Wikipedia:Reference desk/Archives/Science/2007 October 12
| Science desk | ||
|---|---|---|
| < October 11 | << Sep | October | Nov >> | October 13 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
October 12
[edit]Alcohol effects
[edit]Hi there! As 90% of the Portuguese students I've ingested considerable amounts of alcohol during my universitary course, specially - at least in my case - in the form of stout beer. So, the question is: What are the consequences of alcohol ingestion to the brain? I know Wikipedia has an article on "effects of alcohol consumption" and also that you cannot offer medical advice, let's skip that, ok? I want to hear your opinion. Will I be able to conclude my MsC thesis? If that matters, I'm partially drunk, despite being able to write in English. Cheers! 84.91.226.18 00:39, 12 October 2007 (UTC)
- A man was horribly maimed in a farming accident, and had to have major reconstructive surgery on both his hands. He went to see his surgeon for the final time. "Doctor," says the man excitedly and dramatically holds up his heavily bandaged hands. "Will I be able to play the violin when these bandages come off?" "I don't see why not," the doctor responds. "Odd," says the man. "I never could before."
- The odds are, you will be able to conclude your MsC thesis -- if, and only if, you could do so before the stout beer kicked in. - Nunh-huh 01:04, 12 October 2007 (UTC)
- I would say that you will never get your MSc until you learn to spell MSc properly! —Preceding unsigned comment added by 88.109.232.130 (talk) 01:26, 12 October 2007 (UTC)
- It's OK to spell it MsC when it's from a universitary. Cut him a break, it's a second language and he's drunk. Me, I have no excuse... - Nunh-huh 02:40, 12 October 2007 (UTC)
- Really excessive alcohol consumption will in the long run have negative effects. But if you're still a student I assume you're too young for it to have taken enough effect. It is also a matter of what sort of skills you need for it. Memory is affected more than reasoning. The barkeep of my favourite bar in Eindhoven, where I studied, had a 'live fast die young' attitude. Didn't work. He lived pretty 'fast' (which included being drunk every day for pretty much all his life), but he's still alive, about 60 years old now, I guess. He keeps forgetting things, but his mind is still as sharp as it used to be. That is, he can still reason, but there is less in his head to reason with, so to say. DirkvdM 08:16, 12 October 2007 (UTC)
- Party away! Just remember that alcohol has negative health consequences that trend quite clearly with the quantity and temporal nature of its consumption. When it comes to thesis work, don't forget that libations obviously prevent one from working or studying whilst drunk, but can seriously hinder such activities even a couple of days later, depending on hangover severity and sleep deprivation. Also, booze has a way of sapping one's wallet, which can negatively affect the ability to buy healthy, knowledge-supporting meal ingredients or paying rent on a decent apartment.
- On the other end, I believe that socialization is an important part of the education process. Late-night pitchers of stout can facilitate useful discussions, collaborations, and life-long personal and professional relationships that can have positive effects on a career.
