Wikipedia:Reference desk/Archives/Science/2009 December 25
| Science desk | ||
|---|---|---|
| < December 24 | << Nov | December | Jan >> | December 26 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
December 25
[edit]Holes in sandstone
[edit]We have several steps made out of large sandstone slabs. They are nearly flat and essentially oblong. They measure approximately 48"x30"x10". Two of them have many small holes in the top and one edge. These holes vary slightly in size but each one is approximately 5mmx5mm. Each hole is quite round and has straight sides and a nearly flat bottom. I am puzzled as to what might have produced these holes. They do not appear to be man made and they are random in their distribution. There are several hundred holes in each slab. The stones are uncut. They were lifted from their original site with a forklift and transported by truck. Does anybody have a clue as to what might have caused these holes? —Preceding unsigned comment added by 98.149.248.148 (talk) 00:29, 25 December 2009 (UTC)
- Drilling a series of holes and then inserting a wedge to fracture it is a common method of quarrying stone blocks, but this does not sound like what you have. It is probably the fossil holes of a species of Skolithos. SpinningSpark 01:51, 25 December 2009 (UTC)
Marine life and decompression sickness
[edit]Is marine life affected by decompression sickness? As far as I can tell from skimming the Wikipedia article on decompression sickness, it only talks about humans and doesn't say anything about other life forms, and it probably should at least make some mention of how or if it affects other life. —Lowellian (reply) 00:58, 25 December 2009 (UTC)
- Sperm whale bones show pitting which is likely a result of decompression sickness. --Dr Dima (talk) 01:39, 25 December 2009 (UTC)
- I think we just had this question. DRosenbach (Talk | Contribs) 04:46, 25 December 2009 (UTC)
- We did, on the Reference Desk? Could you provide a link? And if there's information, could someone please add it to the "decompression sickness" article? If that article actually had any information on non-humans, then I and others wouldn't have to ask here. —Lowellian (reply) 05:36, 25 December 2009 (UTC)
- I don't recall this question being asked before, and I could not find it in the archives. There was a more general question about respiratory system of marine mammals, here; but not this specific question AFAIR. Yes, I think it may be a good idea to add a cross-link from the decompression sickness article to the sperm whale article. By the way, quick Google Scholar search came up with this paper describing lesions indicative of decompression sickness in a few more species of marine mammals. --Dr Dima (talk) 06:33, 25 December 2009 (UTC)
- There is a lot in the literature which is pinning the blame for decrompression sickness in whales, especially beaked whales but also other species, on naval sonar.[1][2][3] The whale's normal diving behaviour is designed to avoid, or at least keep under control, the effects of decompression. It seems this is disrupted by sonar as the whales attempt to avoid the sonar signals and has led to beachings. On the other hand, the pitting of the bones mentioned above may be normal for whales and does not necessarily indicate that the animal was sick (although it would for a human). SpinningSpark 14:41, 25 December 2009 (UTC)
- I think DRosenbach was referring to Wikipedia:Reference desk/Archives/Science/2009 November 27#Deep sea fish: do we know how the buggers cope with the insane pressures? which touched on this issue somewhat Nil Einne (talk) 15:16, 25 December 2009 (UTC)
And what about non-mammalian marine life? Fish? Crustaceans? Other invertebrates?
—Lowellian (reply) 11:25, 25 December 2009 (UTC)
- Non-mammal marine life does not normally need to surface in order to breathe. This implies that they do not need to rapidly and regularly surface. This may also imply that they have less molecular nitrogen dissolved in their blood. Therefore, under normal conditions, decompression sickness should not be an issue for non-mammal marine animals. Under abnormal conditions - being pulled to the surface from the depth of hundreds of meters - deep sea fauna does not fare very well at all :( --Dr Dima (talk) 01:18, 26 December 2009 (UTC)
- Read recently that many abyssal fish die from hyperthermia not decompression sickness, they are used to a single cold water temperature and if put in water of that temperature they dont die. I looked for a ref but couldn't find it. Polypipe Wrangler (talk) 12:43, 26 December 2009 (UTC)
Is this real?
