User:ReeceVesperman/sandbox
 | This is a user sandbox of ReeceVesperman. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
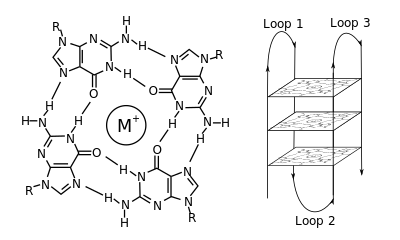
In molecular biology, G-quadruplexes (also known as G4-DNA) are tertiary structures formed in nucleic acids by sequences that are rich in guanine. Four guanine bases can associate through Hoogsteen hydrogen bonding to form a square planar structure called a guanine tetrad, and two or more guanine tetrads can stack on top of each other to form a G-quadruplex held to together by pi interactions.[1] The quadruplex structure is further stabilized by the presence of cations, especially potassium and sodium, which sits in a central channel between each pair of tetrads.[2] They can be formed of DNA, RNA, LNA, and PNA, and may be intramolecular, bimolecular, or tetramolecular.[3] Depending on the direction of the strands or parts of a strand that form the tetrads, structures may be described as parallel or antiparallel.

Quadruplex topology
[edit]G-quadraplex structures vary due to the linear arrangement of guanine bases by sodium and potassium cations through the central G-quadraplex core and loops along its exterior.[4] The length of the nucleic acid sequences involved in tetrad formation determines how the quadruplex folds. Short sequences, consisting of only a single contiguous (adjacent) run of three or more guanine bases, require four individual strands to form a quadruplex. Such a quadruplex is described as tetramolecular, reflecting the requirement of four separate strands. Longer sequences, which contain two contiguous runs of three or more guanine bases, where the guanine regions are separated by one or more bases, only require two such sequences to provide enough guanine bases to form a quadruplex. These structures, formed from two separate G-rich strands, are termed bimolecular quadruplexes. Finally, sequences which contain four distinct runs of guanine bases can form stable quadruplex structures by themselves, and a quadruplex formed entirely from a single strand is called an intramolecular quadruplex.[5]
Depending on how the individual runs of guanine bases are arranged in a bimolecular or intramolecular quadruplex, a quadruplex can adopt one of a number of topologies with varying loop configurations.[6] If the 5' – 3’ direction of all the strands is the same, the quadruplex is termed parallel; that is, all the strands of DNA are proceeding in the same direction. For intramolecular quadruplexes, this means that any loop regions present must be of the propeller type, positioned to the sides of the quadruplex. If one or more of the runs of guanine bases has a 5’-3’ direction opposite to the other runs of guanine bases, the quadruplex is said to have adopted an antiparallel topology. The loops joining runs of guanine bases in intramolecular antiparallel quadruplexes are either diagonal, joining two diagonally opposite runs of guanine bases, or lateral (edgewise) type loops, joining two adjacent runs of guanines.
In quadruplexes formed from double-stranded DNA, possible interstrand topologies have also been discussed [7] .[8] Interstrand quadruplexes contain guanines that originate from both strands of dsDNA.
Telomeric quadruplexes
[edit]Telomeric repeats in a variety of organisms have been shown to form these structures in vitro, and subsequently they have also been shown to form in vivo.[9][10] The human telomeric repeat (which is the same for all vertebrates) consists of many repeats of the sequenced (GGTTAG), and the quadruplexes formed by this structure have been well studied by NMR and X-ray crystallography determination. The formation of these quadruplexes in telomeres has been shown to decrease the activity of the enzyme telomerase, which is responsible for maintaining length of telomeres and is involved in around 85% of all cancers. [11]This is an active target of drug research and discovery, including telomestatin. [1]
Non-telomeric quadruplexes
[edit]Recently, there has been increasing interest in quadruplexes in locations other than at the telomere. For example, the proto-oncogene c-myc was shown to form a quadruplex in a nuclease hypersensitive region critical for gene activity.[12][13] Since then, many other genes have been shown to have G-quadruplexes in their promoter regions, including the chicken β-globin gene, human ubiquitin-ligase RFP2 and the proto-oncogenes c-kit, bcl-2, VEGF, H-ras and N-ras. This list is ever-increasing.
