User:GCentola/sandbox

Histrionicotoxins are a group of related toxins found in the skin of poison frogs from the Dendrobatidae family, notably Oophaga histrionica, which are native to Colombia.[1] It is likely that, as with other poison frog alkaloids, histrionicotoxins are not manufactured by the amphibians, but absorbed from insects in their diet and stored in glands in their skin.[2][3] They are notably less toxic then other alkaloids found in poison frogs, yet their distinct structure acts as a neurotoxin by non-competitive inhibition of nicotinic acetylcholine receptors.[4]
History
[edit]The first record of histrionicotoxins dates to 1823 by Captain Charles Stuart Cochrane.[5] Cochrane was exploring the tropical rainforests around Colombia and Panama. His reports mention tribes of Indians who used poison tipped arrows and blowgun darts for hunting and war. Upon further exploration, Cochrane found that these Indians extracted the poison from the skins of the poison dart frog, Dendrobates histrionicus. An account from his diary reads:
"[...] called rana de veneno by the Spanish, about three inches long, yellow on the back, with very large black eyes....those who use poison catch the frogs in the woods and confine them in a hollow cane where they regularly feed them until they want the poison, when they take the unfortunate reptile and pass a pointed piece of wood down his throat and out of one of his legs. This torture makes the poor frog perspire very much, especially on the back, which becomes covered in a white froth; this is the most powerful poison that he yields, and in this they dip or roll the tips of their arrows, which will preserve their destructive power for a year. Afterwards, below this white substance, appears a yellow oil, which is carefully scraped off, and retains its deadly influence for four to six months, according to the goodness (as they say) of the frog. By this means, from one frog sufficient poison is obtained for about fifty arrows."
Chemical Properties
[edit]Histrionicotoxins are a class rather than a specific poison and this broad spectrum poses synthetic challenges. Structures of histrionicotoxins were characterized in 1971.[6] Since then, several synthetic studies and total syntheses have been carried out. Table 1 describes some of the many variations in histrionicotoxin alkaloids from the parent molecule (283A).[7]
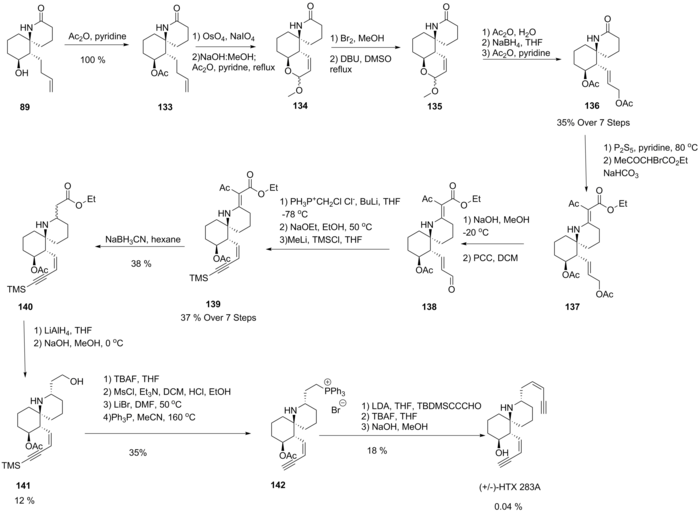
Synthesis
[edit]Since characterization, the development of synthetic pathways to histrionicotoxin has been of interest to research groups due to its unusual functionality. The Kishi group proposed the first total synthesis of the parent 283A in 1985 using 89, a previously synthesized lactam used for the synthesis of other variants.[8] Treatment with acetic anhydride yielded 133 in quantitative yield. The cyclic enol ether 134 was formed through oxidative cleavage promoting intramolecular addition followed by a basic deprotection and dehydration. Bromination followed by dehydrobromination in methanol was then found to give an epimeric mixture of unsaturated 135. Hydrolysis, reduction and acetylation yielded 136. Formation of a thiolactam followed by condensation with ethyl bromoacetate gave 137. Selective deprotection of the allylic alcohol followed by oxidation gave 138. A Wittig reaction then generated a chloroalkene, which, upon base-promoted elimination of HCl, gave a terminal alkyne, which was subsequently protected to form 139. The olefinic function of 139 was first reduced using cyanoborohydride before further reduction of 140 to an epimeric mixture of alcohols. A retro-Michael addition was then performed under basic conditions at low temperature, successfully epimerising this compound to give the desired epimer 141. A reaction with triphenylphosphine then generated the phosphonium salt 142, and a Wittig reaction could then be performed to attach the silyl-protected cis-ene-yne function, which was then deprotected to yield the target (±)-HTX 283A.