[edit]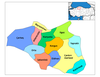

http://www.viddler.com/explore/failblog/videos/211/
That building seems awfully too strong. --99.237.234.104 (talk) 06:42, 25 December 2009 (UTC)
- It is at Cankiri in Turkey and has been widely reported including by the BBC so appears to be real. SpinningSpark 11:02, 25 December 2009 (UTC)
- The building survived the 1999 Izmit earthquake, where many other older building were flattened. However I'm not sure what the effects were in Cankiri Province specifically. ~AH1(TCU) 02:52, 26 December 2009 (UTC)
- Cankiri is over 200 miles to the East of the epicentre and I don't think there was much, if any, damage there. Istanbul, 50 miles to the West, however, did have some builings destroyed. SpinningSpark 16:39, 29 December 2009 (UTC)
- Well that was interesting video! SPOILER ALERT! For anyone who hasnt't see it the building actually rolled over onto it's roof rather than collapsing!! Kudos to the builders, how many structures built today would be able to hold together like that? Now, did they eventually manage to demolish the (now upside down!) structure? What sort of construction, was it, re-inforced concrete slab?--220.101.28.25 (talk) 11:56, 26 December 2009 (UTC)
- That's a factory building (a flour factory, I think). Yes, reinforced concrete. It was built in 1928 and that says it all. Here in Turkey, newer buildings come down on their own. This one was built way back when adhering to building codes and standarts were the norm. 88.242.146.177 (talk) 19:12, 29 December 2009 (UTC)
Viscous flow of granite
[edit]Viscosity, Rheid, Granite. I'm kind-of-confused. I thought granite was a fairly solid rock and that if you pushed on it then it would snap. Rheid suggests that it flows in a viscous manner. Is that really the case? How does this stuff work? -- SGBailey (talk) 09:09, 25 December 2009 (UTC)
- The first sentence of our article says "Almost any type of rock can behave as a rheid under appropriate conditions of temperature and pressure." I think you are correct about the behaviour of cold granite, but perhaps you should wait for a Geologist to give an authoritative reply after their Christmas festivities. Happy Christmas! Dbfirs 09:51, 25 December 2009 (UTC)
- Yet Rheid suggests granite flows at STP -- SGBailey (talk) 10:04, 25 December 2009 (UTC)
- Yes was about to point that out. Specifically "Granite has a measured viscosity at standard temperature and pressure of ~4.5 • 1019 Pa·s [1] so it should be considered a rheid". BTW Christmas is over in 16 minutes Nil Einne (talk) 23:44, 25 December 2009 (NZDT; UTC+13)
- boot polish SpinningSpark 11:10, 25 December 2009 (UTC)
- On further thought, if granite snaps then it's clearly quite inelastic. Remember rheid "is a solid material that deforms by viscous flow. To be considered a rheid, deformation by flow should exceed elastic deformation by at least a factor of three." Something that snaps could easily have deformation by flow exceeding elastic deformation by a factor of 3 I guess. Something like elastic or a rubber band (or basically anything made from vulcanised rubber for example) which obviously has great elastic deformation clearly does not. Remember glass clearly shows viscous flow even if the examples commonly used like old stained glass windows are bullshit. Whether it's rheid, I don't know but I guess it isn't something commonly considered since glass is probably of limited interest to geologists Nil Einne (talk) 23:50, 25 December 2009 (NZDT; UTC+13)
- There are two contrasting types of behaviour, brittle-elastic and plastic (or ductile if you will). Which behaviour dominates in a material depends on three main factors - temperature , increased temperature promotes plasticity - confining pressure (the weight of overlying rock - i.e. how deeply it's buried), increased confining pressure suppresses brittle behaviour and the rate of the applied deformation, or strain rate, high strain rates lead to more brittle and low strain-rates to more plastic behaviour. As an example of contrasting properties depending on physical conditions, rock salt is a brittle elastic solid at surface temperatures unless it is wet, when it flows plastically such as in the salt glaciers of Iran, that move only in the wet season. Granite has been observed to deform plastically under bending (but at very low strain-rate) in a classic series of long-term experiments (decades) in Japan[4]. Mikenorton (talk) 13:09, 25 December 2009 (UTC)
- On further thought, if granite snaps then it's clearly quite inelastic. Remember rheid "is a solid material that deforms by viscous flow. To be considered a rheid, deformation by flow should exceed elastic deformation by at least a factor of three." Something that snaps could easily have deformation by flow exceeding elastic deformation by a factor of 3 I guess. Something like elastic or a rubber band (or basically anything made from vulcanised rubber for example) which obviously has great elastic deformation clearly does not. Remember glass clearly shows viscous flow even if the examples commonly used like old stained glass windows are bullshit. Whether it's rheid, I don't know but I guess it isn't something commonly considered since glass is probably of limited interest to geologists Nil Einne (talk) 23:50, 25 December 2009 (NZDT; UTC+13)
- Yet Rheid suggests granite flows at STP -- SGBailey (talk) 10:04, 25 December 2009 (UTC)
Keeping people alive on Xmas day
[edit]I was reflecting on the fact that it is quite sad for people to die on Xmas day (of course it is sad that they die at all, but it just seems that bit worse for the families). This got me to wondering whether there is any way to keep people alive for a few more days (or even hours) with any kind of extreme medical intervention. I am thinking of people who die of natural causes, and who are reached by medical personnel quickly but who, regardless of treatment, would die very quickly anyway. I am imagining something like giving them large, ongoing shots of adrenalin, or other time of extreme intervention. Of couse, no body would ever do this, at least not as a usual course of action, but I am just wondering what is possible. PaulRicks1983 (talk) 14:26, 25 December 2009 (UTC)
