User:Hikari20XX/Halovir
Halovir[1][2][3] refers to a multi-analogue compound belonging to a group of oligopeptides designated as lipopeptaibols (chemical features including lipophilic acyl chain at the N-terminus, abundant α-aminoisobutyric acid content, and a 1,2-amino alcohol located at the C-terminus)[4] which have membrane modifying capacity[5]and are fungal in origin[1][6]. These peptides display interesting microheterogeneity[5]; slight variation in encoding amino acids gives rise to a mixture of closely related analogues and have been shown to have antibacterial/antiviral properties[2][6].
Background
[edit]Nonribosomal peptides compose a significant group of secondary metabolites in bacterial/fungal organisms (though Drosophila melanogaster and Caenorhabditis elegans both exhibit products of nonribosomal peptide synthetases)[6][7][8]; having been observed functioning as self-defense substances/iron-chelating siderophores, they serve as coping mechanisms for environmental stress, perform as virulence factors/toxins promoting pathogenesis, and act in signalling (enabling communications within and between species)[6][7][8]. In lieu of these functionalities, many nonribosomal peptides have been utilized in development of medical drugs and biocontrol agents (examples of such include β-lactams, daptomycin, echinocandins, emodepside, bleomycin, cyclosporine, and bialaphos)[6][9][10].
Peptaibols are a family of linear, amphipathic polypeptides (typically consisting of 4-21 amino acids residues)[6] that are generated as a result of the assembly of a variety of aminoacyl, ketoacyl or hydroxyacyl monomers by fungal mulitmodular megaenzymes denoted as nonribosomal-peptide synthetases (NRPSs)[11]. Typically, NRPSs are comprised of three highly conserved core domains: an adenylation (A) domain which recognizes, activates and loads monomers onto NRPS, a thiolation (T) domain (also denoted as the peptidyl carrier protein domain) that transports covalently linked monomers/peptidyl intermediates between nearby NRPS domains, and a condensation (C) domain (catalyzes sequential condensation of monomers within the nascent peptide chain)[6][9][12]. In addition, a chain-terminating domain [thioesterase (TE) domain, a terminal C (CT) or a reductase (R) domain] is commonly observed at the end of an NRPS in order to relinquish full-length peptide chains in linear or cyclic forms. Furthermore, often seen are feature tailoring domains [epimerase, N-methyltransferase (M), oxidase (Ox), ketoacyl reductase (KR) and cyclase (Cy)], allowing for further modification of monomers/polypeptide intermediates[6][9][12].
Notable characteristics of peptaibols include: C-terminal alcohol residues (phenylalaninol, leucinol, isoleucinol, valinol)[6][13], an N-acyl terminus (usually acetyl)[13], and high levels of α,α-dialkylated non-proteinogenic amino acids [α-aminoisobutyric acid (Aib), isovaleric acid (Iva), hydroxyproline (Hyp)][13][14]. In most cases, peptaibols form α-helix and β-bend patterns in their 3D structures (α-aminoisobutyric acid is a turn/helix forming agent)[4]. α,α-dialkylated amino acid residues in peptaibols create substantial conformation constrictions in the peptide backbone, resulting in the formation of right-handed α-helical structures[15][16]. Membrane modification abilities can be attributed to the formation of transmembrane voltage-dependent channels[13][17][18]; this occurs as the peptide takes on an α-helical conformation upon contact of lipid bilayers, drilling through and forming ion channels with similar electrophysiological configurations of ion channel proteins[18][19]. The principle functionality of the peptides is to rupture membranes, in turn triggering cytolysis via loss of osmotic balance[19].
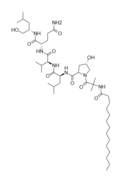
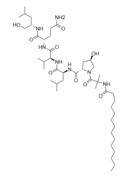
Structurally speaking, lipopeptaibols are peptaibols with a fatty acyl moiety linked to the N-terminal amino acid[4] (thusly named), and have been isolated from a number of soil fungi. Their primary structures all have the L-(S-) configuration at the 2-(α-)carbon[4]. They overwhelmingly display microheterogeneity (being very structurally similar; with a limited pool of conserved variation in natural sample).
Structure
[edit]
-Halovir A: contains L-leucine, L-valine, and L-glutamine
-Halovir B: contains L-alanine, L-leucine, L-glutamine
-Halovir C: contains contains L-leucine, L-valine, L-glutamine,
-Halovir D
-Halovir E
-Halovir I
-Halovir J
-Halovir K
Medical Applications
[edit]The antibiotic capabilities of these compounds can be attributed to membrane insertion and pore-forming functionalities[11], and typically exhibit antimicrobial activity in Gram-positive bacteria and fungi [13][21].
