User:Mr. Ibrahem/Repaglinide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Prandin, GlucoNorm, NovoNorm, Enyglid, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a600010 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Meglitinide[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 56% (by mouth) |
| Protein binding | >98% |
| Metabolism | Liver oxidation and glucuronidation (CYP3A4-mediated) |
| Elimination half-life | 1 hour |
| Excretion | Fecal (90%) and kidney (8%) |
| Identifiers | |
| |
| Chemical and physical data | |
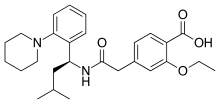
| Formula | C27H36N2O4 |
| Molar mass | 452.595 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 126 to 128 °C (259 to 262 °F) |
| |
| |
| (verify) | |
Repaglinide, sold under the brand name Prandin among others, is a medication used to control blood sugar in type 2 diabetes.[2] It is used together with diet and exercise.[2] It is taken by mouth.[2] Maximum effects occur in about 3 hours.[1]
Common side effects include low blood sugar, headache, joint pain, nausea, and back pain.[2] Use in pregnancy is not generally recommended.[1] It is in the meglitinide class of medication and works by promoting insulin release from the pancreas in response to blood sugar.[1]
Repaglinide was developed in 1983 and approved for medical use in the United States in 1997.[2][3] It is available as a generic medication.[4] In the United Kingdom a month of medication costs around £5 as of 2021.[4] This amount in the United States is about 25 USD.[5]
References[edit]
- ^ a b c d "Repaglinide Monograph for Professionals". Drugs.com. Archived from the original on 17 May 2021. Retrieved 16 October 2021.
- ^ a b c d e f g h "DailyMed - REPAGLINIDE - repaglinide tablet". dailymed.nlm.nih.gov. Archived from the original on 2015-10-19. Retrieved 2015-11-04.
- ^ Engel, Jürgen; Kleemann, Axel; Kutscher, Bernhard; Reichert, Dietmar (14 May 2014). Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the most relevant APIs. Georg Thieme Verlag. p. 1199. ISBN 978-3-13-179275-4. Archived from the original on 17 October 2021. Retrieved 16 October 2021.
- ^ a b BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 740. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ^ "Repaglinide Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 6 August 2020. Retrieved 16 October 2021.
