User:LDP02/sandbox
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Norvasc |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692044 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 64 to 90% |
| Metabolism | Hepatic |
| Elimination half-life | 30 to 50 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
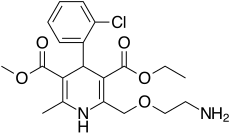
| Formula | C20H25ClN2O5 |
| Molar mass | 408.879 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Amlodipine (as besylate, mesylate or maleate) is a medication used to lower blood pressure and prevent chest pain. It belongs to a group of medications known as dihydropyridine-type calcium channel blockers.[1][2] By widening of blood vessels it lowers blood pressure. In angina, amlodipine increases blood flow to the heart muscle to relieve pain due to angina. It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[3]
Medical uses
[edit]Amlodipine is used in the management of hypertension[4] and coronary artery disease.
Contraindications
[edit]- Breastfeeding
- Cardiogenic shock
- Unstable angina
- Systolic and diastolic blood pressure below 90/60 mmHg
- Aortic stenosis
Adverse effects
[edit]Adverse side effects of the use of amlodipine may include:[5]
- Common: peripheral edema and fatigue
- Uncommon: blood disorders, development of breasts in men (gynecomastia), impotence, depression, insomnia, tachycardia, or gingival enlargement
- Rarely: erratic behavior, hepatitis, jaundice
Overdosage
[edit]Although rare,[6] amlodipine overdose toxicity can result in peripheral vasodilation, severe hypotension, and reflex tachycardia.[7][8]
Toxicity is managed with
- Fluid resuscitation[7][8][9]
- Monitoring of ECG, vitals, respiratory system, glucose, renal function, electrolytes, and urine output[7][8]
- Vasopressor administration (for unresponsive hypotension)[7]
Interactions
[edit]- CYP3A inhibitors: Co-administration of moderate to strong inhibitors of the cytochrome p450 enzyme (CYP3A4) that metabolizes amlodipine in the liver may increase the plasma concentrations of amlodipine.
- CYP3A inducers: No data is currently available for co-administration of CYP3A4 inducers with amlodipine.
- Simvastatin: Co-administration of simvastatin at doses greater than 20 mg can lead to an increase in simvastatin plasma concentrations and increase risk of myopathy.
- Sildenafil: Co-administration of sildenafil can lead to an increased risk of hypotension.
- Tacrolimus: Co-administration of tacrolimus in patients with the CYP3A5*3 genetic polymorphism may increase tacrolimus plasma concentrations.
Mechanism
[edit]Amlodipine inhibits the movement of calcium ions into vascular smooth muscle cells and cardiac muscle cells. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells. Negative inotropic effects can be detected in vitro, but such effects have not been seen in intact animals at therapeutic doses. Serum calcium concentration is not affected by amlodipine. Amlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure. As a calcium channel blocker, amlodipine is expected to inhibit the currents of L-type Cav1.3 channels in the zona glomerulosa.[10][11]
The mechanisms by which amlodipine relieves angina include:
- Stable angina: amlodipine reduces the total peripheral resistance (afterload) against which the heart works and reduces the rate pressure product, thereby lowering myocardial oxygen demand, at any given level of exercise.[12]
- Prinzmetal's angina: amlodipine blocks spasm of the coronary arteries and restores blood flow in coronary arteries and arterioles in response to calcium, potassium, epinephrine, serotonin, and thromboxane A2 analog in experimental animal models and in human coronary vessels in vitro.[13]
Metabolism
[edit]Amlodipine has been studied in healthy volunteers following oral administration of 14C-labelled drug.[14] amlodipine is well absorbed by the oral route with a mean oral bioavailability around 60%. It is metabolized in the liver to inactive metabolites via CYP3A4. The half-life of amlodipine is about 30–50 hours, and steady-state plasma concentrations are achieved after 7 to 8 days of daily dosing. Renal elimination is the major route of excretion with about 60% of an administered dose recovered in urine, largely as inactive pyridine metabolites. However, renal impairment does not significantly influence amlodipine elimination.[15]
Combination therapy
[edit]If monotherapy with Amlodipine or Candesartan is not sufficient to reach the reducing blood pressure target, a combination of Amlodipine 5 mg and Candesartan 8 mg can be effective, lowering blood pressure after 12 weeks in patients not adequately controlled by monotherapy.[16]
History
[edit]Pfizer's patent protection on Norvasc lasted until 2007; total patent expiration occurred later in 2007.[17] A number of generic versions are available. In the United Kingdom, tablets of amlodipine from different suppliers may contain different salts. The strength of the tablets is expressed in terms of amlodipine base, i.e., without the salts. Tablets containing different salts are therefore considered interchangeable.The efficacy and tolerability of a fixed-dose combination of amlodipine 5 mg and perindopril 4 mg, an angiotensin converting enzyme inhibitor, have recently been confirmed in a prospective, observational, multicentre trial of 1250 hypertensive patients.[18]
See also
[edit]References
[edit]- ^ "Amlodipine: MedlinePlus Drug Information". www.nlm.nih.gov. Retrieved 21 May 2015.
