User:Mr. Ibrahem/Glimepiride
 | |
| Clinical data | |
|---|---|
| Trade names | Amaryl, other |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696016 |
| License data | |
| Routes of administration | By mouth (tablets) |
| Drug class | Sulfonylurea[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Protein binding | >99.5% |
| Metabolism | Liver (1st stage through CYP2C9) |
| Elimination half-life | 5–8 hours |
| Excretion | Urine (~60%), feces (~40%) |
| Identifiers | |
| |
| Chemical and physical data | |
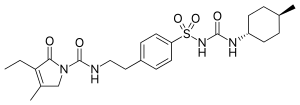
| Formula | C24H34N4O5S |
| Molar mass | 490.62 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 207 °C (405 °F) |
| |
| |
| | |
Glimepiride, sold under the trade name Amaryl among others, is a medication used to treat diabetes mellitus type 2.[2][3] It is less preferred than metformin.[2] Use is recommended together with diet and exercise.[2] It is taken by mouth.[2] Glimepiride takes up to three hours for maximum effect and lasts for about a day.[2]
Common side effects include headache, nausea, and dizziness.[2] Serious side effects may include low blood sugar.[2] Use during pregnancy and breastfeeding is not recommended.[5] It works mainly by increasing the amount of insulin released from the pancreas.[2] It is classified as a second-generation sulfonylurea.[1]
Glimepiride was patented in 1979 and approved for medical use in 1995.[6] It is available as a generic medication.[3] A month supply in the United Kingdom costs the NHS about 7.00 £ per month as of 2019.[3] In the United States, the wholesale cost of this amount is about 2.15 USD.[7] In 2017, it was the 64th most commonly prescribed medication in the United States, with more than twelve million prescriptions.[8][9]
References[edit]
- ^ a b Davis SN (2004). "The role of glimepiride in the effective management of Type 2 diabetes". J. Diabetes Complicat. 18 (6): 367–76. doi:10.1016/j.jdiacomp.2004.07.001. PMID 15531188.
- ^ a b c d e f g h i j "Glimepiride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- ^ a b c d British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 693. ISBN 9780857113382.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 22 January 2021. Retrieved 7 September 2020.
- ^ "Glimepiride Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 449. ISBN 9783527607495. Archived from the original on 2016-12-27. Retrieved 2019-02-28.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ^ "Glimepiride - Drug Usage Statistics". ClinCalc. Archived from the original on 29 November 2020. Retrieved 11 April 2020.
