User:Lack6704/sandbox
| Lack6704/sandbox | |
|---|---|
| Details | |
| Part of | Midbrain |
| Parts | anterior pretectal nucleus, medial pretectal nucleus, nucleus of the optic tract, olivary pretectal nucleus, posterior pretectal nucleus, posterior limitans, commissural pretectal area |
| Identifiers | |
| Latin | area pretectalis |
| Anatomical terms of neuroanatomy | |
The pretectal area, or pretectum, is a midbrain structure composed of seven nuclei and comprises part of the subcortical visual system. Through reciprocal bilateral projections the retina, it is primarily involved in mediating behavioral responses to acute changes in ambient light such as the pupillary light reflex, the optokinetic reflex, the accommodation reflex, and temporary changes to the circadian rhythm.[1][2][3][4][5] In addition to the pretectum's role in the visual system, the anterior pretectal nucleus has been found to mediate somatosensory and nociceptive information.[6][7]
Location and structure[edit]
The pretectum is a bilateral group of highly interconnected nuclei located near the junction of the midbrain and forebrain.[8] The pretectum is generally classified as a midbrain structure, although because of its proximity to the forebrain it is sometimes classified as part of the caudal diencephalon (forebrain).[9] Within vertebrates, the pretectum is located directly anterior to the superior colliculus and posterior to the thalamus. Vertically, it is situated above the periaquaductal grey and nucleus of the posterior commissure.[10]
Several nuclei have been identified within the pretectum, although their borders can be difficult to define and there has been debate over which regions should be included and their precise names.[1][10][11] The five primary nuclei are: the olivary pretectal nucleus (OPN), the nucleus of the optic tract (NOT), and the anterior (APN), medial (MPN), and posterior (PPN) pretectal nuclei. The NOT consists of relatively large cells and is located between the superior colliculi. The OPN is located medial to the NOT and has a tail that extends between the NOT and PPN, which is ventral to the OPN.[10] Two additional nuclei have also been identified: the posterior limitans (PLi) and the commisural pretectal area (CPA).[12] While these two regions have not been examined to the same extent as the five primary nuclei, research has shown both the PLi and CPA to receive retinal input which suggests a role in processing visual information.[13]
Inputs[edit]
The pretectum receives major binocular input from photosensitive ganglion cells in the retina. In primates these afferents mostly come from the ipsilateral retina while in rodents they project from the contralateral retina. The majority of these retino-pretectal projections go to the OPN and NOT while other pretectal nuclei receive minor retinal input in mammals including the posterior, medial, and anterior pretectal nuclei.[1][10]
The NOT receives input from several regions. From the thalamus the NOT recevies inhibitory projections from GABA producing neurons in the ipsilateral lateral geniculate nucleus and bilateral intergeniculate leaflets. The ipsilateral superficial suprachiasmatic nucleus and the medial, dorsal, and lateral terminal nuclei in the midbrain project onto the NOT. Fibers also project to the NOT from the nucleus prepositus hypoglossi in the medulla, the contralateral NOT, and from various cortical regions.[1][14]
Outputs[edit]
Many pretectal nuclei share targets of efferent projections. All pretectal nuclei, except for the OPN, project to nuclei in the thalamus, subthalamus, superior colliculus, reticular formation, pons, and inferior olive.[10] Both the OPN and the CPA have efferent projections to the Edinger-Westphal nucleus. The PPN and APN both project to the pulvinar, the lateral posterior nucleus of the thalamus, and several precerebellar nuclei. Ascending connections to the thalamus.[1]
The NOT has efferent projections to the zona incerta of the subthalamus, several nuclei of the pons, medulla, intralaminar nuclei, midbrain, and dorsal and ventral thalamic nuclei. Its bilateral inhibitory projections to the accessory optic system include connections to the lateral and medial terminal nuclei. Projections to the subthalamus are target towards the lateral geniculate nucleus and pulvinar. The NOT projects bilaterally to the superior colliculus, although the ipsilateral connections appear to be more dominant. In addition to these projections, the NOT projects to the vestibular and vestibulocerebellar relay nuclei.[1]
Function[edit]
As part of the subcortical visual system, neurons within the pretectal nuclei respond to varying intensities of illuminance and are primarily involved in mediating non-conscious behavioral responses to acute changes in light. These responses generally involve the initiations of optokinetic reflexes, although the pretctum can also regulate nociception and REM sleep.[12]