- It depends what you mean and what's the cause of the person dying (natural causes is a very broad concept). What some consider 'extreme medical intervention' are used all the time, e.g. medical ventilator, feeding tubes, artificial pacemakers, defibrillator and other life support measures. These would be of some use in some 'natural causes' cases but may not be used if it's expected there's no chance the person will live. It also depends on what you mean by dead. These measures may keep the body alive, but if the person is already brain dead then in reality all you have is a living body and while that understably be comforting to many, others may say the person is already dead even if they aren't clinically or legally considered dead. Further, as I understand it from TV shows (not a good source but this also not a good source suggests a similar thing [5]) and makes sense I guess that if CPR is being performed the time you stop may be counted as the time of death. Clearly you could go on for ever in the shows you do see them going on for too long (e.g. 40 minutes) or arguing over whether to stop (or even once I think waiting for the family to arrive although it's probably rarely this extreme in real life) but if you take it to an even further extreme, after a while the body is going to go cold and will eventually even start to rot. In other words, you can't say this person is 'alive' in any meaningful way. Incidentally, I'm not sure whether it would be much worse on the families for many of those in e.g. India, China. Even in places where Christmas is widely celebrated in some form, I don't think it'll necessarily be worse then other times, in fact it may be better. Particularly if happens late in the day and the person has already seen many of their families and friends. Or if it's not an instantenous death, family & friends might be more likely to be able to reach the person before death which can help in many cases. P.S. If we include things like cancer, there are of course a variety of measures which in some instances are very unlikely to elimate the cancer and therefore give the chance of a 'normal life' but which may prolong life by weeks, months or days (usually with some side effects i.e. the whole quality vs quantity thing). Nil Einne (talk) 15:53, 25 December 2009 (UTC)
- Caution: a whole load of OR follows. It seems to me, and has been confirmed by my sister in law who's a nurse, that many terminally ill people hang on to die after a holiday or a special event. I'm pretty sure I saw some research a couple of years ago which seemed to back this up, but I have no idea where or what. If someone who's bored on this holiday would like to find it for me then be my guest. (I've got family coming tomorrow and so won't have time.) --TammyMoet (talk) 20:06, 25 December 2009 (UTC)
- Purely anecdotal: On a Craigslist forum someone lost his mother to a blood infection from an acute kidney problem problem early this morning. She had hung on for a day or two. More scientifically, I remember reading that despite common belief, people die at a slightly higher rate during holidays due to perhaps an extended time it takes to take care of them. 67.243.1.21 (talk) 01:05, 26 December 2009 (UTC)
- I seem to recall a certain episode of M*A*S*H that involves a soldier dying shortly before midnight on Christmas and the subsequent faking of the death certificate to say December 26 instead. This seems like a much easier way to "accomplish" this. 75.157.57.12 (talk) 01:32, 26 December 2009 (UTC)
- The Edgar Allen Poe short story, "The Facts in the Case of M. Valdemar" is about a doctor who puts a dying man in a trance as he takes his final breaths. Ideas that human lives can be prolonged meaningfully this way, by Cryonics or by injecting adrenalin (Epinephrine) are all silly. See the article Death. The OP might consider medical personnel whose daily response is to actual needs rather than indulging in a sentimental attachment to a particular holiday. A subsidiary question is whether one has to be sick to die naturally (can one die healthy?). Cuddlyable3 (talk) 02:37, 26 December 2009 (UTC)
- I seem to recall a certain episode of M*A*S*H that involves a soldier dying shortly before midnight on Christmas and the subsequent faking of the death certificate to say December 26 instead. This seems like a much easier way to "accomplish" this. 75.157.57.12 (talk) 01:32, 26 December 2009 (UTC)
Wind mill & water turbine
[edit]is it possible to synchronize and combine the mechanical output of wind mill and water turbine and then use it for power genenration? —Preceding unsigned comment added by 203.175.69.210 (talk) 15:10, 25 December 2009 (UTC)
- I have given your question a seperate header as suggested in the instructions at the top so that it won't be lost, ignored or distracted from the earlier question. Nil Einne (talk) 15:21, 25 December 2009 (UTC)
- Since both output is rotational energy, sure, you can put both axes together in reverse (say by making one axis hollow), and then you have both rotational movements added, i.e., the outer rotation is both rotations added with respect to the inner axis. An application would be to fix a drill bit to one rotating axis, and fix the object to be drilled on the other axis, then lead them together so that their rotation is mutually reverse. --Ayacop (talk) 16:19, 25 December 2009 (UTC)
- Did you not like the last answer you got to this question? SpinningSpark 19:27, 25 December 2009 (UTC)
- It seems straightforward, even though waterwheel output is steady while wind is highly variable. The waterwheel can have its output controlled by the water inlet valve. The windmill can have its output controlled by varying the pitch of the propellor blades. If the wind stops or drops too low, then there would have to be a clutch to disconnect it so the waterwheel does not drive it like a fan. The output of the waterwheel should be continous and uninterrupted as long as the water level does not drop too low. But why would anyone want to combine them mechanically? For many years hydro generators and wind generators have been connected electrically via the power grid. The first such interconnect was at least as far back as the 1940's, and is quite common today, with photovoltaic also electrically synchronized via inverters. See Electrical grid, Sustainable energy. I've seen a mechanical synchronizer from a hydro turbine to maintain constant speed via a pair of rubber cones which were moved together and apart to adjust the gearing continuously. A clutch would still be needed, or the propellor could be adjusted so the blades are flat (no wind moved by propellor rotation) when they spin like a fan, the opposite of feathering the props in high wind, to reduce the energy waste when the wind drops. Edison (talk) 01:51, 26 December 2009 (UTC)
- Attach both machines to a Differential gear. They can then spin at different rates, and you can harvest power from both (or one). Ariel. (talk) 02:00, 27 December 2009 (UTC)
Relativistic mechanical reactionless drive?
