User:Praseodymium-141/Praseodymium compounds
Oxides[edit]
Praseodymium can form oxides in many different ways, although the only oxides that are stable at room temperature are Pr2O3, Pr6O11 and PrO2. Praseodymium(III) oxide is a green powder that forms hexagonal crystals,[1] and crystallizes in the manganese(III) oxide or bixbyite structure.[2] Praseodymium(IV) oxide can be produced by boiling Pr6O11 in water or acetic acid:[3]
- Pr6O11 + 3 H2O → 4 PrO2 + 2 Pr(OH)3
Praseodymium(III,IV) oxide is the most stable form of the praseodymium oxides at ambient temperature and pressure.[4] It is soluble in water[5] and has a cubic fluorite structure.[6] It can be prepared via solid-state methods such as thermolysis, molten salt method, calcination or precipitation.[6][4][7]
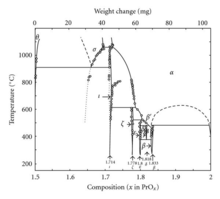
In addition to Pr6O11, praseodymium also forms a system of oxides at different phases:
| Value | Formula | Phase | x in PrOx | Average oxidation state of Pr |
|---|---|---|---|---|
| 4 | Pr2O3 | θ | 1.5 | 3 |
| 7 | Pr7O12 | ι | 1.714 | 3.428 |
| 9 | Pr9O16 | ζ | 1.778 | 3.556 |
| 10 | Pr5O9 | ε | 1.8 | 3.6 |
| 11 | Pr11O20 | δ | 1.818 | 3.636 |
| 12 | Pr6O11 | β | 1.833 | 3.667 |
| ∞ | PrO2 | 2 | 4 |
Halides[edit]

Praseodymium metal reacts with all the stable halogens to form green trihalides:[8]
- 2 Pr (s) + 3 F2 (g) → 2 PrF3 (s)
- 2 Pr (s) + 3 Cl2 (g) → 2 PrCl3 (s)
- 2 Pr (s) + 3 Br2 (g) → 2 PrBr3 (s)
- 2 Pr (s) + 3 I2 (g) → 2 PrI3 (s)
Praseodymium(III) fluoride is the most stable fluoride of praseodymium. It can be prepared the reaction between praseodymium(III) nitrate and sodium fluoride will produce praseodymium(III) fluoride as a green crystalline solid.[9] Praseodymium(III) chloride is a light green solid that can be prepared by treating praseodymium metal with hydrogen chloride.[10][11] It is usually purified by vacuum sublimation.[12] Praseodymium(III) chloride is Lewis acidic, classified as "hard" according to the HSAB concept. Rapid heating of the hydrate may cause small amounts of hydrolysis.[12] PrCl3 forms a stable Lewis acid-base complex K2PrCl5 by reaction with potassium chloride; this compound shows interesting optical and magnetic properties.[13]
Praseodymium(III) bromide is the only stable bromide of praseodymium. It adopts the UCl3 crystal structure.[14] The praseodymium ions are 9-coordinate and adopt a tricapped trigonal prismatic geometry.[15] The praseodymium–bromine bond lengths are 3.05 Å and 3.13 Å.[16] Praseodymium(III) iodide can be prepared by heating praseodymium and iodine in an inert atmosphere produces praseodymium(III) iodide,[17] or by heating praseodymium with mercury(II) iodide.[18] It forms orthorhombic crystals which are hygroscopic.[17] It crystallizes in the PuBr3 type[18][19] with space group Cmcm (No. 63) with a = 4.3281(6) Å, b = 14.003(6) Å and c = 9.988(3) Å.[20]
The tetrafluoride, PrF4, is also known, and is produced by reacting a mixture of sodium fluoride and praseodymium(III) fluoride with fluorine gas, producing Na2PrF6, following which sodium fluoride is removed from the reaction mixture with liquid hydrogen fluoride.[15] Additionally, praseodymium forms a bronze diiodide; like the diiodides of lanthanum, cerium, and gadolinium, it is a praseodymium(III) electride compound.[15]
Other inorganic compounds[edit]
References[edit]
- ^ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 478, 523, ISBN 0-8493-0594-2
- ^ Dabrowski, Jarek; Weber, Eicke R. (2004), Predictive Simulation of Semiconductor Processing, Springer, p. 264, ISBN 978-3-540-20481-7, retrieved 2009-03-18
- ^ Chinese: 《无机化学丛书》.第七卷 钪 稀土元素. 科学出版社. 1.3.4 氧化态+4的化合物. P193~195
- ^ a b Zinatloo-Ajabshir, Sahar; Salavati-Niasari, Masoud (2015). "Novel poly(ethyleneglycol)-assisted synthesis of praseodymium oxide nanostructures via a facile precipitation route". Ceramics International. 41 (1): 567–575. doi:10.1016/j.ceramint.2014.08.105.
