Apatite dissolution with CaF2 formation: Ca10(PO4)6(OH)2 + 20F− → 10 CaF2 + 6PO43− + 2OH−
Iso-ionic exchange by the replacement of F- for OH¯ in apatite and crystal growth of fluorapatite from supersaturated solutions are able to occur during exposure to low levels of fluoride (0.01-10 ppm F) over long periods of time. Reaction of apatite dissolution with CaF2 formation occurs in higher levels of fluoride (100-10,000 ppm F) and the addition of CaF2 or a CaF2 containing compound.[31]
Fluoride's effect on oral microflora and the significance of this effect on fluoride's overall effectiveness against cavities does not currently have a consensus.[37][31] Many studies on bacterial cells in laboratories have shown the fluoride has many effects on them as an antimicrobial agent. The antimicrobial effects require concentrations of fluoride at least 10 ppm F, which only occurs briefly in the mouth with oral fluoride-containing products.[37] A study looked at fluoride's effects on oral microflora and concluded that fluoride may not solely interact as an antimicrobial agent, acting additionally to reduce bacterial adhesion to teeth, along with the primary action of decreasing demineralization. Further investigation will need to be done to verify these claims.[39]
Fluoride can be delivered by many chemical methods (sodium fluoride, stannous fluoride, amine fluoride, monofluorophosphate, and more). The anti-caries performance differences between them have been shown to have less effect than variations in behavior shown by individuals in brushing, using fluoride products and post use behavior. Often the chemical form of fluoride is driven by compatibility with the other elements mixed with, price, and such.[37]
All fluoridation methods provide low concentrations of fluoride ions in saliva, thus exerting a topical effect on the plaque fluid.[40] Fluoride does not prevent cavities but rather controls the rate at which they develop, and so repeated exposure throughout the day is essential for its effective function.[41] The more constant the supply the more beneficial fluoride will be in cavity prevention.[37][31]
Health Benefits[edit]
Preventing Tooth Decay[edit]
The use of fluoride in the US and other countries is the primary factor in the decline of tooth decay and caries. The fluoridation of community water is responsible for a reduction in the rate of tooth decay by 50-60% in the United States since World War II. More recently, this reduction is still between 18-40%.
Health Risks[edit]
Overdose[edit]
Consumption of large amounts of fluoride can lead to fluoride poisoning and death; the lethal dose for most adult humans is estimated at 5 to 10 g (which is equivalent to 32 to 64 mg/kg elemental fluoride/kg body weight).[42][43][44] Ingestion of fluoride can produce gastrointestinal discomfort at doses at least 15 to 20 times lower (0.2–0.3 mg/kg) than lethal doses.[45] Chronic intake and topical exposure may cause dental fluorosis, and excess systematic exposure can lead to systemic effects such as skeletal fluorosis. Young children are at risk for receiving excess fluoride, and the ADA has recently issued an interim guidance on their fluoride consumption.[46]
In 1974 a three-year-old child swallowed 45 milliliters of 2% fluoride solution, estimated to be triple the fatal amount, and then died. The fluoride was administered during his first visit to the dentist, and the dental office was later found liable for the death.[47]
Dental Fluorosis[edit]
- See main article Dental fluorosis.
The use of fluoride toothpaste (with concentrations of 1000 ppm and above) and fluoride supplements in children below the age of six years, and especially within the first three years of life, is associated with a greater risk of dental fluorosis.[33] However, the use of fluoride supplements during the last 6 months of pregnancy has no significant impact on the incidence of fluorosis in children.[48] It has also been estimated that optimal water fluoridation for the prevention of dental caries increases the prevalence of dental fluorosis by 4 to 5%.[12] The observed effects are mild to moderate, usually of minimal aesthetic concern.[12]
Cancer[edit]
Rare cancers such as osteosarcoma, a type of bone cancer, have had an increase incidence in areas that fluoridate their water. Between the two time periods 1973-1980 and 1981-1987 the annual rate of osteosarcoma diagnoses in young men under the age of 20 increased from 3.6 cases per 1,000,000 people to 5.5 cases per 1,000,000 people. These men all had been from areas where the water was being fluoridates. However, there is not yet a discovered correlation and more refined studies must be done to show a true relationship.
Bone Fractures[edit]
There is some suggestion from epidemiological studies that bone fracture incidences have increased with the exposure of fluoride treatment. the increase has happened specifically in areas with naturally high or adjusted fluoride levels in the drinking water. There are a number of confounding factors, however, so the association isn't proven to exist, and therefore requires more research.