[edit]Supposing two small neutron stars could be spun to relativistic speeds using enormous magnetic stirrers. If one neutron star/stirrer transferred all its spin to the other stirrer/star via a rod with an epicyclic reverse reduction gear, the stationary star would have less mass (due to relativistic effects) and could be rotated around the spinning star. The process could be reversed, leading to linear motion in space without reaction, in violation of Newtons Third Law of motion.The reverse reduction gear would stop the whole system from spinning and canceling out the effect. I hope. Incidentally, could this work using gyroscopic effects alone in a normal mechanical system working at non-relativistic speeds? 80.0.105.148 (talk) 15:32, 25 December 2009 (UTC)[Trevor Loughlin]
- "the stationary star would have less mass (due to relativistic effects)" - how does this happen? Why would it have less mass? First you say you have a stirrer, next the star is the stirrer? Which is it? What's a reverse reduction gear? What process can be reversed? Linear motion, after spinning? I think you are assuming you have a place to "attach" the stars to. You don't. If you try to transfer spin from one star to another using a gear, it won't do that - they whole system will spin instead, because you have no place to attach your gear, and the star is not attached to anything either. Ariel. (talk) 19:49, 25 December 2009 (UTC)
- For gyroscopes see Eric Laithwaite and Reactionless drive. 78.151.96.82 (talk) 20:21, 25 December 2009 (UTC)
Stopping the star would cause the whole assembly to spin, but then the other star would be spun up in the opposite direction, which would stop this. In any case the spin of the whole system (a long tube surrounding both stars at each end) would be vertical so this would not move the center of mass, merely spin the tube. When the less massive stationary star is made to orbit the more massive spinning star in the horizontal direction, the center of mass will change and the process can be repeated. However, this is all based on the idea that a star becomes heavier when it spins at relativistic speeds. Einsteins theory says that mass increases at near light speed. So an increase in rotational speed to relativistic speeds of part of a system will alter the systems center of gravity? Or am I misinterpreting the meaning of mass when applied to relativity? incidentally it could just as well be a flywheel rather than a neutron star, but in reality only an object made of degenerate matter would stay in one piece at such speeds.—Preceding unsigned comment added by 80.0.102.45 (talk) 13:17, 27 December 2009 (UTC)
- I think I see what you're getting at - transferring angular momentum from one star to the other will increase the mass of the accelerated star, and move the position of the centre of mass of the system as a whole. There's no need to use relativistic effects to do this - you could just use two tanks of water with a pump between them, and move the position of the centre of mass by pumping water from one tank to the other. The system will indeed move with respect to its centre of mass, and the speed and radius of its overall rotation will change - but, with no external force applied, the centre of mass won't move with respect to the surrounding space. Tevildo (talk) 15:10, 27 December 2009 (UTC)
- You might get linear motion, but it would be oscillatory. The stars would just oscillate relative to their stationary centre of mass, nothing more. --Tango (talk) 15:16, 27 December 2009 (UTC)
I think the crucial point that you are missing is the transfer of energy from one star to the other. If e. g. it is done with an electric generator and an electric motor then the electromagnetic fields carry mass-energy as they move along the tube (or gain energy at one end and lose energy at the other end) - and I'm quite sure that during that process the star which is spun up will move towards the center of gravity. Icek (talk) 08:31, 28 December 2009 (UTC)
Then forget about relativistic effects and neutron stars and instead imagine an old fashioned reel to reel tape recorder in outer space, and assume the tape is extremely heavy,perhaps incorporating lead weights all the way along its length,and there is another reel to reel backing on to it as a mirror image, winding in tape in the same direction. Will the system move whilst it is doing this or stay in the same place? Answer this question before I continue.
o_____o
_____ ----> both tapes wound in the same direction.
o o —Preceding unsigned comment added by 80.2.207.220 (talk) 13:03, 28 December 2009 (UTC)
- Yes, the heavier star/tape reel will move towards the centre of gravity. However, the centre of gravity _won't_ move unless an external force is applied. You can move the system as a whole around its centre of gravity (and not violate Newton's Third Law - the force needed to move the system is reacted against the motor which drives the tape, in your second example), but you can't move the centre of gravity itself. Tevildo (talk) 13:55, 28 December 2009 (UTC)
Unofficial battery charger
[edit]I recently lost the charger to my Nintendo DS and my mother bought me a new one - which can charge a DS, DS Lite or PSP - made by a company called "Quick Act". Now, I know that over time, any battery will gradually wear out and won't last as long as when it was new, no matter how well it's taken care of. My question is, would using an unofficial charger exacerbate this problem? Would the battery run down faster using a poor-quality charger (it's safe to assume it's poor-quality since my mother bought it...) in comparison to the (presumably high-quality) official charger? Vimescarrot (talk) 15:37, 25 December 2009 (UTC)
- There are two problems you can get with cheap chargers. The first is overcharging, which will definitely shorten the battery life if you leave the batteries connected for too long. A good charger will change to trickle charge when the batteries are getting near full charge. If your charger does not do this you must make sure it is only left charging for the prescribed time. The second problem is that some chargers (in order to avoid the first problem and to make them even cheaper because they do not have to deliver high currents) only work as trickle chargers and will take longer to charge the batteries than it takes to discharge them in operation. No idea what you have got - read the instructions :) SpinningSpark 19:35, 25 December 2009 (UTC)