Pupillary light reflex[edit]
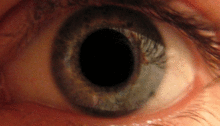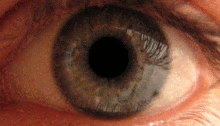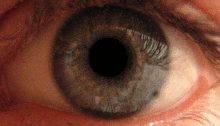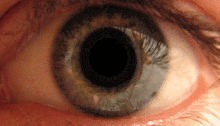
The pupillary light reflex is mediated by the pretectum.[2] This reflex is responsible for the constriction of the pupils upon light entering the eye. Several pretectal nuclei, particularly the OPN receive illuminance information from the ipsilateral retina via the optic tract. Nuclei in the OPN are known to gradually increase in activation in response to increasing levels of illuminance. This information is then relayed directly to the Edinger-Westphal nucelus which proceeds to relay the command to constrict the pupils to the pupillary sphincter via the ciliary ganglion.[4][15]
Smooth pursuit[edit]
Pretectal nuclei, particularly the NOT, are involved in coordinating eye movements during smooth pursuit. These movements allow the eye to cloesely follow a moving object and to catch up to an object after an unexpected change in direction or velocity. Direction sensitive retinal slip neurons within the NOT provide ipsiversive horizontal retinal error information to the cortex through the inferior olive. During the day this information is sensed and relayed by neurons with large receptive fields while parafoveal neurons with small receptive fields do so in the dark. It is through this pathway that the NOT is able to provide retinal error information to guide eye movements. [14][16][1] In addition to its role in maintaining smooth pursuit, the pretectum is activated during the optokinetic nystagmus in which the eye returns to a central, forward-facing position after an object it was following passes out of the field of vision.[17]
Accommodation reflex[edit]
Part of the pretectum, particularly the NOT and PPN, are implicated in the accommodation reflex by which the eye maintains focus.[18] Prioprioceptive information from the retina reaches the pretectum via the occulomotor nerve and the trigeminal nerve. From that point the mechanism by which the eye maintains focus through muscular contractions of the retina is similar to that of the pupillary light reflex.[4]
Antinociception[edit]
The APN is participates in the active diminishing of the perception of pain stimuli (antinociception).[7] Although the mechanism by which the APN alters an organism's response to painful stimuli is not fully known, research has shown that activity in the ventral APN triggers cholinergic and serotonergic neurons. These neurons activate descending pathways that synapse in the spinal cord and inhibit nociceptive cells in the dorsal horn.[19][20] In addition to its direct antionociceptive mechanism, the APN projects onto brain regions that, through connections to the somatosensory cortex, regulate the perception of painful stimuli. Two of these regions that the APN is known to project to are the zona incerta and posterior thalamic nucleus. Regions of the APN may be specialized to respond to different types of pain. Research has found that the dorsal APN best diminished the perception of brief pain whereas the ventral APN reduced the perception of chronic pain.[21] Because of its role in the reduction of chronic pain, abnormal activity of the APN is thought to be implicated in central pain syndrome.[22]
REM sleep[edit]
Multiple pretectal nuclei may be involved in regulating REM sleep and sleep behaviors. Research has shown that the pretectum, in conjuction with the superior colliculus, may be responsible for causing non-circadian changes in REM sleep behaviors.[23] Pretectal nuclei receiving retinal input, in particular the NOT and the PPN, have been shown to be partially responsible for initiating REM sleep in albino rats.[5] The discovery of projections from the pretectum to several thalamic nuclei involved in cortical activation during REM sleep, specifically the projection to the superchiasmatic nucleus which is part of a known REM sleep regulatory mechanism, supports this hypothesis.[12]
References[edit]
- ^ a b c d e f g Gamlin, PD (2006). "The pretectum: connections and oculomotor-related roles". Progress in brain research. 151: 379–405. PMID 16221595.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b MAGOUN, H. W. (1). "THE CENTRAL PATH OF THE LIGHT REFLEX: A STUDY OF THE EFFECT OF LESIONS". Archives of Ophthalmology. 13 (5): 791–811. doi:10.1001/archopht.1935.00840050069006.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Neuhuber, Winfried (16). "Autonomic control of the eye and the iris". Autonomic Neuroscience. 165 (1): 67–79. doi:10.1016/j.autneu.2010.10.004. Retrieved 15 May 2012.