Halovirs A-E (isolated from Scytidium sp.) has displayed potent antiviral activity against HSV-1, and has been observed performing replication inhibition of HSV-1 and HSV-2[2] in standard plaque reduction assay without cytotoxicity (at concentrations upwards of 0.85μM)[2] Mechanistic studies suggest that halovirs kills virus in direct contact and in a time-dependent manner before it can affect host cells.
Halovirs I and J were analyzed for antibacterial and cytotoxic activities, and displayed significant growth inhibition against two Gram-positive bacteria (Staphylococcus aureus and Enterococcus faecium), but not Gram-negative Escherichia coli[6]. Notably, these two halovirs were found to be effective against methicillin-resistant S. aureus (MRSA) and vancomycin-resistant E. faeccium, indicating that the activity against them to be persistent[6]. Additionally, strong cytotoxic activity was observed against a panel of cancer cell lines, including: human lung carinoma A549, human breast carcinoma MCF-7, and human cervical carcinoma HeLa cells (however it should be noted that cytotoxicity was not specific to cancer cells in referenced study)[6].
References:
[edit]- ^ a b "Halovir, an antiviral marine natural product, and derivatives thereof - Patent US-2003013659-A1 - PubChem". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-10-09.
- ^ a b c d Rowley, David C.; Kelly, Sara; Kauffman, Christopher A.; Jensen, Paul R.; Fenical, William (2004-02-10). "Halovirs A-E, New Antiviral Agents from a Marine-Derived Fungus of the Genus Scytalidium". ChemInform. 35 (6). doi:10.1002/chin.200406159. ISSN 0931-7597.
- ^ Rowley, David C.; Kelly, Sara; Jensen, Paul; Fenical, William (2004-09). "Synthesis and structure–activity relationships of the halovirs, antiviral natural products from a marine-derived fungus". Bioorganic & Medicinal Chemistry. 12 (18): 4929–4936. doi:10.1016/j.bmc.2004.06.044.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c d Toniolo, C.; Crisma, M.; Formaggio, F.; Peggion, C.; Epand, R.F.; Epand, R.M. (2001-08). "Lipopeptaibols, a novel family of membrane active, antimicrobial peptides:". Cellular and Molecular Life Sciences. 58 (9): 1179–1188. doi:10.1007/PL00000932. ISSN 1420-682X.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Auvin-Guette, Catherine; Rebuffat, Sylvie; Prigent, Yann; Bodo, Bernard (1992-03). "Trichogin A IV, an 11-residue lipopeptaibol from Trichoderma longibrachiatum". Journal of the American Chemical Society. 114 (6): 2170–2174. doi:10.1021/ja00032a035. ISSN 0002-7863.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c d e f g h i j k l Xiao, Dongliang; Zhang, Mei; Wu, Ping; Li, Tianyi; Li, Wenhua; Zhang, Liwen; Yue, Qun; Chen, Xinqi; Wei, Xiaoyi; Xu, Yuquan; Wang, Chen (2022-05). "Halovirs I–K, antibacterial and cytotoxic lipopeptaibols from the plant pathogenic fungus Paramyrothecium roridum NRRL 2183". The Journal of Antibiotics. 75 (5): 247–257. doi:10.1038/s41429-022-00517-7. ISSN 0021-8820.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Richardt, Arnd; Kemme, Tobias; Wagner, Stefanie; Schwarzer, Dirk; Marahiel, Mohamed A.; Hovemann, Bernhard T. (2003-10-17). "Ebony, a novel nonribosomal peptide synthetase for beta-alanine conjugation with biogenic amines in Drosophila". The Journal of Biological Chemistry. 278 (42): 41160–41166. doi:10.1074/jbc.M304303200. ISSN 0021-9258. PMID 12900414.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Shou, Qingyao; Feng, Likui; Long, Yaoling; Han, Jungsoo; Nunnery, Joshawna K.; Powell, David H.; Butcher, Rebecca A. (2016-10). "A hybrid polyketide-nonribosomal peptide in nematodes that promotes larval survival". Nature Chemical Biology. 12 (10): 770–772. doi:10.1038/nchembio.2144. ISSN 1552-4469. PMC 5030153. PMID 27501395.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c Süssmuth, Roderich; Müller, Jane; Döhren, Hans von; Molnár, István (2010-12-17). "Fungal cyclooligomer depsipeptides: From classical biochemistry to combinatorial biosynthesis". Natural Product Reports. 28 (1): 99–124. doi:10.1039/C001463J. ISSN 1460-4752.