- What Spinningspark said. :) It's also possible, however, that the charging circuit is actually in the DS itself, and all the "charger" does is to provide power to it - this is the situation with mobile phones. In this case, the "quality" of the charger doesn't matter, as it's just providing fixed DC. See also Battery charger and Lithium-ion battery for more details. Tevildo (talk) 22:01, 25 December 2009 (UTC)
- Ahh, neat - how might I find out if the DS has such a thing? Vimescarrot (talk) 22:38, 25 December 2009 (UTC)
- I don't have a DS myself, but, looking at some pictures, it seems that the charging monitor light is on the DS itself rather than the charger. This would suggest to me that the charging circuit is in the DS. I also note that you can buy "chargers" for £1.75. :) That will barely cover the cost of the _plug_, let alone anything but the most basic circuitry. I've also seen some forum postings that suggest it's possible to recharge the DS by connecting it directly to a USB port. It's not a definitive answer, but... Tevildo (talk) 00:29, 26 December 2009 (UTC)
- Hmm. Alrighty, thanks. I think I'll risk it! :p Vimescarrot (talk) 00:39, 26 December 2009 (UTC)
- I don't have a DS myself, but, looking at some pictures, it seems that the charging monitor light is on the DS itself rather than the charger. This would suggest to me that the charging circuit is in the DS. I also note that you can buy "chargers" for £1.75. :) That will barely cover the cost of the _plug_, let alone anything but the most basic circuitry. I've also seen some forum postings that suggest it's possible to recharge the DS by connecting it directly to a USB port. It's not a definitive answer, but... Tevildo (talk) 00:29, 26 December 2009 (UTC)
- Ahh, neat - how might I find out if the DS has such a thing? Vimescarrot (talk) 22:38, 25 December 2009 (UTC)
Elements and the three states of matter
[edit]Can all naturally occuring elements be heated or frozen into the three basic states of matter? Lova Falk (talk) 17:32, 25 December 2009 (UTC)
- Not at atmospheric pressure, but if you can change the pressure then yes, I believe so. --Tango (talk) 17:47, 25 December 2009 (UTC)
- To be specific, the ones you can't get at atmospheric pressure are solid helium, liquid carbon, and liquid arsenic. Despite apperances, iodine _does_ liquify at atmospheric pressure. Tevildo (talk) 18:13, 25 December 2009 (UTC)
- Thank you! Lova Falk (talk) 18:30, 25 December 2009 (UTC)
- Is that it? I would have thought there were more than that. --Tango (talk) 19:02, 25 December 2009 (UTC)
- The vast majority of elements are solid metals which can all be melted. SpinningSpark 19:22, 25 December 2009 (UTC)
- Incidentally, although the arsenic and sublimation articles say that arsenic sublimes at atmospheric pressure, List of elements by melting point says it doesn't. I'll do some reference checking and make the appropriate corrections. Tevildo (talk) 21:35, 25 December 2009 (UTC)
- Done. List of elements by melting point is now correct. Tevildo (talk) 21:51, 25 December 2009 (UTC)
- Don't forget the classic example of carbon dioxide, in which a liquid state does not exist until pressures exceed 5.1 atmospheres. ~AH1(TCU) 02:34, 26 December 2009 (UTC)
- True - many substances undergo sublimation at atmospheric pressure. However, carbon and arsenic are the only elements that do so. Tevildo (talk) 02:58, 26 December 2009 (UTC)
- Don't forget the classic example of carbon dioxide, in which a liquid state does not exist until pressures exceed 5.1 atmospheres. ~AH1(TCU) 02:34, 26 December 2009 (UTC)
- Done. List of elements by melting point is now correct. Tevildo (talk) 21:51, 25 December 2009 (UTC)
El Niño-Southern Oscillation
[edit]Hi. When the El Niño warm pool of water cuts off the Humboldt Current during a strong El Nino event (more than 1.5C above normal in the Niño 3.4 region), where does the upwelling of cold water resurface? Does this occur in the Western equatorial and north Pacific, or in the Northeastern Pacific, or in the Southwestern Pacific? Let's say the current is both cut off at the southern end, as well as blocked at the equator, the northern coast (Peru-Chile border), and from the west, as it is now[6], where does it resurface, or does it have significant effects on the global Thermohaline circulation? Thanks. ~AH1(TCU) 18:14, 25 December 2009 (UTC)
Fires in the Amazon rainforest and ENSO
[edit]This is a related question to my original one. In which years were forest fire activity higher than normal, due to higher-than-average temperatures and drought (and possibly other factors such as lightning, arson, and slash-and-burn)? Was El Nino present in those years, as it was in 2002 and 1997, when forest fires threatened forests and peat bogs in Borneo? ~AH1(TCU) 03:23, 26 December 2009 (UTC)
replacing -COOH with -CH3
[edit]How would I approach this? I was thinking reduction .... use lithium aluminum hydride then proceed to elimination, forming an alkene that I can then reduce? (The proton is prolly a problem, so I guess use the ester...)
(Also, how is LiAlH4 used in the reduction of protic amides? Even if you use an excess, isn't the formation of anionic amide nucleophile kind of a problem?)
Or maybe, use decarboxylation with some methyl halide in it? (If there's an amine group in the compound, e.g. an amino acid, do I have to protect the amine, or can I just use strongly acidic conditions? Would that inhibit decarboxylation?) It's actually okay if the amino group picks up a methyl group, but two methyl groups would be bad. Help??? John Riemann Soong (talk) 18:26, 25 December 2009 (UTC)
- Anyone? I know decarboxylation is a step required for methyltransferase to work... any way of duplicating the same idea in vitro? John Riemann Soong (talk) 06:08, 26 December 2009 (UTC)
- Reduction of a carboxylic acid with LAH would work fine...the first equivalent of hydride does react with the acidic proton, but LAH is a strong enough reducing agent to reduce that carboxylate. But given your alternative is decarboxyliation, your whole scenario is not well-formed...one way shortens the carbon chain, one does not. You'll need to ask specific, well-explained situations rather than vague ideas...given your history here, you'd better know that the devil is in the details and the details you leave out are the ones that cause you trouble later! DMacks (talk) 04:01, 28 December 2009 (UTC)
- This involves reduction of amino acids. (I'm using the skeleton of the amino acids as building blocks for larger compounds, but the COOH group is in the way.) What's the most feasible/practical way of going about this? I was thinking doing a low-temp reduction with DiBalH, stopping at the aldehyde and then reducing further with Clemmensen. (Generally, Clemmensen is cheaper / less messy than Wolf-Kishner, right? Or is W-K easier to work up since the waste product is N2?)