- It's just a matter of moderation... — Scientizzle 20:40, 12 October 2007 (UTC)
- If you drink somuch you can't pay the rent then you've really got a problem. And healthy food (nothing special, just healthy) is generally cheaper, certainly cheaper than junk food (hamburgers, crisps and the like). DirkvdM 09:52, 13 October 2007 (UTC)
- I think you're missing the bigger problem. How are you supposed to finish you thesis when your too busy drinking to do the research and start the writeup? (And when you're not drinking you're too drunk to get any significant work done) Nil Einne 12:25, 13 October 2007 (UTC)
- Well, no, he asked if his previous alcohol consumption will have had an effect on his abilities. Anyway, he says he can write English even when he's drunk (at least, I think that's the point he was making - then again, if he can't make that sufficiently clear, then maybe he couldn't write a decent thesis, but on the other hand, we can't know if that has anything to do with his alcohol consumption ... all very complicated. :) ). DirkvdM 07:27, 14 October 2007 (UTC)
Cutting vinyl discs
[edit]On the old vinyl discs, was there some advantage or technical limitation that made recording engineers produce discs with minimum (or inadequate) stereo separation? —Preceding unsigned comment added by 88.109.232.130 (talk) 01:02, 12 October 2007 (UTC)
- My understanding was that it was a limitation of the cartridge in the replay head that limited the stereo separation quite sharply. Gramophone_record#Shortcomings seems to agree with me. SteveBaker 04:46, 12 October 2007 (UTC)
- One reason is that the stereo recording format was backward-compatable with the earlier monaural format. The mono fromay moced the needle perpendicular to the surface: the groove varied in depth only. The stereo format moved the needle in two orthogonal dimensions, both 45 degrees to the perpendicular. In any actual physical system, this leads to cross-talk. -Arch dude 19:57, 12 October 2007 (UTC)
time dilations at high g
[edit]do particals in sychrotrons experience time dilations because of the high g forces involved, i.e. have longer than expected half lives? basically i have read of large gravitational bodies bending space time and was wondering if artificial gravity causes similar effects.209.204.181.5 05:52, 12 October 2007 (UTC)
- The particles in synchrotrons experience a lot of time dilation due to their speed, as they are generally accelerated to nearly the speed of light. However I am not sure if acceleration due to other sources than gravity produces the same time dilation effects. Einstein used gravity-acceleration equivalence thought experiments before discovering general relativity which suggests that, yes, you'd expect the same effect, but I can't be sure. Cyta 06:56, 12 October 2007 (UTC)
- The time dilation factor is the Lorentz gamma, which is also the ratio of the particle's rest energy to its speed. E.g. the Tevatron accelerates protons (~1 GeV) to energies of around 1 TeV, so the time dilation factor is about 1000 (not that it matters for protons, which don't decay as far as anyone knows). The acceleration, as such, has nothing to do with it, even in the gravitational case. Time dilation is about the geometry of the worldline. For motion in a circular accelerator, it's analogous to the fact that the wire that goes into a spring of a given length is longer than the length of the spring. For gravitational time dilation, it's analogous to the fact that circular arcs subtending a given angle have different lengths depending on their radii. -- BenRG 11:22, 12 October 2007 (UTC)
How would the [lub dub] of the human heart be represented musically?
[edit]If you had to give a drummer a piece of music to represent a healthy heartbeat, how would you do it? What's the beat, is there a taradiddle?
Thanks in advance Adambrowne666 05:59, 12 October 2007 (UTC)
- Damn, a taradiddle would be one big-time arrhythmia. Nothing the heart does is that complicated. So the drummer's going to be bored. For a look at notation, see the opening measures of the Mahler Ninth Symphony. The cellos have the heart-beat. Actually, music notation doesn't really convey the info doctors listen for in auscultation very well...it's better to listen to recordings like this one (the most normal found on this site devoted to auscultation. - Nunh-huh 06:38, 12 October 2007 (UTC)
- Why don't we have an article on taradiddle? Now I don't know why that would be an arrhythmia. Anyway, heartbeat is very important in music. The number of beats per minute is very determining for the mood of music because it emulates the heart rate. A normal heart rate at rest is around 80 b/m, so that should sound relaxed (not sure about the actual value - just know the principle). A faster beat should get you more excited. Maybe if the beat actually sounds like a heartbeat, the effect will be even stronger. Just listen to the beginning of Pink Floyd's Dark side of the Moon. There's a second, softer, beat after every beat. Actually, it's a 3/4 beat (or is that a triplet?), with the 'afterbeat' on the second beat. DirkvdM 08:40, 12 October 2007 (UTC)
- Taradiddle would be a dictionary entry, but paradiddle we have... - Nunh-huh 18:58, 12 October 2007 (UTC)
- Dirk says "The number of beats per minute is very determining for the mood of music" — which is certainly true — "because it emulates the heart rate". To this I say, to put it politely, "citation needed". Most people are not aware of their heart rate most of the time, so why would it affect the experience of listening to music? Do people with bradycardia find music more exciting, and people with tachycardia find it less exciting, than people with normal heart rates? --Anonymous, 02:02 UTC, October 13, 2007.