International Stance on Fluoride Treatment[edit]
European Directive for the use of Fluorides[edit]
The European Academy of Pediatric Dentistry recommends that all of the population, even pregnant women, should use preventive fluoride treatments in order to prevent caries. These measures include parents brushing their children's teeth with fluoride toothpaste as soon the child's first tooth erupt. the concentration and quantity of the toothpaste that is recommended by the EAPD and parents should assist their children in brushing their teeth with this too paste until the age of at least 7.
Water Fluoridation[edit]
In Europe only Ireland, Poland, Serbia, Spain, and the UK fluoridate their water. Japan and other western European countries do not fluoridate their public drinking supply.
[49] [50]creation of fluoridation
[51] Flouride Varnish
[52] Mouth wash
Reworking the sections:
instead of delivery-> Dental Uses & non dental uses
Instead of medical uses-> Health benefits & Health Risks
 | This is a user sandbox of Elovic.a. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
- ^ Mosby's Review Questions for the National Board Dental Hygiene Examination. Elsevier Health Sciences. 2013. p. 231. ISBN 9780323226318. Archived from the original on 18 September 2017.
- ^ Weiner, Eugene R. (2008). Applications of Environmental Aquatic Chemistry: A Practical Guide, Second Edition (2 ed.). CRC Press. p. 389. ISBN 9781420008371. Archived from the original on 18 September 2017.
- ^ a b WHO Model Formulary 2008 (PDF). World Health Organization. 2009. pp. 501–502. ISBN 9789241547659. Archived (PDF) from the original on 13 December 2016. Retrieved 8 January 2017.
- ^ a b c d e f British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. pp. 699–700. ISBN 9780857111562.
- ^ a b c d "Fluorides". The American Society of Health-System Pharmacists. Archived from the original on 3 June 2016. Retrieved 8 January 2017.
- ^ Murray, John J.; Nunn, June H.; Steele, James G. (2003). The Prevention of Oral Disease. OUP Oxford. p. 53. ISBN 9780192632791. Archived from the original on 18 September 2017.
- ^ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- ^ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 217. ISBN 9781284057560.
- ^ Centers for Disease Control and Prevention (2001). "Recommendations for using fluoride to prevent and control dental caries in the United States". MMWR Recomm Rep. 50 (RR-14): 1–42. PMID 11521913. Archived from the original on 8 February 2007.
- ^ Ripa LW (1993). "A half-century of community water fluoridation in the United States: review and commentary". J Public Health Dent. 53 (1): 17–44. doi:10.1111/j.1752-7325.1993.tb02666.x. PMID 8474047.
- ^ a b Cheng KK, Chalmers I, Sheldon TA; Chalmers; Sheldon (2007). "Adding fluoride to water supplies". BMJ. 335 (7622): 699–702. doi:10.1136/bmj.39318.562951.BE. PMC 2001050. PMID 17916854. Archived from the original on 17 June 2008.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c National Health and Medical Research Council (Australia) (2007). "A systematic review of the efficacy and safety of fluoridation" (PDF). Archived (PDF) from the original on 13 January 2012. Retrieved 24 February 2009. Summary: Yeung CA (2008). "A systematic review of the efficacy and safety of fluoridation". Evid Based Dent. 9 (2): 39–43. doi:10.1038/sj.ebd.6400578. PMID 18584000.
{{cite journal}}: Unknown parameter|lay-date=ignored (help); Unknown parameter|lay-source=ignored (help); Unknown parameter|lay-url=ignored (help) - ^ Armfield JM (2007). "When public action undermines public health: a critical examination of antifluoridationist literature". Aust New Zealand Health Policy. 4 (1): 25. doi:10.1186/1743-8462-4-25. PMC 2222595. PMID 18067684. Archived from the original on 17 June 2008.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Association, Wisconsin Dental. "How much does community water fluoridation cost?". Wisconsin Dental Association - Oral & Dentistry Advocates | WDA. Retrieved 2018-08-04.
- ^ Nóbrega, DF; Fernández, CE; Del Bel Cury, AA; Tenuta, LM; Cury, JA (2016). "Frequency of fluoride dentifrice use and caries lesions inhibition and repair". Caries Research. 50 (2): 133–140. doi:10.1159/000444223. PMID 26992247. Archived from the original on 21 December 2016.
- ^ a b c American Dental Association. "Learn more about toothpastes". Archived from the original on 2 December 2016. Retrieved 30 November 2016.
- ^ a b c d e f Carey, CM (2014). "Focus on fluorides: update on the use of fluoride for the prevention of dental caries". Journal of Evidence Based Dentistry. 14: 95–102. doi:10.1016/j.jebdp.2014.02.004. PMC 4058575. PMID 24929594.