Mechanism of Action
[edit]The structure of histrionicotoxins are similar to acetylcholine.

Due to these similarities, HTX acts as a noncompetitive antagonist of acetylcholine receptors, which are implicated in neural signaling. As a non-competitive antagonist, HTX binds to a subunit of the nicotinic acetylcholine receptor.3 This actually increases the affinity for the agonist acetylcholine and stabilizes the desensitized receptor.[9] This essentially blocks action potentials and slows neural function. Interestingly, high concentrations of HTX have been demonstrated to have antagonistic effects on batrachotoxin.[10]
Toxicity
[edit]Interestingly, histrionicotoxin is not nearly as toxic as other alkaloids found from poison frogs. Typical levels of up to 200 micrograms per frog are found, and tests in mice have shown that even 1000 micrograms were not lethal to mice (LD50 ≈ 50 mg/kg).
References
[edit]- ^ Daly, J. W. (1982). "Alkaloids of neotropical poison frogs (Dendrobatidae)". Fortschritte der Chemie organischer Naturstoffe. Progress in the chemistry of organic natural products. 41: 205-340. PMID 7049875.
- ^ Daly, J. W. (1995). ""The chemistry of poisons in amphibian skin". Proceedings of the National Academy of Sciences of the United States of America. 92 (1): 9-13. doi:10.1073/pnas.92.1.9. PMID 7816854.
- ^ Jones, T.H.; Adams, R. M. M.; Spande, T. F.; Garraffo, H. M.; Kaneko, T.; Schultz, T. R. (2012). "Histrionicotoxin alkaloids finally detected in an ant". Journal of Natural Products. 75 (11): 1930-1936. doi:10.1021/np300485v. PMID 23088730.
- ^ Oberthur, W.; Muhn, P.; Bauman, H.; Lottspeich, F.; Wittman-Liebold, B.; Hucho, F. (1986). "The reaction site of a non-competitive antagonist in the delta-subunit of the nicotinic acetylcholine receptor". The EBMO Journal. 5 (8): 1815-1819. PMID 3758027.
- ^ Edwards, N.; Reed, M. "Histrionicotoxin from South American Poison-Dart Frogs". The Chemical Laboratories. Retrieved 2015.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Daly, J. W.; Karle, I.; Meyers, C. W.; Tokuyama, T.; Waters, J. A.; Witkop, B. (1971). "Histrionicotoxins: roentgen-ray analysis of the novel allenic and acetylenie spiroalkaloids isolated from a Colombian dart frog, Dendrobates histrionicus". Preceedings of the National Academy of Sciences of the United States of America. 68 (8): 1870-1875. PMID 5288773.
- ^ Sinclair, A.; Stockman, R. A. (2007). "Thirty-five years of synthetic studies directed towards the histrionicotoxin family of alakaloids". Natural Product Reports. 24 (2): 298-326. doi:10.1039/b604203c. PMID 17389999.
- ^ Carey, S. C.; Aratani, M.; Kishi, Y. (1985). "A total synthesis of d,1-histrionicotoxin". Tetrahedron Letters. 26 (48): 5887-5890. doi:10.1016/S0040-4039(00)98253-4.
- ^ Burgermeister, W.; Catterall, W. A.; Witkop, B (1977). "Histrionicotoxin enhances agonist induced desensitization of acetylcholine receptor". Proceedings of the National Academy of Sciences of the United States of America. 74 (12): 5754-5758. PMID 272000.
- ^ Bartels-Bernal, E.; Diaz, E.; Cadena, R.; Ramos, J.; Daly, J. W, (1983). "Effect of histrionicotoxin on ion channels in synaptic and conductiong membranes of electroplax of Electrophorus electricus". Cellular and Molecular Neurobiology. 3 (3): 203-212. PMID 6322994.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link)