- I wondered about that too when I heard it (sorry, can't remember where). But it is rather striking that really fast dance music has the same beat frequency as a heart of someone excited (about 120 b/m and over). DirkvdM 09:57, 13 October 2007 (UTC)
- Buy the music to Heart of Rock and Roll on Huey Lewis' Sports. Delmlsfan 21:34, 12 October 2007 (UTC)
Although music is abstract, I have always assumed it's an unconscious mimicking of the music of the human voice - pathetic music mimics sobs; jolly music mimics laughter - that sort of thing. I would say too that although we generally don't hear our heartbeat, we are aware of it in other ways, especially when it's racing - so I side with Dirk on this, Anonymous, though it might be my flaky theorising; I can't provide citations. Thanks, everyone, for your interesting and useful answers. The article on the Mahler Ninth is a fantastic bonus, Nunh. Adambrowne666 03:12, 13 October 2007 (UTC)
- OT: Weird. I must have seen this thread unconsciously earlier in my recent changes, because I just woke up from a nap where I dreamt that I pressed so hard on my carotid artery that my blood backed up in my chest and I could hear my heart trying to clear it. Anchoress 03:53, 13 October 2007 (UTC)
dot and cross product
[edit]why we use cos in dot product and sin in cross product? —Preceding unsigned comment added by 202.69.33.15 (talk) 06:43, 12 October 2007 (UTC)
- see Dot_product#Proof_of_the_geometric_interpretation
- and ask on the maths desk for a derivation of the cross product .. if you have a vector C that is at right angles to A and B vectors - you can work out for yourself what this vector will be using the dot products C.A=0 and C.B=0 - if you do the maths you should be able to see where the sin comes from - the cross product is the result of solving these two dot products..eg http://hemsidor.torget.se/users/m/mauritz/math/vect/xprodpro.htm (note in this derivation it only goes as far as getting the orthogonal vector - but doesn't show that the magnitude is ab sin(theta) - if you can get this far you should be able to do that? or ask on the maths desk87.102.87.36 12:54, 12 October 2007 (UTC)
- Sorry I can't be clearer on the cross product but I can't find a link to the derivation - you best bet is to derive the cross product for yourself and then look at what the magnitude of the vector is...87.102.87.36 13:20, 12 October 2007 (UTC)
Wait for answers at Wikipedia:Reference_desk/Mathematics#dot_and_cross_product —Preceding unsigned comment added by 87.102.87.36 (talk) 13:23, 12 October 2007 (UTC)
beams that reflect from bones
[edit]thank you mr. SteveBaker and mr. Daniel for your response on the q i asked on 6th. that's true that my application matters a lot. i asked for some beams/rays/waves that reflect from bones. my application is like i'm trying to make a control system for automobiles in which i want these beams/rays to sense the living object in front of and no other object should bother the control system. for that i need some method that will sense bones only and not metal, plastic or concrete... also at these stage for me the cost and size of the method do not matter eventhough it's a non-stationary application... Neel shah556 10:46, 12 October 2007 (UTC)
- Whatever beams you use will have to pass through flesh to get from your transmitter to the bone and back to a receiver. I, for one, wouldn't want that to happen to me. I'm thinking you could use echolocation to find objects in front of the vehicle and then aim a parabolic microphone at each one to listen for a heartbeat. You could electronically filter the incoming sound quite a bit, and use software to identify the repeating pattern of the heartbeat. Sounds crazy, I know, but there just might be a way to make it work. --Milkbreath 12:37, 12 October 2007 (UTC)
- Also, it would be very difficult to distinguish between bone and cement/concrete, as they are pretty much made out of the same thing.Tuckerekcut 13:39, 12 October 2007 (UTC)
- I very much doubt you could to this - I don't think there is any unique human 'signature' that could distinguish flesh and bone from a lot of other natural and man-made substances. SteveBaker 14:00, 12 October 2007 (UTC)
- Why not measure infrared radiation (heat)? You could at least distinguish between living and non-living in that way without too much difficulty based on the shape and size of the thing in question, and I'm betting that non-living things look quite differently in an infrared spectrum. --24.147.86.187 14:34, 12 October 2007 (UTC)