- Are there any enzyme complexes (that would work in vitro) that would transform COOH to CH3? I was thinking decarboxylation effectively creates a carbanion nucleophile, which would go on to attack an alkyl halide ... but prolly quite problematic with an amino group? Also, is there any way to encourage monosubstituted product? John Riemann Soong (talk) 04:40, 28 December 2009 (UTC)
- Decarboxylation/methylation is incredibly difficult to control. N-alkylation, loss of stereochemistry, etc., and even very difficult to decarboxylate at all unless the carbanion is stabilized. You're safer and saner to just reduce the COOH to CH3. Generally, both W-K and Clemmensen are messy—extreme acid or base, possible use of mercury or high-temperature—you have to decide what your substrate can tolerate (if either). And the success of Dibal reduction that is controlled enough to stop at the aldehyde is substrate-dependent—"looks great on paper". There are lots of ways of reducing alcohols to alkanes (deoxygenation via radical reactions is especially popular there...several "name reactions" for several variations). Lots of people like to use a tool called "google" to look up information...you can type in a phrase and it will give you some references (and even try to find something close if there is no exact hit). For example, "reduce carboxylic acid to methyl" first result is exactly what you want. DMacks (talk) 05:08, 28 December 2009 (UTC)
- I did google it .... but I used abbreviations. Oh hmm. John Riemann Soong (talk) 05:26, 28 December 2009 (UTC)
- Decarboxylation/methylation is incredibly difficult to control. N-alkylation, loss of stereochemistry, etc., and even very difficult to decarboxylate at all unless the carbanion is stabilized. You're safer and saner to just reduce the COOH to CH3. Generally, both W-K and Clemmensen are messy—extreme acid or base, possible use of mercury or high-temperature—you have to decide what your substrate can tolerate (if either). And the success of Dibal reduction that is controlled enough to stop at the aldehyde is substrate-dependent—"looks great on paper". There are lots of ways of reducing alcohols to alkanes (deoxygenation via radical reactions is especially popular there...several "name reactions" for several variations). Lots of people like to use a tool called "google" to look up information...you can type in a phrase and it will give you some references (and even try to find something close if there is no exact hit). For example, "reduce carboxylic acid to methyl" first result is exactly what you want. DMacks (talk) 05:08, 28 December 2009 (UTC)
Attaching a light to a wall
[edit]Hi. I have a light that I need to attach to an interior wall (the wall is painted plaster, no wallpaper). The light probably weighs about 500g. Because I am living in rented accomodation I cannot drill into the wall (even if I agree to laster fix it), so I need a way to fix it to the wall without damaging the wall or the paint. I imagined that there would be some kind of "sticky pad" that would hold the weight but allow itself to be peeled away eventually, but I have tried all kinds of Google searches and searches on shopping sites but can't find anything matching that description. Could anybody please recommend how I could attach this light? Thanks StickyProblem (talk) 18:32, 25 December 2009 (UTC)
- Yes, you can buy various double-sided tapes, self-adhesive Velcro and "adhesive foam pads" (try a Google search) which are possibly strongest, but you should be advised that any such method of fixing may not be suitable for heavy electrical fittings because, however strong the adhesive, the painted surface of the wall may not have the equivalent strength. Dbfirs 19:52, 25 December 2009 (UTC)
- To hold 500g you will need a very big pad - that way the weight is spread over lots of paint and it should be fine. --Tango (talk) 20:01, 25 December 2009 (UTC)
- Try these: 3M Command Strips Ariel. (talk) 19:54, 25 December 2009 (UTC)
- I was thinking some type of suction cup? If the surface is smooth enough --220.101.28.25 (talk) 20:02, 25 December 2009 (UTC)
- Wedge a suitably shaped piece of wood ceiling to floor. Attach light to wood. Smaller and lighter the wood the better from an aesthetic viewpoint. Advantage: little to no attaching to the wall. Disadvantage: it looks stupid. Bus stop (talk) 20:11, 25 December 2009 (UTC)
- Why did someone delete this contribution? Here it is again: The landlord will not be pleased as the tape will almost certainly damage the surface when removed, particularly if it is tough enough to support 500g. I suggest putting the light on a pole, or asking the landlords permission to pay for an electrician to fit a proper light. Merely sticking it to a wall will be unsafe and dangerous. 78.151.96.82 (talk) 20:07, 25 December 2009 (UTC)
- (after edit conflicts) Even on smooth glass, suction cups eventually fall off. If your fitting is low voltage and there is something soft underneath for it to fall on, then Tango's large pad of Ariel's 3M strips (or Foam tape) will do the job, but be aware that when you remove the fitting you might take paint off the wall. Dbfirs 20:26, 25 December 2009 (UTC)
- [7] I would guess there was an edit conflict and it didn't inform Tango Nil Einne (talk) 09:17, 26 December 2009 (UTC)
- A small screw with a small rawplug is likely to be less unsightly after the light is removed, then a large blemished patch where a large amount of sticky tape was. Safer too. 78.151.96.82 (talk) 21:03, 25 December 2009 (UTC)
- The largest 3M Command strips are claimed to be able to support 3.4kg - that's more than six times as much as our OP requires - they also claim not to leave a mark on the wall when they are ultimately removed. This seems by far the most logical solution here. After all, there are only three options:
- Hang it from the ceiling: Which involves either something adhesive or something with suction or making a hole.
- Hang it from the wall: Same deal - adhesive, suction or hole.
- Make a stand so it can be supported from the floor - a table lamp or a taller 'Torchiere' - or BusStop's idea of wedging a piece of wood floor-to-ceiling and bolting the lamp to that.
- If the fitting is designed to be wall-mounted then it's likely that neither (1) nor (3) would be acceptable. If we can't make holes in the wall then you're left with adhesive or suction. You need a removable adhesive and IMHO, 3M leads the world in that kind of product - so using their latest gadget makes the most sense. If you wanted to consider suction cups (and presuming the wall is smooth, shiney and flat) then these claim to support up to 10lbs. Personally, I'd go with the 3M gadget.
