User:Hanatakei/sandbox
History of the reaction[edit]
Kazuhiko Takai is a professor of applied chemistry at Okayama University and is the recipient of the 2013 Chemical Society of Japan Award.[1] He primarily studies the use catalytic metals in synthesis reactions. He studied at Kyoto University and the University of California, Berkeley. The Takai olefination reaction was first described in a 1986 paper in the Journal of American Chemical Society, Simple and selective method for aldehydes--> (E)-haloalkenes (RCH:CHX) conversion by means of a haloform-chromous chloride system. Written by Kazuhiko Takai, Kenji Nitta, Kiitiro Utimoto, the paper discusses the limitations of existing reagents, such as the Wittig reagent, in creating solely E isomers.[2] [3] Nitta, a graduate student working in the Utimoto group, carried out the reaction.
Similar olefination reactions have been performed, using a variety of reagents such as zinc and lead chloride[4]; however, these olefination reactions often lead to the formation of McMurry diols, not methylenation or alkylidenation of aldehydes[5]. To circumvent this issue, Takai researched the potential to of chromium (II) salts.
The reaction primarily employs the use of aldehydes, but ketones may be used. However, ketones do not react as well as aldehydes; thus, for a compound with both aldehyde and ketone groups, the reaction can target just the aldehyde group and leave the ketone group intact. E-selectivity increases [3]
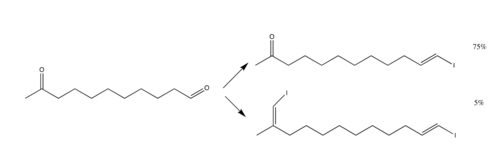
The drawbacks to the reaction include the fact that stoichiometrically, 4 equivalents of chromium chloride must be used, since there is a reduction of two halogen atoms[6]. Ways to limit the amount of chromium chloride exist, namely by utilization of zinc equivalent[7], but this method remains unpopular.
- ^ "Development of Novel Synthetic Organic Reactions by the Use of Group 4-7 Metals". The Chemical Society of Japan. Retrieved 2020-04-30.
- ^ achem.okayama-u.ac.jp http://achem.okayama-u.ac.jp/omc/cv-takai-e.html. Retrieved 2020-04-13.
{{cite web}}: Missing or empty|title=(help) - ^ a b Takai, K.; Nitta, K.; Utimoto, K. (1986-11). "Simple and selective method for aldehydes (RCHO) .fwdarw. (E)-haloalkenes (RCH:CHX) conversion by means of a haloform-chromous chloride system". Journal of the American Chemical Society. 108 (23): 7408–7410. doi:10.1021/ja00283a046. ISSN 0002-7863.
{{cite journal}}: Check date values in:|date=(help) - ^ Okazoe, Takashi; Takai, Kazuhiko; Oshima, Koichiro; Utimoto, Kiitiro (1987-09-01). "Alkylidenation of ester carbonyl groups by means of a reagent derived from RCHBr2, Zn, TiCl4, and TMEDA. Stereoselective preparation of (Z)-alkenyl ethers". The Journal of Organic Chemistry. 52 (19): 4410–4412. doi:10.1021/jo00228a055. ISSN 0022-3263.
- ^ Mukaiyama, Teruaki; Sato, Toshio; Hanna, Junichi (1973-10-05). "REDUCTIVE COUPLING OF CARBONYL COMPOUNDS TO PINACOLS AND OLEFINS BY USING TiCl4 AND Zn". Chemistry Letters. 2 (10): 1041–1044. doi:10.1246/cl.1973.1041. ISSN 0366-7022.
- ^ Murai, Masahito; Taniguchi, Ryuji; Hosokawa, Naoki; Nishida, Yusuke; Mimachi, Hiroko; Oshiki, Toshiyuki; Takai, Kazuhiko (2017-09-20). "Structural Characterization and Unique Catalytic Performance of Silyl-Group-Substituted Geminal Dichromiomethane Complexes Stabilized with a Diamine Ligand". Journal of the American Chemical Society. 139 (37): 13184–13192. doi:10.1021/jacs.7b07487. ISSN 0002-7863.
- ^ Takai, Kazuhiko; Ichiguchi, Tetsuya; Hikasa, Shintaro (1999-01-01). "A practical transformation of aldehydes into (E)-iodoalkenes with geminal dichromium reagents". Synlett (8): 1268–1270. doi:10.1055/s-1999-2829. ISSN 0936-5214.