- ^ Srinivasan, M; Schimmel, M; Riesen, M; Ilgner, A; Wicht, MJ; Warncke, M; Elwood, RP; Nitschke, I; Müller, F; Noack, MJ (2014). "High-fluoride toothpaste: a multicenter randomized controlled trial in adults". Community Dentistry and Oral Epidemiology. 42 (4): 333–340. doi:10.1111/cdoe.12090. PMC 4282025. PMID 24354454.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: url-status (link) - ^ Yeung, CA (2014). "Some beneficial effect on root caries from use of higher concentration fluoride toothpaste (5000 ppm F)". Evidenced Based Dentistry. 15 (1): 8–9. doi:10.1038/sj.ebd.6400981. PMID 24763166.
- ^ West, NX; Seong, J (2015). "Management of dentine hypersensitivity: efficacy of professionally and self-administered agents". Journal of Clinical Periodontology. 42 (S16): S256–S302. doi:10.1111/jcpe.12336. PMID 25495777.
- ^ Selwitz, RH (2009). "Twice Daily Toothbrushing With a Stabilized Stannous Fluoride/Sodium HexametaPhosphate Dentifrice May Reduce Gingivitis, Gingival Bleeding, and Dental Plaque". Journal of Evidence-Based Dental Practice. 9 (1): 28–29. doi:10.1016/j.jebdp.2008.12.007. PMID 19269614.
- ^ Twetman, S; Keller, MK (2016). "Fluoride rinses, gels and foams: an update of controlled clinical trials". Caries Research. 50 (1): 38–44. doi:10.1159/000439180. PMID 27101002. Archived from the original on 21 December 2016.
- ^ a b c d e f Centers for Disease Control and Prevention (17 August 2001). "Recommendations for using fluoride to prevent and control dental caries in the United States". Archived from the original on 8 February 2007. Retrieved November 30, 2016.
- ^ a b Marinho, VCC; Chong, LLY; Worthington, HV; Walsh, T (2016). "Fluoride mouthrinses for preventing dental caries in children and adolescents". The Cochrane Database of Systematic Reviews. 7 (11): CD002284. doi:10.1002/14651858.cd002284.pub2. PMC 6457869. PMID 27472005.
- ^ a b "Perio Med Uses, Side Effects & Warnings - Drugs.com". Drugs.com. Retrieved 2018-08-04.
- ^ Darby, ML; Walsh, MM (2015). Dental Hygiene Theory and Practice 4th edition. St. Louis: Saunders/Elsevier. pp. 580–597. ISBN 978-1-4557-4548-7.
- ^ American Dental Association Division of Communications (2007). "Fluoride dental treatments in the dental office". Journal of the American Dental Association. 138 (3): 420. doi:10.14219/jada.archive.2007.0175. PMID 17332048. Archived from the original on 11 December 2016.
- ^ a b c d American Dental Association Council on Scientific Affairs (2006). "Professionally applied topical fluoride: evidence-based clinical recommendations" (PDF). Journal of the American Dental Association. 137 (8): 1151–1159. doi:10.14219/jada.archive.2006.0356. PMID 16873333. Archived (PDF) from the original on 4 November 2016.
- ^ Hancock, PJ; Epstein, JB; Sadler, GR (2003). "Oral and dental management related to radiation therapy for head and neck cancer". Journal (Canadian Dental Association). 69 (9): 585–590. PMID 14653934.
- ^ a b Lenzi, TL; Fernandes Montagner, A; Zovico Maxnuck Soares, F; de Oliveira Rocha, R (2016). "Are topical fluorides effective for treating incipient carious lesions?". Journal of the American Dental Association. 147 (2): 84–91. doi:10.1016/j.adaj.2015.06.018. PMID 26562737.
- ^ a b c d e f Rošin-Grget, K; Peroš, K; Sutej, I; Bašić, K (November 2013). "The cariostatic mechanisms of fluoride". Acta Medica Academica. 42 (2): 179–88. doi:10.5644/ama2006-124.85. PMID 24308397. Archived from the original on 7 April 2014. Retrieved 31 March 2014.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ B.G. Bibby; Esther Wilkins; Evelyn Witol (February 1995). "A preliminary study of the effects of fluoride lozenges and pills on dental caries, republished 1995". OOOO. 8 (2): 213–216. Retrieved January 19, 2015.
- ^ a b c Ismail AI, Hasson H; Hasson (2008). "Fluoride supplements, dental caries and fluorosis: a systematic review". J Am Dent Assoc. 139 (11): 1457–68. doi:10.14219/jada.archive.2008.0071. PMID 18978383. Archived from the original on 30 June 2012.
