Wikipedia:Reference desk/Archives/Science/2010 May 8
| Science desk | ||
|---|---|---|
| < May 7 | << Apr | May | Jun >> | May 9 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
May 8
[edit]Reaction of chromates with acid
[edit]I am still analyzing chromates in my home lab. When hydrochloric acid is added to chromates, it turns black instantly. About 2 seconds later, it fizzes Cl2 furiously and turns green (the color of CrCl3) again. When sodium hypochlorite is added, it turns yellow again, showing the formation of chromates. My question is: What is the intermediate unstable (seems like strong oxidizer, produces Cl2) black substance? It looks similar to CrO5, chromium peroxide, but I didn't add any peroxide to the solution, just HCl. Thanks.--Chemicalinterest (talk) 00:41, 8 May 2010 (UTC)
- Could you please give me the formula for the chromate you react HCl with? --The High Fin Sperm Whale 02:12, 8 May 2010 (UTC)
- Na2CrO4 --Chemicalinterest (talk) 11:22, 8 May 2010 (UTC)
- Could it be CrO2Cl2? Just an idea. 67.170.215.166 (talk) 05:10, 8 May 2010 (UTC)

Manganese dioxideGraeme Bartlett (talk) 08:10, 8 May 2010 (UTC)- And where, may I ask, did the manganese come from? 67.170.215.166 (talk) 08:13, 8 May 2010 (UTC)
- No Mn in the solution, just Na2CrO4 and NaCl with a very little H2O2, not enough to make the dark black color. --Chemicalinterest (talk) 11:25, 8 May 2010 (UTC)
- In the chromyl chloride article it talks about reaction of potassium chromate with hydrochloric acid, followed by sulfuric acid as a desiccant. What happens if the sulfuric acid is not added, which is similar to what I did? --Chemicalinterest (talk) 11:52, 8 May 2010 (UTC)
- A possible reaction to form CrO2Cl2 is: Na2CrO4 + 2 HCl → CrO2Cl2 + 2 NaOH --Chemicalinterest (talk) 11:55, 8 May 2010 (UTC)
- Then: 2 CrO2Cl2 + 8 HCl → 2 CrCl3 + 4 H2O + 3 Cl2 --Chemicalinterest (talk) 18:02, 8 May 2010 (UTC)
- The big issue I have with that mechanism is that you seem to be proposing that HCl forms chromic acid. Then apparently Cl- is strong enough of a nucleophile to attack the Cr(VI) centre and push out OH- as a leaving group. OH- is a horrible leaving group. Now, perhaps what happens is that the oxygens get "doubly protonated" and leave as water. Is Cr(VI) that weak of a Lewis acid that Cl- can displace HOH from it? John Riemann Soong (talk) 03:26, 10 May 2010 (UTC)
- Btw, in water the reverse reaction is strongly favoured. Chromyl chloride is basically a Cr(VI) version of an acyl chloride or a phosphoryl chloride -- it hydrolyses to give HCl. John Riemann Soong (talk) 03:34, 10 May 2010 (UTC)
- No Mn in the solution, just Na2CrO4 and NaCl with a very little H2O2, not enough to make the dark black color. --Chemicalinterest (talk) 11:25, 8 May 2010 (UTC)
- And where, may I ask, did the manganese come from? 67.170.215.166 (talk) 08:13, 8 May 2010 (UTC)
I've been trying to work out a possible mechanism on paper... and here's what I think happens.
- Chromate gets protonated in water to form various protonated species of chromic acid.
- Chloride doesn't attack the Cr(VI) centre directly to form chromyl chloride. Cl- is too soft of a nucleophile to do that. Instead it donates into an oxygen (does the Cr=O bond involve pi bonds, or no?), reducing Cr(VI) to Cr(IV), and forming a "chromium (IV) hypochlorite".
- This Cr(IV) formation indirectly allows for the displacement of a water molecule as a leaving group.