- The good news for your application is that if human beings can do it, at least it's theoretically possible. I'd go for a combination of systems. A simple camera image will give you some information on whether there's a living being in front of the car (based on shape, movement, etc.) An infrared image should give you a lot of information. And of course any setup with multiple cameras or other wave sensors will give you some spatial (3d) information as well. You can then combine this information into a machine learning algorithm, so that the system can learn to identify living things by itself. I think that just a simple camera could get you a pretty accurate result already. I any case, I think there are plenty of options left before you decide to start throwing x-rays around the place. :)
- risk 01:34, 13 October 2007 (UTC)
- So you want to avoid pedestrians, but crashing into a brick wall doesn't matter? What you need is something that will give the mass of the object and reflect back. Electromagnetic waves won't work, because they only reflect off of electrically conductive objects. I think sonar might work, but I'm not sure. You could also use some way to recognize objects. That way, you detect objects above a certain size, and see if they look like an object on a list of stuff you can safely hit (like a tumble weed). If you don't recognize it, brake. One thought that just occurred to me is to hit it with a pulse of sound and see how much it moves. The problem is that, unless have an extremely precise way to tell acceleration, it would have to be a very loud sound. — Daniel 01:44, 13 October 2007 (UTC)
?
[edit]I need instruction on how to draw the molecular structure of elements —Preceding unsigned comment added by Cali08P (talk • contribs) 13:14, 12 October 2007 (UTC)
- It depends - some elements have a molecular structure, others are not molecules.. Sulphur exists as a molecule, argon doesn't - could you be more specific.
- Molecular graphics has stuff about different ways to show molecules, also try Molecular model —Preceding unsigned comment added by 87.102.87.36 (talk) 13:32, 12 October 2007 (UTC)
- You could also look at simple Lewis structures. Someguy1221 17:21, 12 October 2007 (UTC)
Gay twin
[edit]Is it possible for one identical twin to be gay and not the other? --124.254.77.148 13:42, 12 October 2007 (UTC)
- Yes. It's not generally believed to be a genetic matter. SteveBaker 13:55, 12 October 2007 (UTC)
- Of course, if they're conjoined twins, there may be some logistical problems. GeeJo (t)⁄(c) • 16:24, 12 October 2007 (UTC)
- I understand it's not that simple - there's not a single determining factor. There are advocates for nature or nurture as causes, which, alas, has a lot to do with what people want to be true. When a Dutch researcher found indications for a biological cause, the reactions by homosexuals in the Netherlands and the US were opposite. I can't remember which way around that was, but it surprised me to hear that some homosexuals disliked this finding. I supose they interpreted it like they were 'abnormal' (which, literally, is the case of course, but that doesn't make it a bad thing - I consider myself to be quite abnormal and am proud of it :) ). But if it were nurture, then that would suggest that they're just imagining it. Like they're really heterosexual and somehow they deny their nature. Of course, none of this says anything about what the real causes of homosexuality are. DirkvdM 16:57, 12 October 2007 (UTC)
- In the US it is a very complicated identity-politics issue, primarily because "biological vs. non-biological" has been interpreted to mean "inevitable and unchangeable vs. totally arbitrary and easily changeable choice." This is of course a not very scientifically-informed approach to the nature/nurture dichotomy, but it is the way this issue (and many others relating to sexuality and/or genetics) are handled in terms of US politics. --24.147.86.187 17:11, 12 October 2007 (UTC)
- It is not particularly unusual. One study ([ PMID 8494487]) that sampled gay individuals who were also twins found "thirty-eight pairs of monozygotic [identical] twins (34 male pairs and 4 female pairs) were found to have a concordance rate of 65.8% for homosexual orientation. Twenty-three pairs of dizygotic [non-identical] twins were found to have a concordance rate of 30.4% for homosexual orientation." That means 13 of the identical twins had one homosexual, with the other being straight. Their conclusion is that this data supports a genetic influence for homosexuality, but obviously not an absolutely determinant one. This conclusion is generally held up by similar studies. [1] Rockpocket 17:20, 12 October 2007 (UTC)
Fear suppressing drugs?