Genome-wide surveys based on a quadruplex folding rule have been performed, which have identified 376,000 Putative Quadruplex Sequences (PQS) in the human genome, although not all of these probably form in vivo.[14] A similar study has identified putative G-quadruplexes in prokaryotes.[15] There are several possible models for how quadruplexes could influence gene activity, either by upregulation or downregulation. One model is shown below, with G-quadruplex formation in or near a promoter blocking transcription of the gene, and hence de-activating it. In another model, quadruplex formed at the non-coding DNA strand helps to maintain an open conformation of the coding DNA strand and enhance an expression of the respective gene.

Quadruplex function
[edit]Nucleic acid quadruplexes have been described as "structures in search of a function",[5] as for many years there was minimal evidence pointing towards a biological role for these structures. It has been suggested that quadruplex formation plays a role in immunoglobulin heavy chain switching.[17] As cells have evolved mechanisms for resolving (i.e., unwinding) quadruplexes that form, quadruplex formation may be potentially damaging for a cell; for example, the helicases WRN and Bloom syndrome protein have a high affinity for resolving G4 DNA.[18] More recently, there are many studies that implicate quadruplexes in both positive and negative transcriptional regulation, and in allowing programmed recombination of immunologlobin heavy genes and the pilin antigenic variation system of the pathogenic Neisseria.[19] It has been suggested that G-quadruplexes play a role in gene regulation due to their prevalence in differing gene regulating elements and because of their ability to promote the recruitment of specific transcription factors.[20]The roles of quadruplex structure in translation control are not as well explored, but it is believed that 5'-UTR G-quadruplexes act to inhibit, and in some cases promote, cap-dependent and cap-independent translation initiation by altering RNA stability. [21] The direct visualization of quadruplex structures in human cells [22] has provided an important confirmation of their existence. The potential positive and negative roles of quadruplexes in telomere replication and function remains controversial. T-loops and G-quadruplexes are described as the two tertiary DNA structures that protect telomere ends and regulate telomere length.[23]
Ligands which bind quadruplexes
[edit]One way of inducing or stabilizing G-quadruplex formation, is to introduce a molecule which can bind to the G-quadruplex structure, and a number of ligands, both small molecules and proteins, have been developed which can do so. In addition, several naturally occurring small organic molecules and ligands have also been found to non-cavalently interact with G-quadruplexes in biological processes such as tetrad stacking, loop-binding, and loop binding. [24][25]This has become an increasingly large field of research.
A number of naturally occurring proteins have been identified which selectively bind to G-quadruplexes. These include the helicases implicated in Bloom's and Werner's syndromes and the Saccharomyces cerevisiae protein RAP1. An artificially derived three zinc finger protein called Gq1, which is specific for G-quadruplexes has also been developed, as have specific antibodies.
Cationic porphyrins have been shown to bind intercalatively with G-quadruplexes, as well as the molecule telomestatin.[25]
The proteins and ligands that bind to G-quadruplexes affect the overall stability of the structures. In-vitro and established system studies have revealed that G-quadruplex structures change dramatically depending on the relative concentration of other quadruplexes, nearby proteins, and binding ligands. Because of this, it has been suggested that G-quadruplex structures bear a dynamic stability when in living organisms due to the constantly changing chemical, topographical, physiological environment found in established systems. [26]
Quadruplex prediction techniques
[edit]Identifying and predicting sequences which have the capacity to form quadruplexes is an important tool in further understanding their role. Generally, a simple pattern match is used for searching for possible intrastrand quadruplex forming sequences: d(G3+N1-7G3+N1-7G3+N1-7G3+), where N is any base (including guanine).[27] This rule has been widely used in on-line algorithms.
Notes
[edit]- ^ "Off-Campus Access | FSU Libraries". www.sciencedirect.com.proxy.lib.fsu.edu. Retrieved 2017-02-17.
- ^ Largy, Eric; Mergny, Jean-Louis; Gabelica, Valérie (2016). "Chapter 7. Role of Alkali Metal Ions in G-Quadruplex Nucleic Acid Structure and Stability". In Astrid, Sigel; Helmut, Sigel; Roland K.O., Sigel (eds.). The Alkali Metal Ions: Their Role in Life. Metal Ions in Life Sciences. Vol. 16. Springer. pp. 203–258. doi:10.1007/978-4-319-21756-7_7.
