User:Mr. Ibrahem/Linaclotide
 | |
| Clinical data | |
|---|---|
| Trade names | Linzess, Constella |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613007 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Guanylate cyclase-C (GC-C) agonist[2] |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| Chemical and physical data | |
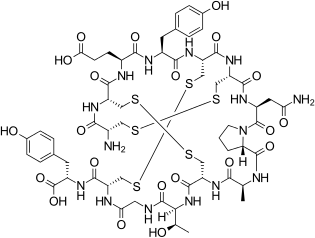
| Formula | C59H79N15O21S6 |
| Molar mass | 1526.73 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Linaclotide, sold under the brand name Linzess and Constella, is a medication used to treat irritable bowel syndrome with constipation and chronic constipation of unknown cause.[2] It is taken by mouth.[2]
Common side effects include diarrhea, abdominal pain, and intestinal gas.[3][4] Rarely this may lead to dehydration, low potassium, and low blood pressure with standing.[3] It works by attaching to guanylate cyclase C receptors in the intestines which results in greater fluid release.[3]
Linaclotide was approved for medical use in the United States and Europe in 2012.[3][2] In 2017, it was the 257th most commonly prescribed medication in the United States, with more than one million prescriptions.[5][6] In the United Kingdom 4 weeks of medication costs the NHS about £38 as of 2021.[7] This amount in the United States costs about 430 USD.[8]
References[edit]
- ^ Oh, See Arr (August 17, 2011). "Macrocycle Milestone for Ironwood Pharma". The Haystack. Archived from the original on 5 June 2019. Retrieved 11 February 2017 – via CENBlog.org.
- ^ a b c d e "Linaclotide Monograph for Professionals". Drugs.com. Archived from the original on 19 October 2021. Retrieved 23 November 2021.
- ^ a b c d e "Constella". Archived from the original on 22 June 2021. Retrieved 23 November 2021.
- ^ a b c "DailyMed - LINZESS- linaclotide capsule, gelatin coated". dailymed.nlm.nih.gov. Archived from the original on 29 March 2021. Retrieved 23 November 2021.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ^ "Linaclotide - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
- ^ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 52. ISBN 978-0857114105.
- ^ "Linaclotide Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 23 November 2021.