- ^ Cash, Jill C.; Glass, Cheryl Anne (10 February 2014). Family Practice Guidelines, Third Edition. Springer Publishing Company. ISBN 9780826197825.
- ^ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ^ Wang, JG (2009). "A combined role of calcium channel blockers and angiotensin receptor blockers in stroke prevention". Vascular Health and Risk Management. 5: 593–605. doi:10.2147/vhrm.s6203. PMC 2725792. PMID 19688100.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Munoz, Ricardo; Vetterly, Carol G.; Roth, Stephen J.; Cruz, Eduardo da (18 October 2007). Handbook of Pediatric Cardiovascular Drugs. Springer Science & Business Media. ISBN 9781846289538.
- ^ Aronson, J (2014). Side Effects of Drugs Annual 35. Elsevier. ISBN 978-0-444-62635-6.
- ^ a b c d "Amlodipine Package Insert" (PDF). Retrieved 2 November 2015.
- ^ a b c Pillay, V (2013). Modern Medical Toxicology 4th Ed. Jaypee. ISBN 978-93-5025-965-8.
- ^ Hui, David (2015). Approach to Internal Medicine: A Resource Book for Clinical Practice Fourth Edition. Springer. ISBN 978-3-319-11820-8.
- ^ Arcangelo, Virginia Poole; Peterson, Andrew M. (2006). Pharmacotherapeutics for Advanced Practice: A Practical Approach. Lippincott Williams & Wilkins. ISBN 9780781757843.
- ^ Ritter, James; Lewis, Lionel; Mant, Timothy; Ferro, Albert (11 December 2012). A Textbook of Clinical Pharmacology and Therapeutics, 5Ed. CRC Press. ISBN 9781444113006.
- ^ Li, Y. Robert (6 April 2015). Cardiovascular Diseases: From Molecular Pharmacology to Evidence-Based Therapeutics. John Wiley & Sons. ISBN 9780470915370.
- ^ LEARNING, JONES & BARTLETT; Bartlett, Jones and (15 July 2012). 2013 Nurse's Drug Handbook. Jones & Bartlett Publishers. ISBN 9781449642846.
- ^ Beresford AP, McGibney D, Humphrey MJ, Macrae PV, Stopher DA (February 1988). "Metabolism and kinetics of amlodipine in man". Xenobiotica. 18 (2): 245–54. doi:10.3109/00498258809041660. PMID 2967593.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brittain, Harry G. (9 May 2012). Profiles of Drug Substances, Excipients and Related Methodology. Academic Press. ISBN 9780123977564.
- ^ Kazuaki Nishio, Takeshi Kondo, Youichi Kobayashi. [www.scirp.org/Journal/PaperInformation.aspx?paperID=5091[%5b%5bPredatory publishing|predatory publisher%5d%5d] "Efficacy and Tolerability of Candesartan Cilexetil and Amlodipine in Patients with Poorly Controlled Essential Hypertension"]. Retrieved 21 July 2015.
{{cite web}}: Check|url=value (help)CS1 maint: multiple names: authors list (link) - ^ Kennedy VB (22 March 2007). "Pfizer loses court ruling on Norvasc patent". MarketWatch.
- ^ Bahl VK, Jadhav UM, Thacker HP (2009). "Management of hypertension with the fixed combination of perindopril and amlodipine in daily clinical practice: results from the STRONG prospective, observational, multicenter study". Am J Cardiovasc Drugs. 9 (3): 135–42. doi:10.1007/BF03256570. PMID 19463019. S2CID 74260893.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
[edit]Category:Amines Category:Calcium channel blockers Category:Carboxylate esters Category:Dihydropyridines Category:Ethers Category:Chloroarenes Category:World Health Organization essential medicines Category:Ethyl esters Category:Methyl esters