- Another Cl- ion comes along and reduces the hypochlorite into an oxide, forming chlorine gas.
- Anhydrous Cr(IV) is chromium dioxide ... an insoluble black precipitate. Industrially it is formed at high temperatures at 30000 psi but it would form a plausible reactive intermediate in acidic conditions.
- However, Cr(IV) dioxide exists in equilibrium with its protonated, soluble form, but dissolution is not complete.
- The oxidation of Cl- via the reduction Cr(IV) to Cr(III) is slow. I say this because it's hard to find a plausible mechanism for it. Cr(IV) is less electronegative. Two options: Cr(IV) to Cr(II), which is instantly oxidised to Cr(III), or Cr(IV) to Cr(III) via single electron transfer. John Riemann Soong (talk) 04:42, 10 May 2010 (UTC)
- I might not understand all of your terms since I just finished 11th grade, but I guess what you're saying in the first part is that HCl does not react with Na2CrO4 to form H2CrO4 and NaCl.
- Why is the chromate protonated in water? I added HCl, which would protonate it easier than water. Normally neutral solutions, such as Na2CrO4 and NaCl, do not hydrolyze to form chromic acid and sodium hydroxide, or hydrochloric acid and sodium hydroxide respectively. It would probably be protonated by the HCl.
- Hypochlorites aren't too easy to form. I don't think Cr(VI) is strong enough.
- But why does it fizzle so rapidly when HCl is added? Maybe the reaction mechanism is impure. If you want to try it, you can. Dissolve nichrome heating element in HCl (dark green solution). React it with alkali and filter the precipitate(dark green precipitate, turns brown). React the precipitate with household bleach (some fizzing, yellow solution). Add small amounts of hydrogen peroxide to get rid of the remainder of bleach so the bleach doesn't react with the acid directly(some fizzing, if too much H2O2 then it turns dark purple). Filter the solution and collect the filtrate. Add about an equal amount of acid to the filtrate. It should turn black instantly, then start fizzing furiously, clearing up to a green solution again. Add more bleach and it will turn yellow again, with the possible release of Cl2. --Chemicalinterest (talk) 12:16, 10 May 2010 (UTC)
- Some reactions I figured out, John Riemann Soong:
- 2 Na2CrO4 + 4 HCl → 2 CrO2 + 2 NaOCl + 2 NaCl + 2 H2O [black precipitate]
- 2 CrO2 + 8 HCl → 2 CrCl3 + Cl2 + 4 H2O [fizzing, clearing to green solution]
- These reactions are not proven. --Chemicalinterest (talk) 14:45, 10 May 2010 (UTC)
- Well, CrO4- is a very weak base. I meant it gets protonated by HCl, of course. Chromic acid has a pKa of 0.74. H3O+ has a pKa of approximately -1.7 -- H3O+ will protonate chromate outright. No indeed, it might form some equivalent of NaCl and chromic acid.
- The thing to notice is the reduction potential of Cl2 (1.36) versus Cr(VI) (+1.33 for dichromate). Calculate the net redox potential for the entire reaction. In fact the potentials are so close that I think you could very well have an equilibrium reaction. An excess of hypochlorite will lead to formation of Cr(VI) from Cr(III). An excess of Cr(VI) (or chloride!) will lead to the formation of hypochlorite and the reduction of Cr(VI) to Cr(III). Also hypochlorite + acid <== (eq) ==> chlorine + water.
- Try varying the excesses. Try lots of chromate and a modest amount of acid, or a modest amount of chromate and a generous amount of acid. Just how much hypochlorite did you add? John Riemann Soong (talk) 19:31, 10 May 2010 (UTC)
- What would be really telling, if after you reoxidised Cr(III) into Cr(VI) with hypochlorite, is if you added some plain ole NaCl. If it is indeed an equilibrium reaction, an excess of Cl- will reduce some of the Cr(VI) into Cr(IV) or Cr(III).John Riemann Soong (talk) 19:39, 10 May 2010 (UTC)
Is it possible to shoot a bear in the head?