- ^ Felnagle, Elizabeth A.; Jackson, Emily E.; Chan, Yolande A.; Podevels, Angela M.; Berti, Andrew D.; McMahon, Matthew D.; Thomas, Michael G. (2008-04-01). "Nonribosomal Peptide Synthetases Involved in the Production of Medically Relevant Natural Products". Molecular Pharmaceutics. 5 (2): 191–211. doi:10.1021/mp700137g. ISSN 1543-8384. PMC 3131160. PMID 18217713.
{{cite journal}}: CS1 maint: PMC format (link) - ^ a b Hou, Xuewen; Sun, Ruonan; Feng, Yanyan; Zhang, Runfang; Zhu, Tianjiao; Che, Qian; Zhang, Guojian; Li, Dehai (2022-09-01). "Peptaibols: Diversity, bioactivity, and biosynthesis". Engineering Microbiology. 2 (3): 100026. doi:10.1016/j.engmic.2022.100026. ISSN 2667-3703.
- ^ a b Bills, Gerald; Li, Yan; Chen, Li; Yue, Qun; Niu, Xue-Mei; An, Zhiqiang (2014-09-11). "New insights into the echinocandins and other fungal non-ribosomal peptides and peptaibiotics". Natural Product Reports. 31 (10): 1348–1375. doi:10.1039/C4NP00046C. ISSN 1460-4752.
- ^ a b c d e Wiest, Aric; Grzegorski, Darlene; Xu, Bi-Wen; Goulard, Christophe; Rebuffat, Sylvie; Ebbole, Daniel J.; Bodo, Bernard; Kenerley, Charles (2002-06-07). "Identification of Peptaibols from Trichoderma virens and Cloning of a Peptaibol Synthetase *". Journal of Biological Chemistry. 277 (23): 20862–20868. doi:10.1074/jbc.M201654200. ISSN 0021-9258. PMID 11909873.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Whitmore, Lee; Wallace, B. A. (2004-01-01). "The Peptaibol Database: a database for sequences and structures of naturally occurring peptaibols". Nucleic Acids Research. 32 (Database issue): D593–D594. doi:10.1093/nar/gkh077. ISSN 0305-1048. PMID 14681489.
- ^ Marshall, G R; Hodgkin, E E; Langs, D A; Smith, G D; Zabrocki, J; Leplawy, M T (1990-01). "Factors governing helical preference of peptides containing multiple alpha,alpha-dialkyl amino acids". Proceedings of the National Academy of Sciences of the United States of America. 87 (1): 487–491. ISSN 0027-8424. PMID 2296604.
{{cite journal}}: Check date values in:|date=(help) - ^ Schweitzer-Stenner, Reinhard; Gonzales, Widalys; Bourne, Gregory T.; Feng, Jianwen A.; Marshall, Garland R. (2007-10-01). "Conformational Manifold of α-Aminoisobutyric Acid (Aib) Containing Alanine-Based Tripeptides in Aqueous Solution Explored by Vibrational Spectroscopy, Electronic Circular Dichroism Spectroscopy, and Molecular Dynamics Simulations". Journal of the American Chemical Society. 129 (43): 13095–13109. doi:10.1021/ja0738430. ISSN 0002-7863.
- ^ Rebuffat, S.; Duclohier, H.; Auvin-Guette, C.; Molle, G.; Spach, G.; Bodo, B. (1992-09). "Membrane-modifying properties of the pore-forming peptaibols saturnisporin SA IV and harzianin HA V". FEMS microbiology immunology. 5 (1–3): 151–160. doi:10.1111/j.1574-6968.1992.tb05886.x. ISSN 0920-8534. PMID 1384595.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Sansom, Mark S. P.; Kerr, Ian D.; Mellor, Ian R. (1991-11-01). "Ion channels formed by amphipathic helical peptides". European Biophysics Journal. 20 (4): 229–240. doi:10.1007/BF00183460. ISSN 1432-1017.
- ^ a b Huang, Huey W. (1996), Merz, Kenneth M.; Roux, Benoît (eds.), "Structural Basis and Energetics of Peptide Membrane Interactions", Biological Membranes: A Molecular Perspective from Computation and Experiment, Boston, MA: Birkhäuser, pp. 281–298, doi:10.1007/978-1-4684-8580-6_9, ISBN 978-1-4684-8580-6, retrieved 2022-10-10
- ^ "Support | pymol.org". pymol.org. Retrieved 2022-11-27.
- ^ Jen, W. -C.; Jones, G. A.; Brewer, D.; Parkinson, V. O.; Taylor, A. (1987-10). "The antibacterial activity of alamethicins and zervamicins". Journal of Applied Bacteriology. 63 (4): 293–298. doi:10.1111/j.1365-2672.1987.tb02705.x.
{{cite journal}}: Check date values in:|date=(help)