[edit]Are there any drugs that suppress or numb fear? 64.236.121.129 13:46, 12 October 2007 (UTC)
- Some types of fear are described as phobia and treated medically. An example of recently published research: Glucocorticoids reduce phobic fear in humans. --JWSchmidt 14:16, 12 October 2007 (UTC)
- Alcohol has traditionally been used to suppress fear. DuncanHill 14:17, 12 October 2007 (UTC)
- True, but they probably want something without major effects other than suppressing fear. PCP will suppress fear too, but that effect is coupled with a number of other effects that make it unsuitable for most purposes. --24.147.86.187 15:27, 12 October 2007 (UTC)
- Every drug has side-effects, or, more precisely, has multiple effects. If you desire one of the effects, then the other effects will be 'side-effects'. The suppression of fear by any drug, including alcohol and PCP, can also be quite undesirable, notably in traffic (such as when you are driving a car or others around you are). DirkvdM 17:06, 12 October 2007 (UTC)
- Cocaine certainly, opiates and stimulants probably. DirkvdM 17:06, 12 October 2007 (UTC)
- Beta blockers are traditionally used for stage fright. - Nunh-huh 01:47, 13 October 2007 (UTC)
It would be a useful drug to give to soldiers at the front - I bet there's been some research on it Adambrowne666 04:01, 13 October 2007 (UTC)
- They used to dose them with amphetamines for this purpose, didn't they? --Kurt Shaped Box 08:50, 13 October 2007 (UTC)
- Or shell the enemy with marijuana-bombs, so they'll all turn pacifist. :) I recently heard the US army experimented with something like this. DirkvdM 10:01, 13 October 2007 (UTC)
- I'm not so sure that suppressing fear is a good thing in a battlefield situation. You don't want your men rushing into dangerous situations when they'd be better off staying in cover. It's noticable in video games (where people have very little fear because the consequences of death or injury is minor) - if you watch how players behave in the virtual battlefield, it's totally different to how soldiers behave in the real world - and the casualties are always very much higher. SteveBaker 11:45, 13 October 2007 (UTC)
- Simple solution - dose them with PCP at the same time, so that they don't feel the bullets and also get a neat little aggression boost as part of the package... :) --Kurt Shaped Box 19:50, 13 October 2007 (UTC)
- 'They' (i.e. the finest minds of US military science) were trying to invent a gas shell that would instantly turn enemy soldiers gay (and presumably drop their guns and immediately start buggering each other) at one point, weren't 'they'? --Kurt Shaped Box 19:45, 13 October 2007 (UTC)
Sure! Antidepressants such as SSRIs work to increase the availability of serotonin in the brain which makes you feel confident/horny/manic/aggressive etc. —Preceding unsigned comment added by 88.111.61.118 (talk) 23:44, 13 October 2007 (UTC)
Graduate degree titles
[edit]If you are a nursing graduate student, when can you use the title MSN(c) for Master's in Nursing Candidate? —Preceding unsigned comment added by 71.193.119.109 (talk) 15:48, 12 October 2007 (UTC)
- when you have passed the exam? —Preceding unsigned comment added by 88.109.232.130 (talk) 21:54, 12 October 2007 (UTC)
beta sheets
[edit]what is the distance between every second residue on a beta sheet? —Preceding unsigned comment added by 144.173.6.67 (talk) 15:59, 12 October 2007 (UTC)
- I think what you are looking for is at Beta sheet. --JWSchmidt 16:32, 12 October 2007 (UTC)
LED flickering question.