- ^ Takahashi, R; Ota, E; Hoshi, K; Naito, T; Toyoshima, Y; Yuasa, H; Mori, R; Nango, E (2017). "Fluoride supplementation (with tablets, drops, lozenges or chewing gum) in pregnant women for preventing dental caries in the primary teeth of their children". Cochrane Database of Systematic Reviews. 10 (10): CD011850. doi:10.1002/14651858.CD011850.pub2. PMC 6485723. PMID 29059464.
- ^ Pessan JP, Al-Ibrahim NS, Buzalaf MAR, Toumba KJ; Al-Ibrahim; Buzalaf; Toumba (2008). "Slow-release fluoride devices: a literature review". J Appl Oral Sci. 16 (4): 238–46. doi:10.1590/S1678-77572008000400003. PMC 4327531. PMID 19089254. Archived from the original on 7 October 2012.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chong, Lee-Yee; Clarkson, Janet E.; Dobbyn‐Ross, Lorna; Bhakta, Smriti (2018-03-01). "The Cochrane Library". Cochrane Database of Systematic Reviews. 3: CD005101. doi:10.1002/14651858.cd005101.pub4. PMC 6494221. PMID 29495063.
- ^ a b c d e ten Cate, JM (Feb 2013). "Contemporary perspective on the use of fluoride products in caries prevention". British Dental Journal. 214 (4): 161–7. doi:10.1038/sj.bdj.2013.162. PMID 23429124.
- ^ Featherstone JD (1999). "Prevention and reversal of dental caries: role of low level fluoride". Community Dent Oral Epidemiol. 27 (1): 31–40. doi:10.1111/j.1600-0528.1999.tb01989.x. PMID 10086924.
- ^ Loskill, Peter; Zeitz, Christian; Grandthyll, Samuel; Thewes, Nicolas; Müller, Frank; Bischoff, Markus; Herrmann, Mathias; Jacobs, Karin (7 May 2013). "Reduced Adhesion of Oral Bacteria on Hydroxyapatite by Fluoride Treatment". Langmuir. 29 (18): 5528–5533. doi:10.1021/la4008558. PMID 23556545.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "ADA.org:A-Z Topics: Fluoride and Fluoridation" (PDF). American Dental Association. Archived from the original (PDF) on 15 March 2016.
- ^ ten Cate FM. Contemporary perspective on the use of fluoride products in caries prevention Archived 7 April 2014 at the Wayback Machine British Dental Journal 214, 161 - 167 (2013) PMID 23429124
- ^ Gosselin, RE; Smith RP; Hodge HC (1984). Clinical toxicology of commercial products. Baltimore (MD): Williams & Wilkins. pp. III–185–93. ISBN 978-0-683-03632-9.
- ^ Baselt, RC (2008). Disposition of toxic drugs and chemicals in man. Foster City (CA): Biomedical Publications. pp. 636–40. ISBN 978-0-9626523-7-0.
- ^ IPCS (2002). Environmental health criteria 227 (Fluoride). Geneva: International Programme on Chemical Safety, World Health Organization. p. 100. ISBN 978-92-4-157227-9.
- ^ Bradford D. Gessner; Michael Beller; John P. Middaugh; Gary M. Whitford (13 January 1994). "Acute fluoride poisoning from a public water system". New England Journal of Medicine. 330 (2): 95–99. doi:10.1056/NEJM199401133300203. PMID 8259189. Archived from the original on 15 July 2009.
- ^ (2006). Interim Guidance on Fluoride Intake for Infants and Young Children Archived 25 December 2014 at the Wayback Machine
- ^ New York Times. (1979). $750,000 Given in Child's Death in Fluoride Case: Boy, 3, Was in City Clinic for Routine Cleaning. NYT archive, free full-text available at NYT here.
- ^ Takahashi, R; Ota, E; Hoshi, K; Naito, T; Toyoshima, Y; Yuasa, H; Mori, R; Nango, E (2017). "Fluoride supplementation (with tablets, drops, lozenges or chewing gum) in pregnant women for preventing dental caries in the primary teeth of their children". The Cochrane Database of Systematic Reviews. 10: CD011850. doi:10.1002/14651858.CD011850.pub2. PMC 6485723. PMID 29059464.
- ^ "The Story of Fluoridation | National Institute of Dental and Craniofacial Research". www.nidcr.nih.gov. Retrieved 2018-10-02.
- ^ "The Invention of Fluoride Toothpaste". Moment of Indiana History - Indiana Public Media. Retrieved 2018-10-02.
- ^ Collins, Fiona M. (May 2011). "The development and Utilization of Flouride Varnish" (PDF). PennWell.
- ^ Adair, Steven M. (1998). "The tole of Flouride mouthiness in the control of dental caries: a brief review" (PDF). Pediatric Dentistry. 20:2 – via American Academy of Pediatric Dentistry.