[edit]Hello all, I'm a writer and I've recently completed a novel in which a weatherman gets attacked by a bear and the Civil Air Patrol has to drop a surgeon to him by parachute to save his life. Well anyway, in the opening scene the bear attacks the weatherman, whose own shotgun backfires in his face (I believe some of you might remember me asking about it under a different IP address), and the weatherman (having been partially, but not completely, blinded but still fully conscious) tries to fight off the bear with his bare hands and by hitting it with the gun butt until his partner arrives and shoots the bear in the head. So far, most of the folks who have read the novel seem to like the action, but many of them have also pointed out some bad details to me (mostly in the dialogues). One reviewer, however, objected to something entirely different: he told me that a bullet would not pierce a bear's skull because it's too thick and also sloped like the armor on a tank, so the bullet would just bounce off. Is it true, what he said? And if so, does it depend on the aspect angle of the hunter with respect to the bear (e.g. possible to shoot from the side but not from the front)? I'm not a hunter, so I'd really like to know. Thanks in advance! 67.170.215.166 (talk) 05:08, 8 May 2010 (UTC)
- Well, if it's a brown bear (which I presume it is, since black bears don't attack people that way), the really implausible thing is that a person could put up meaningful resistance using bare hands and a rifle butt -- your weatherman better be one hell of an athlete. But that being said, whether a bullet would penetrate the skull depends a lot on the gun. Is is a pistol or a high-powered rifle? If it's a pistol, not only would the bullet probably not penetrate the skull, but the brain is so small compared to the head that you'd be likely to miss it in any case. Shooting for the heart is a much more viable proposition. Looie496 (talk) 05:50, 8 May 2010 (UTC)
- The preferred way to take a bear is to shoot it in the shoulder, with a high-powered, fairly small-bore rifle (say, a .30-06 caliber). These rounds are small and fast, and can penetrate the thick skin and fur (and bone), and shatter the shoulder. This incapacitates the bear; it can then be approached and finished with a larger-caliber weapon, e.g. a pistol. Even a high-powered rifle round will (supposedly) bounce off the skull; at the very least, it is not considered fatal and is considered unnecessarily cruel and harmful to the bear (induces slow death). (I am not a hunter and don't really approve of taking bears for sport, but I know a bit about the subject. During radio-science field-work in the great Alaskan interior and Kodiak Island, I regularly stayed in hunting lodges, a few of which were operated by commercial bear hunt guides - there aren't always motels where you want to study radio signals!) Nimur (talk) 06:00, 8 May 2010 (UTC)
- You probably have underestimated the power a gun can generate. It is nearly one mile per second! Yes, small bores like .22 or .25 or may be even a 9mm can not do much damage, but bores greater than that can really incapacitate a bear etc. in no time. A buckshot from a short-barrled 12 gauge will tear bear's skull apart. Anything larger, a 10-gauge if you can manage will blow the skull literally off. But the recoil may hurt even you (the shooter)People have killed elephants using big guns.Besides there are special revolvers made by Smith and Wesson to kill bears [[1]] Jon Ascton (talk) 07:50, 8 May 2010 (UTC)
- For this discussion, assume Colt 45 automatic to the side of the head at point-blank range. And FYI, the bear is a polar bear. 67.170.215.166 (talk) 08:01, 8 May 2010 (UTC)
- Oh, and did I say that the bullets are of the hollow-point type? 67.170.215.166 (talk) 08:09, 8 May 2010 (UTC)
- Hilaire Belloc recommended platinum bullets. Gandalf61 (talk) 10:07, 8 May 2010 (UTC)
- Even if the bullet didn't penetrate the bear's skull, it would have caused it a lot of pain. The sound produced by the gun would scare it. Both effects would hamper the bear's attack and may even cause it to run away122.169.221.144 (talk) 12:51, 8 May 2010 (UTC)