[edit]A lot of commercial vehicles here, and some cars (continental US) now use LED based taillights and marker lights. When I look at them straight-on, they are (when lit) a steady red or amber color, depending on the light. When I'm rapidly moving my eyes or head, say from one side to the other, and an LED light is thus moving across my field of vision, it will appear to flicker, and I'll see a row of lit dots, not a continuous "smear" of light. Why is this? Do the LED units need to switch on-and-off at some rapid rate? Is it some characteristic of the vehicle electrical system? 71.112.9.77 19:10, 12 October 2007 (UTC)
- I've noticed that too. Just a guess: I think that the LEDs are modulated (Pulse-width_modulation?) by flashing so there can be different brightness levels, so the brake lights can get brighter when the brake is applied. -- Diletante 20:20, 12 October 2007 (UTC)
- Exactly correct, and at least some of the designers have picked too damned low a frequency for the PWM. I hope this gets regulated soon.
- How hard is it to hook up a few capacitors? LED's use tiny amount of current... --antilivedT | C | G 22:59, 12 October 2007 (UTC)
- Strobing effect —Preceding unsigned comment added by 88.109.232.130 (talk) 21:53, 12 October 2007 (UTC)
- No, the reason is that they are multiplexed displays. Cacycle 04:26, 13 October 2007 (UTC)
- Neither of the explanations seen here makes sense to me. Taillights may operate at different brightness levels, but marker lights don't, so why wouldn't they be at full "on" all the time? And multiplexed displays are used when the elements need to be individually switchable, which doesn't apply to these kinds of lights either.
- The IC that regulates the current flow to the LEDs is still a pulse width modulator, whether we're talking about the tail lights or the marker lights. It's gotten to the point where its cheaper and far more energy-efficient to use an active circuit than a power-consuming resistor. After all, these LEDs used as auto lamps are operating on a substantial amount of current, not the 5-20 mA thta's used for "indicator" LEDs.
- If we were talking about LED traffic lights I'd guess that they were strobing at twice the power line frequency, the way fluorescents do, but that doesn't make sense for vehicle lights that would run on DC.
- Does anyone have an actual reference on this? --Anonymous, 06:57 UTC, October 13, 2007.
- I know this has been asked before with a good answer given, but I can't find it in the archives. 68.231.151.161 01:36, 14 October 2007 (UTC)
- Here is a description of the electronics involved in automotive LED lighting. It seems they regulate the current to the LEDs by switching a MOSFET, so they all strobe all the time. You can't power LEDs right from the battery, even with analog regulation, for several reasons, one of which is the transients you get when stuff switches on or off in the rest of the electrical system. They use a DC-DC converter driver to power the LEDs. --Milkbreath 02:00, 14 October 2007 (UTC)
- Thanks! --Anon, 04:25 UTC, October 14, 2007.
- LED brakelights turn on quicker than incandescent bulbs, allowing a following driver to apply his brakes quicker and avoid rear-ending the braking car. That is great. But present LED lights on cars are typically flashed on and off at high speed, above the critical flicker limit, to appear continuously on when you look right at them, but resulting in the scattered afterimages if there are eyemovements (as there normally are), which, like the questioner, I find distracting and annoying. An array of LEDs in a brakelight array could very easily be set up to operate on the 12 volts (or other voltage) supplied from the battery, without the rapid flashing on and off which results in the scattered afterimages. Of course you can power a series connection of LEDs directly from the battery! If the forward biased voltage drop for one LED is 2 volts, then connect 6 of them in series across the battery! Two or more such series arrays would allow the light to contimue operating if one series array failed. Suitable adjustments could be made for other forward biased voltage drops or supply voltages. No need to annoy everyone else on the road by flashing them 30 (or however many) times per second. (edited to add) The ref supplied by Milkbreath discusses the challenges which may have led to the strobing and afterimage problem. The designers worry about an idiot jumpstarting your car's 12 volt system with a 24 or even 36 volt battery, perhaps with reverse polarity. Crowbar circuits with a fuse could stop that sort of moronic insult to the car's electrical system. Then the designers legitimately worry about keeping constant brightness with varying battery voltage, so that your taillights and other exterior safety lights stay on even when you crank the engine for a prolonged period and the battery voltage gets down to 7 volts. You don't want someone running into your car at night. They also legitimately worry about the transient energy which might get dumped through the LEDs when the battery (a fine voltage clamp itself) is isolated from the electrical system by the ignition being shut off or a fuse blowing. This dictates DC-DC conversion incorporating protective circuitry, but note that said convertor puts out DC. The problem is they apparently found it useful to turn the DC on and off rapidly 30 or 60 times per second or sime such, I would guess. The article cited does not even mention this frequency. Looks fine in the showroom, I guess. No afterimage problem there like there is on the highway at night. If the headlights of cars also switch to ultrabright LEDs and the designers similarly use circuits which switch them on and off rapidly, the multiple afterimage problem will get even worse and more distracting. I have used solid state DC-DC convertors for decades which produce a very steady DC output, even though internally the current is switched rapidly to allow voltage multipliers etc. Edison 02:15, 14 October 2007 (UTC)
- Efficiency might be an important factor. A failure mode of LED chips is overheating. Thus you have to keep the power dissipated below a certain level. At the same time, you want the LEDs to be as bright as possible for a given current.