- ^ "Praseodymium Oxide (Pr6O11)". www.reade.com. Retrieved 2018-03-15.
- ^ a b Matović, Branko; Pantić, Jelena; Prekajski, Marija; Stanković, Nadežda; Bučevac, Dušan; Minović, Tamara; Čebela, Maria (2013). "Synthesis and characterization of Pr6O11 nanopowders". Ceramics International. 39 (3): 3151–3155. doi:10.1016/j.ceramint.2012.09.098.
- ^ Shamshi Hassan, M., Shaheer Akhtar, M., Shim, KB. et al. Morphological and Electrochemical Properties of Crystalline Praseodymium Oxide Nanorods. Nanoscale Res Lett 5, 735 (2010). https://doi.org/10.1007/s11671-010-9547-8
- ^ "Chemical reactions of Praseodymium". Webelements. Retrieved 9 July 2016.
- ^ Lin Ma, Wei-Xiang Chen, Yi-Fan Zheng, Jie Zhao, Zhude Xu (May 2007). "Microwave-assisted hydrothermal synthesis and characterizations of PrF3 hollow nanoparticles". Materials Letters. 61 (13): 2765–2768. doi:10.1016/j.matlet.2006.04.124. Retrieved 2019-03-26.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ J. Cybinska, J. Sokolnicki, J. Legendziewicz, G. Meyer, Journal of Alloys and Compounds, 341, 115–123 (2002).
- ^ L. F. Druding, J. D. Corbett, "Lower Oxidation States of the Lanthanides. Neodymium(II) Chloride and Iodide", J. Am. Chem. Soc. 83, 2462 (1961); J. D. Corbett, Rev. Chim. Minerale 10, 239 (1973),
- ^ a b F. T. Edelmann, P. Poremba, in: Synthetic Methods of Organometallic and Inorganic Chemistry, (W. A. Herrmann, ed.), Vol. 6, Georg Thieme Verlag, Stuttgart, 1997.
- ^ J. Cybinska, J. Sokolnicki, J. Legendziewicz, G. Meyer, Journal of Alloys and Compounds, 341, 115–123 (2002).
- ^ Wells, A. F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford University Press. p. 421. ISBN 978-0-19-965763-6.
- ^ a b c Greenwood and Earnshaw, p. 1240–2
- ^ Schmid, B.; Hälg, B.; Furrer, A. (1987). "Structure and crystal fields of PrBr3 and PrCl3: A neutron study". J. Appl. Phys. 61: 3426–3428. doi:10.1063/1.338741.
- ^ a b Haynes, William M. (2016-06-22). CRC Handbook of Chemistry and Physics. CRC Press. pp. 2016–2652. ISBN 978-1-4987-5429-3.
- ^ a b Asprey, L. B.; Keenan, T. K.; Kruse, F. H. (1964). "Preparation and Crystal Data for Lanthanide and Actinide Triiodides". Inorg. Chem. 3 (8): 1137–1141. doi:10.1021/ic50018a015.
- ^ Wells, A. F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford University Press. p. 421. ISBN 978-0-19-965763-6.
- ^ E. Warkentin, H. Bärnighausen (1979), "Die Kristallstruktur von Praseodymdiiodid (Modifikation V)", Zeitschrift für anorganische und allgemeine Chemie (in German), vol. 459, no. 1, pp. 187–200, doi:10.1002/zaac.19794590120