- ^ Bochman, Matthew L.; Paeschke, Katrin; Zakian, Virginia A. (2012). DNA secondary structures: stability and function of G-quadruplex structures. Nature Reviews Genetics. Vol. 13. Nature Publishing Group. pp. 770–780. doi:10.1038/nrg3296.
- ^ Balasubramanian, Shankar; Hurley, Laurence H.; Neidle, Stephen (2017-02-17). "Targeting G-quadruplexes in gene promoters: a novel anticancer strategy?". Nature reviews. Drug discovery. 10 (4): 261–275. doi:10.1038/nrd3428. ISSN 1474-1776. PMC 3119469. PMID 21455236.
{{cite journal}}: CS1 maint: PMC format (link) - ^ a b Simonsson, T. (2001). "G-Quadruplex DNA Structures Variations on a Theme". Biological Chemistry. 382 (4): 621–628. doi:10.1515/BC.2001.073. PMID 11405224.
- ^ Burge, S.; Parkinson, G. N.; Hazel, P.; Todd, A. K.; Neidle, S. (2006). "Quadruplex DNA: Sequence, topology and structure". Nucleic Acids Research. 34 (19): 5402–5415. doi:10.1093/nar/gkl655. PMC 1636468. PMID 17012276.
- ^ Cao, K.; Ryvkin, P.; Johnson, FB. (2012). "Computational detection and analysis of sequences with duplex-derived interstrand G-quadruplex forming potential". Methods. 57 (1): 3–10. doi:10.1016/j.ymeth.2012.05.002. PMC 3701776. PMID 22652626.
- ^
Kudlicki, A. (2016). "G-Quadruplexes Involving Both Strands of Genomic DNA Are Highly Abundant and Colocalize with Functional Sites in the Human Genome". PLoS ONE. 11 (1): e0146174. doi:10.1371/journal.pone.0146174. PMC 4699641. PMID 26727593.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Schaffitzel, C; Berger, I; Postberg, J; Hanes, J; Lipps, H. J.; Plückthun, A (2001). "In vitro generated antibodies specific for telomeric guanine-quadruplex DNA react with Stylonychia lemnae macronuclei". Proceedings of the National Academy of Sciences. 98 (15): 8572–7. doi:10.1073/pnas.141229498. PMC 37477. PMID 11438689.
- ^ Paeschke, K.; Simonsson, T.; Postberg, J.; Rhodes, D.; Lipps, H. J. (2005). "Telomere end-binding proteins control the formation of G-quadruplex DNA structures in vivo". Nature Structural & Molecular Biology. 12 (10): 847–854. doi:10.1038/nsmb982. PMID 16142245.
- ^ Neidle, Stephen (2010-03-01). "Human telomeric G-quadruplex: The current status of telomeric G-quadruplexes as therapeutic targets in human cancer". FEBS Journal. 277 (5): 1118–1125. doi:10.1111/j.1742-4658.2009.07463.x. ISSN 1742-4658.
- ^ Simonsson, T.; Pecinka, P.; Kubista, M. (1998). "DNA tetraplex formation in the control region of c-myc". Nucleic Acids Research. 26 (5): 1167–1172. doi:10.1093/nar/26.5.1167. PMC 147388. PMID 9469822.
- ^ Siddiqui-Jain, A.; Grand, C. L.; Bearss, D. J.; Hurley, L. H. (2002). "Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription". Proceedings of the National Academy of Sciences. 99 (18): 11593–11598. doi:10.1073/pnas.182256799. PMC 129314. PMID 12195017.
- ^ Huppert, J. L.; Balasubramanian, S. (2005). "Prevalence of quadruplexes in the human genome". Nucleic Acids Research. 33 (9): 2908–2916. doi:10.1093/nar/gki609. PMC 1140081. PMID 15914667.
- ^ Rawal, P.; Kummarasetti, V. B.; Ravindran, J.; Kumar, N.; Halder, K.; Sharma, R.; Mukerji, M.; Das, S. K.; Chowdhury, S. (2006). "Genome-wide prediction of G4 DNA as regulatory motifs: Role in Escherichia coli global regulation". Genome Research. 16 (5): 644–655. doi:10.1101/gr.4508806. PMC 1457047. PMID 16651665.