- SteveBaker (talk) 01:29, 26 December 2009 (UTC)
- The 3M hooks are widely used as long as the surface is suitable. The adhesive removes by pulling a tab. Wedging a long plank or pilaster from floor to ceiling would be the next best solution. The wiring could be concealed behind the plank or pilaster. The bottom could be trimmed to fit snugly around the baseboard. A pair of wedges could be tapped in to secure it, with a protective pad to protect the ceiling surface. Care is needed to avoid denting the surface by too much pressure. Trim could then be attached to conceal the wedges. I might do this if I were planning to live there a long time. Edison (talk) 03:40, 26 December 2009 (UTC)
- As a landlord I have bad experiences with stick-on hooks. I often find many of them - they must be sold in multiple packs - all over the house after the tenants leave, and they always damage the surface when you remove them, requiring the surface to be smoothed and the room to be redecorated. Bear in mind that you will be sticking anything to the paint over the plaster, and not the plaster. In my experience the paint will invariably come off when you remove them, and perhaps even some of the plaster too. 92.24.73.139 (talk) 15:16, 27 December 2009 (UTC)
- As a landlord myself (I have one house in the UK and another in the US that are rented out), I suggest you invest in a better grade of paint and plaster! I rarely have this problem in either of the houses I rent out (although I have plenty of other problems with inconsiderate tenants). Besides, these 3M hooks are very new and they say (in an unqualified manner) that they won't damage the surface. If your tenants use these new kinds of hook and the wall suffers then I think it's fair to say that they did 'due diligence' and you should seriously consider whether it's your own fault. However - I doubt very much that you are talking about these new-style 3M hooks - some of the older kinds were indeed likely to damage the wall. SteveBaker (talk) 17:58, 27 December 2009 (UTC)
- As a landlord I have bad experiences with stick-on hooks. I often find many of them - they must be sold in multiple packs - all over the house after the tenants leave, and they always damage the surface when you remove them, requiring the surface to be smoothed and the room to be redecorated. Bear in mind that you will be sticking anything to the paint over the plaster, and not the plaster. In my experience the paint will invariably come off when you remove them, and perhaps even some of the plaster too. 92.24.73.139 (talk) 15:16, 27 December 2009 (UTC)
- The 3M hooks are widely used as long as the surface is suitable. The adhesive removes by pulling a tab. Wedging a long plank or pilaster from floor to ceiling would be the next best solution. The wiring could be concealed behind the plank or pilaster. The bottom could be trimmed to fit snugly around the baseboard. A pair of wedges could be tapped in to secure it, with a protective pad to protect the ceiling surface. Care is needed to avoid denting the surface by too much pressure. Trim could then be attached to conceal the wedges. I might do this if I were planning to live there a long time. Edison (talk) 03:40, 26 December 2009 (UTC)
Gears
[edit]How to design a simple gear —Preceding unsigned comment added by 202.162.160.246 (talk) 19:31, 25 December 2009 (UTC)
- We're going to need way more detail than that. But, read gear, and all the pages linked to it about various types of gears. Ariel. (talk) 19:50, 25 December 2009 (UTC)
- Also, if you plan to design the gear to articulate with another gear (or anything else), you might read gear ratio. Nimur (talk) 23:11, 25 December 2009 (UTC)
- A very simple gear is a lantern gear, which is not covered very well in the Gear article, nor does it have its own well deserved article. These were used in early machines from the 1500's, in clocks as well as large machines like windmills and waterwheels. One gear consists of pegs projecting out from a rotating shaft, either radially from the shaft or parallel to the shaft, extending from a disc attached to the shaft. The other gear is a sort of cage, with two discs having between them a series of staves parallel to the shaft the discs are connected to. The rotation of either shaft causes the projecting pegs to engage the staves of the cage. No precise machining of gear teeth is required as for later gear arrangements. You could make one out of dowel rods and plywood. You might be able to make one out of Tinker Toys. Varying gear ratios are had by varying the number of pegs on one shaft versus staves on cage on the other shaft. Here is another version, where the pegs project upward from a disc attached to one shaft, rather than projection radially. This version is illustrated here in a mule powered irrigation system. Here is a modern drawing of a lantern gear driven by wind power to pump water. Here is one from American colonial days, where a water wheel drives a mill. Here is another illustration from 1661. A gear ratio and 90 degree axis change are accomplished. If I recall correctly, Diderot discussed them in his early Encyclopédie in the 1750s. They were commonly used for hundreds of years in machines large and small, and do not require a foundry or precision machine tools. There is likely much more friction than in fancier metal gear teeth. Edison (talk) 01:21, 26 December 2009 (UTC)
- Also, if you plan to design the gear to articulate with another gear (or anything else), you might read gear ratio. Nimur (talk) 23:11, 25 December 2009 (UTC)
Why is Swine flue in decline this winter?