- To use an example, if you have a constant current of 10 ma going through an LED, you will dissipate a certain power. If, instead, you pulse it at 100ma at a 10% duty cycle, you’ll dissipate the same power, to a first approximation. However, the pulses will be a lot brighter.
- It so happens that the human eye is a good peak detector. So the pulsed LEDs will appear a lot brighter, while not dissipating any more power. Bunthorne 03:47, 15 October 2007 (UTC)
- I disagree with the claim that the human eye responds to the peak brightness. Bloch's Law says itt integrates pulses that occur at a frequency higher than the critical flicker frequency. Designers of LED lights [2] claim to utilize the Broca-Sulzer effect that for certain durations, flashing the LED on and off may produce an enhanced brightness impression, but apparently the frequency has to be relatively low, producing the cluster of afterimages, and annoying people who notice it. There is no inherent reason that the LED signal lights on cars could not be used in a continuous rather than flashing mode, by giving up this gimmick. Automakers couls spend a few more bucks and produce a more pleasing light. Edison 16:02, 16 October 2007 (UTC)
- I'm not even sure it's a question of price. The LED controller ICs often use an inductor and generally, the higher the frequency, the smaller the inductor. I think it may be as simple as "the problem wasn't apparent when we tested the taillights in the lab". That's why I hope this will be regulated soon.
When Hydrogen Peroxide is doing its thing (oxidising something), is ozone ever a by-product? Anchoress 20:14, 12 October 2007 (UTC)
- Not usually. Ozone is a more oxidized form of oxygen than peroxide is and is less stable. DMacks 21:18, 12 October 2007 (UTC)
- This explores the idea that hydrogen peroxide might be generated from ozone. This article says, "H2O2 catalyzes the decomposition of O3 via the peroxone process". --JWSchmidt 21:19, 12 October 2007 (UTC)
- Ever is a pretty strong word. It is unlikely to do so as shown by the oxidation potential chart in the H2O2 article. See where ozone is more positive than hydrogen peroxide? That means that going from ozone to H2O2 is a favorable reaction and going the other way is unfavorable. But I wouldn't say never. Delmlsfan 21:21, 12 October 2007 (UTC)
- Thanks all for the info and the links!! Anchoress 23:02, 12 October 2007 (UTC)
What is the name of this plant?
[edit]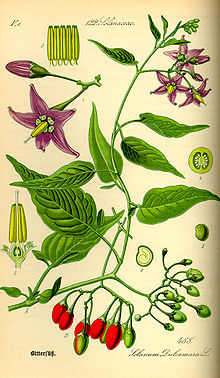
Cacycle 16:26, 13 October 2007 (UTC)


It is a low shrub, found in the U.S. mid-Atlantic states. In the spring it has little purple flowers with yellow centers. In the summer the flowers are joined by green berries which ripen to orange and then bright red. Here it is in the autumn, when the flowers are gone.