- ^ Bugaut A, Balasubramanian S (2012). "5'-UTR RNA G-quadruplexes: translation regulation and targeting". Nucleic Acids Res. 40 (11): 4727–41. doi:10.1093/nar/gks068. PMC 3367173. PMID 22351747.
- ^ Sen, D.; Gilbert, W. (1988). "Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis". Nature. 334 (6180): 364–366. doi:10.1038/334364a0. PMID 3393228.
- ^ Kamath-Loeb, A.; Loeb, L. A.; Fry, M. (2012). Cotterill, Sue (ed.). "The Werner Syndrome Protein is Distinguished from the Bloom Syndrome Protein by Its Capacity to Tightly Bind Diverse DNA Structures". PLoS ONE. 7 (1): e30189. doi:10.1371/journal.pone.0030189. PMC 3260238. PMID 22272300.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Maizels, N.; Gray, L. T. (2013). Rosenberg, Susan M (ed.). "The G4 Genome". PLoS Genetics. 9 (4): e1003468. doi:10.1371/journal.pgen.1003468. PMC 3630100. PMID 23637633.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Kaplan, Oktay I.; Berber, Burak; Hekim, Nezih; Doluca, Osman (2016-11-02). "G-quadruplex prediction inE. coligenome reveals a conserved putative G-quadruplex-Hairpin-Duplex switch". Nucleic Acids Research. 44 (19). doi:10.1093/nar/gkw769. ISSN 0305-1048. PMC 5100583. PMID 27596596.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Bugaut, Anthony; Balasubramanian, Shankar (2017-02-17). "5′-UTR RNA G-quadruplexes: translation regulation and targeting". Nucleic Acids Research. 40 (11): 4727–4741. doi:10.1093/nar/gks068. ISSN 0305-1048. PMC 3367173. PMID 22351747.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Biffi, G.; Tannahill, D.; McCafferty, J.; Balasubramanian, S. (2013). "Quantitative visualization of DNA G-quadruplex structures in human cells". Nature Chemistry. 5 (3): 182–186. doi:10.1038/nchem.1548. PMC 3622242. PMID 23422559.
- ^ Rice C, Skordalakes E (2016). "Structure and function of the telomeric CST complex". Computational and Structural Biotechnology Journal. 14: 161–167. doi:10.1016/j.csbj.2016.04.002. PMC 4872678. PMID 27239262.
- ^ Le, D. D.; Antonio, M. Di; Chan, L. K. M.; Balasubramanian, S. (2015-01-01). "G-quadruplex ligands exhibit differential G-tetrad selectivity". Chemical Communications. 51 (38). doi:10.1039/C5CC02252E.
- ^ a b Raju, Gajjela; Srinivas, Ragampeta; Santhosh Reddy, Vangala; Idris, Mohammed M.; Kamal, Ahmed; Nagesh, Narayana (2012-04-27). "Interaction of Pyrrolobenzodiazepine (PBD) Ligands with Parallel Intermolecular G-Quadruplex Complex Using Spectroscopy and ESI-MS". PLoS ONE. 7 (4). doi:10.1371/journal.pone.0035920. ISSN 1932-6203. PMC 3338766. PMID 22558271.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Wang, Shi-Ke; Su, Hua-Fei; Gu, Yu-Chao; Lin, Shu-Ling; Tan, Jia-Heng; Huang, Zhi-Shu; Ou, Tian-Miao (2016-03-01). "Complicated behavior of G-quadruplexes and evaluating G-quadruplexes' ligands in various systems mimicking cellular circumstance". Biochemistry and Biophysics Reports. 5: 439–447. doi:10.1016/j.bbrep.2015.09.022.
- ^ Todd, A. K.; Johnston, M.; Neidle, S. (2005). "Highly prevalent putative quadruplex sequence motifs in human DNA". Nucleic Acids Research. 33 (9): 2901–2907. doi:10.1093/nar/gki553. PMC 1140077. PMID 15914666.
References
[edit]- Jiangtao Ren; Jiahai Wang*; Lei Han; Erkang Wang*; Jin Wang* (2011). "Kinetically grafting G-qaudruplex onto DNA nanostructure as structure and function encoding via DNA machine". Chem. Commun. 47 (38): 10563–10565. doi:10.1039/c1cc13973h.