[edit]The number of cases in the UK and in the US has been declining this winter. Why is this despite the increasingly cold weather of winter, when a layperson's view would expect things to get worse? 78.151.96.82 (talk) 19:58, 25 December 2009 (UTC)
- Because lots of people have already had it and are now immune. There is also lots of Tamiflu (and similar antivirals) and even some vaccines available. The cold weather would tend to increase infection rates (indirectly - it's people spending more time indoors with each other, not the coldness itself that does it) but it seems the opposite effect from increased immunity and drug availability is having a greater effect. --Tango (talk) 20:07, 25 December 2009 (UTC)
I thought that only a small proportion of the population had had it. If that is the case, then immunity would niot be the reason. 78.151.96.82 (talk) 20:15, 25 December 2009 (UTC)
- It's a few percent, I think (there are no accurate numbers now since they aren't testing most people). That is enough to make a difference, particularly when you considered things at a more local level where percentages in some areas will be much higher. --Tango (talk) 20:28, 25 December 2009 (UTC)
I have to say I'm doubtful a few percent would make a difference, as then epidemics like the Black Death would never happen. 78.151.96.82 (talk) 21:05, 25 December 2009 (UTC)
- A few percent immunity won't prevent an epidemic on its own, but it does make a difference, particularly, as I said, on a small scale. A few percent nationally will mean there are places with very high immunity, which makes a difference. Also, those that are immune will, disproportionately, be those most at risk of getting infected, since they are the most likely to have already been infected. --Tango (talk) 21:57, 25 December 2009 (UTC)
- That sounds very unlikely, do you have a source? I believe immunisation programs require a take up rate of 60-80% to be effective. SpinningSpark 23:58, 25 December 2009 (UTC)
- For something like smallpox where the aim was to eliminate the disease entirely, you need something like 95%, I think. When you are just trying to reduce the chance of an epidemic, much smaller amounts are useful. To effectively eliminate the chance of an epidemic you need really high rates, but it is still beneficial to reduce the risk even if you don't eliminate it. --Tango (talk) 15:01, 26 December 2009 (UTC)
- Another reason could be that many people in developed countries have also had the swine flu vaccine, and are now immune to the virus. Getting the virus once does not give a person complete immunity from swine flu, just as getting the seasonal flu once does not make you immune to it, and there was little natural immunity in the human population to begin with. However, currently the rates of contraction of the virus by month during the year seem similar to the historical H1N1 1918 flu pandemic, only without the high death rate. ~AH1(TCU) 02:17, 26 December 2009 (UTC)
- (edit conflict)Well, first off, Tango said a difference, not an effective one. Second of all, 2009 flu pandemic in the United States says about 1/6 of Americans has potentially contracted it - definitely overly significant, especially when you consider herd immunity. ~ Amory (u • t • c) 02:19, 26 December 2009 (UTC)
- That sounds very unlikely, do you have a source? I believe immunisation programs require a take up rate of 60-80% to be effective. SpinningSpark 23:58, 25 December 2009 (UTC)
- It's possible that many more people have had swine flu than the reported figures suggest. There was a news report last week in the UK which claimed that, for most people, swine flu was no worse than a bad cold, and many people in the UK have had bad colds this autumn which could actually have been swine flu - as nobody is being tested except people who are really, really ill with flu symptoms this is speculation. (OR both myself and my husband had symptoms which match those given for swine flu: they weren't bad enough to keep us at home, never mind in bed!) Also the recent cold snap will have killed off many bugs, swine flu included. --TammyMoet (talk) 10:12, 26 December 2009 (UTC)
- The key difference in the symptoms between a cold and flu (and swine flu has identical symptoms to other strains of flu) is the fever. Colds don't usually have an associated fever, flu almost always does. If people aren't taking there temperatures then there is no way they can really know what is wrong with them. Also, even those that have definitely had flu could have had any strain - the UK stopped routinely testing to see what strain people had months ago. That means all the numbers are extremely imprecise. I expect that 1/6 figure is an upper estimate - it seems rather higher than I'd expect for a mid-range estimate. --Tango (talk) 14:57, 26 December 2009 (UTC)
- It is not a few percent: The U.S. CDC estimates that by the middle of November, 34 million and 67 million Americans had already had it (and around 10,000 had died from it).[8] That is around 10-20% of the U.S. population. About 60 million have been vaccinated[9]. Although there is certainly overlap in the two groups and large error bars on the estimates, as many as 40% of the U.S. could already be immune to it. 75.41.110.200 (talk) 15:04, 26 December 2009 (UTC)
- According to the Public Health Agency of Canada[10] and Google Flu Trends[11], the flu epidemic peaked in Canada around the first or second week of November, and has been since on the decline. Since the vaccine was distributed in Canada in late October, it's possible that higher rates of immunity to the virus, as well as the virus being circulated in the vaccine being much less virulent (according to the article, "the virus is first adapted to grow at 25°C and then grown at this temperature until it loses the ability to cause illness in humans, which would require the virus to grow at our normal body temperature of 37°C. Multiple mutations are needed for the virus to grow at cold temperatures, so this process is effectively irreversible and once the virus has lost virulence (become "attenuated"), it will not regain the ability to infect people."). However, the H1N1 flu seems to have become the dominant strain in Canada, as close to 100% of the tested cases were pandemic H1N1 since late October, although total flu activity has now declined to ordinary seasonal levels. ~AH1(TCU) 19:12, 26 December 2009 (UTC)
- Another possibility, though I freely admit it's rampantly cynical, is that the media have moved on to other things. Repeating what's effectively the same story ("some people have had flu, it's a DEADLY NEW STRAIN but there's no need to panic") over and over again doesn't sell newspapers. Tonywalton Talk 01:17, 28 December 2009 (UTC)
Color Of Rosiglitazone Maleate (Avandia)
[edit]I have seen brown and pink or light pink rosiglitazone maleate (Avandia) tablets. Does this drug come in all white?174.3.102.6 (talk) 23:52, 25 December 2009 (UTC)
- According to this, the genuine product (in the UK, at least) will only come in pink 2mg, orange 4mg or brown 8mg tablets, marked "GSK" Glaxo SmithKlein. It's not out-of-patent yet, so nobody can legally manufacture generic equivalents. Does "only get prescription drugs from a real pharmacist using a real prescription that a real doctor has written for you" count as medical advice? I hope not - if it does, though, please can someone delete it? Tevildo (talk) 00:47, 26 December 2009 (UTC)
- It's good advice.Cuddlyable3 (talk) 01:32, 26 December 2009 (UTC)
- Concur 101% with Cuddlyable3. You don't need a medical degreee or MD registration (I hope) to give bit of old fashioned common-sense. --220.101.28.25 (talk) 05:08, 26 December 2009 (UTC)