- Break the details down into believable chunks. 1) Most guns carried into the wilderness -even if there are bears about- would I think - be light and practical ( I'm using 'light' in the relative sense). Therefore, his partner could have an ordinary and popular type firearm, which the reader may have well fired himself down a the local range. This takes the emphases away from the hardware and focuses it more on the action. I.e., instead of making the gun do all the hard-work, it makes his partner the brave hero instead. 2) Gun magazines for decades have been encouraging people to buy bigger guns by pointing out that rounds can be deflected by a bears scull. So don't go against popularly held beliefs -and in this case it does seem true in some cases. 3) Its bad form for a writer to let the any of the good guys kill with the first shot (unless the hero is down to his very last BB). So let the first round ricochet off into the blue yonder. 4) The suggestions posted above, give a good idea where to put the other slugs. If you look at the these polar bear and grizzly bear skulls one can easily believe the partner in a final act of desperation (perhaps the bear swipes his rifle from is hands) pulling out his grandfather's old Colt 36 and severing the spinal cord with a lucky shot in through the mouth. If the partner gets this close, don't forget to comment on weather this bear also has Halitosis . If the shot is at point blank, don't forget to consider the hot flash going out sideways and the smell of a bit of singed fur. Films and CSI aside, who really carries around a heavy Colt 45 ? Finally: Writer Cory Doctorow has discovered, he sells more books if he also releases them under a Creative Commons licence. HowTo Negotiate a Creative Commons License. --Aspro (talk) 12:55, 8 May 2010 (UTC)
- Purely on your last point, Aspro, while that works well for Cory Doctorow who already has a doubly-established reputation, as a fiction writer, and as a champion of Creative Commons, it might not work so well for the (presumably as-yet unknown) OP. 87.81.230.195 (talk) 17:10, 8 May 2010 (UTC)
- Break the details down into believable chunks. 1) Most guns carried into the wilderness -even if there are bears about- would I think - be light and practical ( I'm using 'light' in the relative sense). Therefore, his partner could have an ordinary and popular type firearm, which the reader may have well fired himself down a the local range. This takes the emphases away from the hardware and focuses it more on the action. I.e., instead of making the gun do all the hard-work, it makes his partner the brave hero instead. 2) Gun magazines for decades have been encouraging people to buy bigger guns by pointing out that rounds can be deflected by a bears scull. So don't go against popularly held beliefs -and in this case it does seem true in some cases. 3) Its bad form for a writer to let the any of the good guys kill with the first shot (unless the hero is down to his very last BB). So let the first round ricochet off into the blue yonder. 4) The suggestions posted above, give a good idea where to put the other slugs. If you look at the these polar bear and grizzly bear skulls one can easily believe the partner in a final act of desperation (perhaps the bear swipes his rifle from is hands) pulling out his grandfather's old Colt 36 and severing the spinal cord with a lucky shot in through the mouth. If the partner gets this close, don't forget to comment on weather this bear also has Halitosis . If the shot is at point blank, don't forget to consider the hot flash going out sideways and the smell of a bit of singed fur. Films and CSI aside, who really carries around a heavy Colt 45 ? Finally: Writer Cory Doctorow has discovered, he sells more books if he also releases them under a Creative Commons licence. HowTo Negotiate a Creative Commons License. --Aspro (talk) 12:55, 8 May 2010 (UTC)
- A writer's biggest problem is obscurity. Who is going to buy a book that they don't know exists, or a book from an author who is unknown to them? By distributing some work under a CC licence, they attract a wider audience. The same has happened, to people who thought of themselves as non writers but have been surprised with offers of book deals based on what the publisher has read on their blogs. Pop-stars understand this. You have to be 'seen' if you want to be noticed. So, this exposer could be even more valuable to any of the little know story tellers out there. --Aspro (talk) 18:54, 8 May 2010 (UTC)
