User:Mr. Ibrahem/Colistin
 | |
| Clinical data | |
|---|---|
| Trade names | Xylistin, Coly Mycin M, others |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Topical, by mouth, intravenous, inhaled |
| Drug class | Antibiotic |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 0% |
| Elimination half-life | 5 hours |
| Identifiers | |
| |
| Chemical and physical data | |
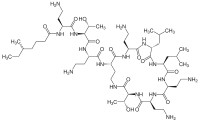
| Formula | C52H98N16O13 |
| Molar mass | 1155.455 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Colistin, also known as polymyxin E, is an antibiotic used as a last-resort for multidrug-resistant Gram negative infections including pneumonia.[1][2] These may involve bacteria such as Pseudomonas aeruginosa, Klebsiella pneumoniae, or Acinetobacter.[5] It comes in a form which can be injected into a vein or muscle or inhaled, known as colistimethate sodium and one which is applied to the skin or taken by mouth, known as colistin sulfate.[6] Resistance to colistin is beginning to appear as of 2017.[7]
Common side effects of the injectable form include kidney and neurological problems.[2] Other serious side effects may include anaphylaxis, muscle weakness, and Clostridium difficile-associated diarrhea.[2] The inhaled form may result in constriction of the bronchioles.[2] It is unclear if use during pregnancy is safe for the baby.[8] Colistin is in the polymyxin class of medications.[2] It works by breaking down the cytoplasmic membrane which generally results in bacterial cell death.[2]
Colistin was discovered in 1947 and colistimethate sodium was approved for medical use in the United States in 1970.[5][2] It is on the World Health Organization's List of Essential Medicines.[9] It is available as a generic medication.[10] Dosing is complicated and inconsistent.[11] In the United Kingdom it costs the NHS £18 for 10 vials of the injectable form (1 million units in each vial) as of 2021.[10] In the United States the dose is expressed as 'Colistin base' 150mg and costs about US$24 as of 2019.[12] It is derived from bacteria of the Paenibacillus type.[6]
References[edit]
- ^ a b Pogue, JM; Ortwine, JK; Kaye, KS (April 2017). "Clinical considerations for optimal use of the polymyxins: A focus on agent selection and dosing". Clinical Microbiology and Infection. 23 (4): 229–233. doi:10.1016/j.cmi.2017.02.023. PMID 28238870.
- ^ a b c d e f g h i "Colistimethate Sodium Monograph for Professionals". Drugs.com. Archived from the original on 6 November 2019. Retrieved 6 November 2019.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 7 August 2020. Retrieved 10 September 2020.
- ^ a b "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 27 November 2020. Retrieved 10 September 2020.
- ^ a b Falagas ME, Grammatikos AP, Michalopoulos A (October 2008). "Potential of old-generation antibiotics to address current need for new antibiotics". Expert Review of Anti-infective Therapy. 6 (5): 593–600. doi:10.1586/14787210.6.5.593. PMID 18847400.
- ^ a b Kaye, Keith S; Kaye, Donald (2010). "32. Polymyxins (polymyxin B and colistin)". In Bennett., John E.; Dolin, Raphael; Blaser, Martin J.; Mandell, Gerald L. (eds.). Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. Vol. 1 (7th ed.). Philadelphia: Elsevier Churchill Livingstone. pp. 469–470. ISBN 9780443068393. Archived from the original on 2021-08-28. Retrieved 2020-06-05.
- ^ Caniaux, I; van Belkum, A; Zambardi, G; Poirel, L; Gros, MF (March 2017). "MCR: modern colistin resistance" (PDF). European Journal of Clinical Microbiology & Infectious Diseases. 36 (3): 415–420. doi:10.1007/s10096-016-2846-y. PMID 27873028. Archived (PDF) from the original on 2018-07-23. Retrieved 2019-11-23.
- ^ "Colistimethate (Coly Mycin M) Use During Pregnancy". Drugs.com. Archived from the original on 6 November 2019. Retrieved 11 November 2019.
- ^ Organization, World Health (2019). "World Health Organization model list of essential medicines: 21st list 2019" (Document). hdl:10665/325771.
{{cite document}}: Cite document requires|publisher=(help) - ^ a b BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 588-589. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ^ Nation, Roger L; Li, Jian (December 2009). "Colistin in the 21st Century". Current opinion in infectious diseases. 22 (6): 535–543. doi:10.1097/QCO.0b013e328332e672. ISSN 0951-7375. PMID 19797945. Archived from the original on 2018-02-06. Retrieved 2021-02-02.
- ^ "Colistimethate Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 6 November 2019. Retrieved 11 November 2019.