{{cite journal}}: Check|doi=value (help); Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b c Donkelaar, Hans (2012). Clinical neuroanatomy brain circuitry and its disorders (1. Ed. ed.). Berlin: Springer. p. 343. ISBN 978-3642191336.
{{cite book}}:|edition=has extra text (help) - ^ a b Miller, AM (1999). "The pretectum mediates rapid eye movement sleep regulation by light". Behavioral neuroscience. 113 (4): 755–65. PMID 10495083.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Bosman, LW (1). "Anatomical pathways involved in generating and sensing rhythmic whisker movements". Frontiers in integrative neuroscience. 5: 53. doi:10.3389/fnint.2011.00053. PMID 22065951.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)CS1 maint: unflagged free DOI (link) - ^ a b Reis, GM (2012 Apr 30). "μ(1)- and 5-HT(1)-dependent mechanisms in the anterior pretectal nucleus mediate the antinociceptive effects of retrosplenial cortex stimulation in rats". Life sciences. PMID 22575824.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Millodot, Michel (2009). Dictionary of optometry and visual science (7th ed. ed.). Edinburgh: Elsevier/Butterworth-Heinemann. ISBN 978-0-7020-2958-5.
{{cite book}}:|edition=has extra text (help) - ^ Ramachandran, Vilayanur S. (2002). Encyclopedia of the human brain. Amsterdam: Acad. Press. ISBN 0122272102.
- ^ a b c d e Nicholson, R. Nieuwenhuys ; H. J. ten Donkelaar ; C. (1998). The central nervous system of vertebrates. Berlin [u.a.]: Springer. pp. 1812–1817. ISBN 3540560130.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Borostyánkői-Baldauf, Z (1 March 2002). "Parcellation of the human pretectal complex: a chemoarchitectonic reappraisal". Neuroscience. 110 (3): 527–540. doi:10.1016/S0306-4522(01)00462-6.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Prichard, J.R (1). "Fos immunoreactivity in rat subcortical visual shell in response to illuminance changes". Neuroscience. 114 (3): 781–793. doi:10.1016/S0306-4522(02)00293-2.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Morin, LP (1997). "Neuropeptide Y and enkephalin immunoreactivity in retinorecipient nuclei of the hamster pretectum and thalamus". Visual neuroscience. 14 (4): 765–77. PMID 9279004.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b Ono, S (2010). "Visual error signals from the pretectal nucleus of the optic tract guide motor learning for smooth pursuit". Journal of neurophysiology. 103 (5): 2889–99. PMID 20457849.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Gamlin, PD (1995). "Luminance neurons in the pretectal olivary nucleus mediate the pupillary light reflex in the rhesus monkey". Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 106 (1): 169–76. PMID 8542972.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Collewijn, H (1975). "Oculomotor areas in the rabbits midbrain and pretectum". Journal of neurobiology. 6 (1): 3–22. PMID 1185174.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Dieterich, M. (1). "Brain stem and cerebellar activation during optokinetic stimulation". Clinical Neurophysiology. 118 (12): 2811–2812. doi:10.1016/j.clinph.2007.09.019.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Konno, S (1997 Jan-Feb). "Accommodation and pupilloconstriction areas in the cat midbrain". Japanese journal of ophthalmology. 41 (1): 43–8. PMID 9147188.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Villarreal, Cristiane Flora (1). "Modulation of persistent nociceptive inputs in the anterior pretectal nucleus of the rat". Pain. 132 (1–2): 42–52. doi:10.1016/j.pain.2007.01.021.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Villarreal, Cristiane F (1). "Involvement of the anterior pretectal nucleus in the control of persistent pain: a behavioral and c-Fos expression study in the rat". Pain. 103 (1–2): 163–174. doi:10.1016/S0304-3959(02)00449-9.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Villarreal, CF (2004). "Antinociception induced by stimulating the anterior pretectal nucleus in two models of pain in rats". Clinical and experimental pharmacology & physiology. 31 (9): 608–13. PMID 15479168.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Murray, PD (2010). "Abnormal anterior pretectal nucleus activity contributes to central pain syndrome". Journal of neurophysiology. 103 (6): 3044–53. PMID 20357063.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Miller, AM (1998 Jul 21). "The superior colliculus-pretectum mediates the direct effects of light on sleep". Proceedings of the National Academy of Sciences of the United States of America. 95 (15): 8957–62. PMID 9671786.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help)