-- Dominus 23:00, 12 October 2007 (UTC)
- That looks to me like an ornamental pepper plant. So, I don't think they are berries. If you search for ornamental pepper plant, you'll see many plants that look similar. -- kainaw™ 02:50, 13 October 2007 (UTC)
- I thought it was a pepper of some kind when I saw the picture. The shape of the fruit and the three colors are suggestive. I don't think it's a native plant where I live (southern New Jersey); I think I'd have seen it. I googled on lots of stuff like "capiscum" and "wild pepper" and came up with zilch. --Milkbreath 03:05, 13 October 2007 (UTC)
- I just saw a pic of a plant that looks like it - goji. Here's the pic. Anchoress 05:07, 13 October 2007 (UTC)
- After reading the goji article, I'm pretty sure that's what it is. BTW goji is a member of the nightshade family, which also contains peppers. Anchoress 05:10, 13 October 2007 (UTC)
I think Cacycle wins this one. Thank you very much! I have been wondering about this plant for years. -- Dominus 03:41, 14 October 2007 (UTC)
Is it abstract or not?
[edit]ok, someone last week tried to tell me that physics and chemistry were abstract. To me that doesnt make any sense because something that is abstract is the opposite of "concrete" and i have always been told that science is very concrete?
so could you consider physics and chemistry to be abstract?
(sense this might be more of an opinion than a definite answer, input from man people would be appreciated)
!NOTE! he was calling it abstract because you cant see it (I.E. you cant see atoms) but to me just because something isn't visible to the naked eye doesn't mean its abstract
also how can you consider a theory to be science/ i thought science was suppose to be based on fact not speculation?
—Preceding unsigned comment added by 71.98.105.119 (talk) 23:19, 12 October 2007 (UTC)
- It's somewhat meaningless to apply abstract/concrete to an entire field of science. Both chemistry and physics can predict and explain very detailed phenomena, which would make them "concrete." However, they can also produce broad predictions about a wide range of phenomena, which would be somewhat abstract. Someguy1221 00:02, 13 October 2007 (UTC)
- It would have probably been more clear to try and press whomever told you this exactly what they meant by the description at the time. "Abstract" has many meanings. Maybe they just meant they dealt with "abstractions"—rough representations—which most thoughtful practitioners would agree with. Perhaps he meant something else. Who knows? Science deals with representations, in any case. The goal is to make these representations match as closely as possible to how things "actually are," but that has always been tough and the ultimate ability for humans to do that has often been questioned. --24.147.86.187 01:08, 13 October 2007 (UTC)
Science goes from the specific to the general and then back again. It takes many examples (through observation, preferably by experimentation) to create an abstract image of reality, which it can then apply back to specific instances to predict what will happen. So it uses abstraction to describe the specific. DirkvdM 10:05, 13 October 2007 (UTC)
- I didn't read the comment but perhaps what was meant was that in many way, theoretical physics and theoretical chemistry nowadays tends to seem very abstract. Stuff like subparticles may be important, but it can be fairly hard for the average person to see the importance or relevance in real life. While this applies to all areas of science to some extent, I think it's easier for the average person to see the relevance and perhaps even important of even the more theoretical aspects of biology Nil Einne 12:15, 13 October 2007 (UTC)
Physics and chemistry can be real OR abstract - theoretical models are abstract, doing reactions is real.
In a addition the scientific models used (eg atomic theory etc) can be considered abstractions - so the answer is yes and no.83.100.254.51 14:57, 13 October 2007 (UTC)
- I said that. :) DirkvdM 07:48, 14 October 2007 (UTC)
- Calling something concrete and abstract is often a matter of how familiar you are with that kind of thing. As an example, I remember very well when, on one of the first functional analysis classes, our professor said "Let's take a concrete example: let x be a self-adjoint operator". At that point, we laughed hard, because we didn't think something starting like that could be concrete enough. We were familiar with expressions containing variables which represent complex numbers and simple functions and things like that, so we could think of an expression with such variable as concrete (even if it might look as an abstract expression to someone else). However, we didn't have a grasp of functional analysis at that time so we couldn't imagine an operator as a concrete thing. On the other hand, a did a matured physician who works with such operators every day have heard this, he wouldn't even have winked, for he can accept such things as concrete. – b_jonas 21:26, 16 October 2007 (UTC)