- Johnson JE, Smith JS, Kozak ML, Johnson FB (2008). "In vivo veritas: using yeast to probe the biological functions of G-quadruplexes". Biochimie. 90 (8): 1250–1263. doi:10.1016/j.biochi.2008.02.013. PMC 2585026. PMID 18331848.
- Huppert JL, Balasubramanian S (2005). "Prevalence of quadruplexes in the human genome". Nucleic Acids Research. 33 (9): 2908–2916. doi:10.1093/nar/gki609. PMC 1140081. PMID 15914667.
- Todd AK, Johnston M, Neidle S (2005). "Highly prevalent putative quadruplex sequence motifs in human DNA". Nucleic Acids Research. 33 (9): 2901–2907. doi:10.1093/nar/gki553. PMC 1140077. PMID 15914666.
- Burge S, Parkinson GN, Hazel P, Todd AK, Neidle S (2006). "Quadruplex DNA: sequence, topology and structure". Nucleic Acids Research. 34 (19): 5402–5415. doi:10.1093/nar/gkl655. PMC 1636468. PMID 17012276.
- Siddiqui-Jain A, Grand CL, Bearss DJ, Hurley LH (2002). "Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription". PNAS. 99 (18): 11593–8. doi:10.1073/pnas.182256799. PMC 129314. PMID 12195017.
- Rawal P, Kummarasetti VB, Ravindran J, Kumar N, Halder K, Sharma R, Mukerji M, Das SK, Chowdhury S (2006). "Genome-wide prediction of G4 DNA as regulatory motifs: Role in Escherichia coli global regulation". Genome Res. 16 (5): 644–55. doi:10.1101/gr.4508806. PMC 1457047. PMID 16651665.
- Xu Hou; Wei Guo; Fan Xia; Fu-Qiang Nie; Hua Dong; Ye Tian; Liping Wen; Lin Wang; Liuxuan Cao; Yang Yang; Jianming Xue; Yanlin Song; Yugang Wang; Dongsheng Liu; Lei Jiang (2009). "A biomimetic potassium responsive nanochannel: G-quadruplex DNA conformational switching in a synthetic nanopore". J. Am. Chem. Soc. 131 (22): 7800–7805. doi:10.1021/ja901574c. PMID 19435350.
- Neidle & Balasubramanian, ed. (2006). Quadruplex Nucleic Acids. ISBN 0-85404-374-8.
- Rowland, Gerald B.; Barnett, Kerry; DuPont, Jesse I.; Akurathi, Gopalakrishna; Le, Vu H.; Lewis, Edwin A (1 December 2013). "The effect of pyridyl substituents on the thermodynamics of porphyrin binding to G-quadruplex DNA". Bioorganic & Medicinal Chemistry. 21 (23): 7515–. doi:10.1016/j.bmc.2013.09.036.
External links
[edit]- Nanopore and Aptamer Biosensor group{NAB group}
Quadruplex websites
[edit]- G-Quadruplex World – a website to discuss publications and other information of interest to those working in the field of G-quadruplexes
- Quadbase – downloadable data on predicted G-quadruplexes
- Greglist – a database listing potential G-quadruplex regulated genes
- Database on Quadruplex information: QuadBase from IGIB
- GRSDB- a database of G-quadruplexes near RNA processing sites.
- GRS_UTRdb- a database of G-quadruplexes in the UTRs.
- G-quadruplex Resource Site
- non-B Motif Search Tool at non-B DB- a web server to predict G-quadruplex forming motifs and other non-B DNA forming motifs from users' DNA sequences.
Tools to predict G-quadruplex motifs
[edit]- Quadparser: Downloadable program for finding putative quadruplex-forming sequences from Balasubramanian's group.
- QGRS Mapper: a web-based application for predicting G-quadruplexes in nucleotide sequences and NCBI genes from Bagga's group.
- Quadfinder: Tool for Prediction and Analysis of G Quadruplex Motifs in DNA/RNA Sequences from Maiti's group, IGIB, Delhi, India
- [2] G4Hunter from Mergny's group but user need to run the code in R.