- Fair points, Aspro, but people like Cory Doctorow and blogger/author John Scalzi spent many years slowly building on-line reputations, and had the abilities necessary to do so; random unpublished authors like the OP may not possess the latter or want to spend the time necessary for the former, so the traditional publishing route via direct (or agent-mediated) submissions to a Publishing House who will exercise its long-honed professional skills and do much of the marketing for them from the off (and who may or may not incorporate the strategies discussed), is still in my view a better bet. My view however is shaped by a former career in traditional-model bookselling and publishing, so may be increasingly irrelevant. 87.81.230.195 (talk) 01:12, 10 May 2010 (UTC)
- A writer's biggest problem is obscurity. Who is going to buy a book that they don't know exists, or a book from an author who is unknown to them? By distributing some work under a CC licence, they attract a wider audience. The same has happened, to people who thought of themselves as non writers but have been surprised with offers of book deals based on what the publisher has read on their blogs. Pop-stars understand this. You have to be 'seen' if you want to be noticed. So, this exposer could be even more valuable to any of the little know story tellers out there. --Aspro (talk) 18:54, 8 May 2010 (UTC)
- A polar bear attacking an adult man is comparable to an adult man attacking a four year old child. In your case it would be a blind child waving a small stick. To make the story even halfway plausible you need something to slow down the bear. Why not give your guy some pepper spray? That wouldn't necessarily drive off the bear, but might slow it down enough to make the encounter last longer than 5 seconds. Youtube has a bunch of videos of bears attacking various things -- you might be able to write a more realistic account if you watched a few of them. Looie496 (talk) 22:07, 8 May 2010 (UTC)
- Hey everyone, I see a lot of good ideas here on how to make this scene more dramatic (it's pretty dramatic as it is, but from reading your replies, I see that I could make it even more dramatic) as well as more plausible. Now, I have to remind y'all that I'm not a hunter, and for this reason sorting out all this plausible but contradictory info will require some qualified advice. (I think I'll talk to the guy at the local gun store, he should know this kind of stuff in some detail.) Thank y'all for your ideas, and clear skies to you! 67.170.215.166 (talk) 07:14, 10 May 2010 (UTC)
Conventional Explosive v/s Plastic explosives
[edit]Conventional explosive like gunpowder cannot do damage if not held air-tightly. If we open a 12-gauge shot cartidge (NEVER, NEVER TRY IT AT HOME. I AM JUST GIVING EXAMPLE), and put all the smokless "powder", which is of course not plastic explosive, and set it light it will burn away with a SWOOSH and a very brillant flame, but there will not be any explosion. Even a fire-cracker has to be contained in something from which gases cannot escape without destroying it. But as far as plastic explosives are concerned, if I am not very much wrong, they do not need to be air-tightened. A piece of C-4 or semtex can explode in open when properly detonated (can't do it with fire, methinks). But is that also true about nitroglycine. Is it not necessery to hold nitroglycrine in a tight container to cause explosion ? Jon Ascton (talk) 08:51, 8 May 2010 (UTC)
- I believe the difference is that gunpowder is a "low explosive" which, technically, deflagrates rather than detonates. This makes it suitable as a propellant in munitions and fireworks, but not damaging unless in a confined space. On the other hand, C-4, Semtex and nitroglycerin are all "high explosives", which undergo true detonation. Incidentally, you don't need always need fire to initiate detonation - some explosives are very sensitive and can be detonated by pressure, friction, electric shock, sound or even light. However, such sensitive explosives are usually used in only small amounts in detonators to trigger a larger quantity of more stable "seondary" explosive. See our article on explosive material for more details. Gandalf61 (talk) 10:01, 8 May 2010 (UTC)
- Yeah, that is what I wanna know : Will a small quantity of nitroglycrine, let's say 1/10 litre - which is an oily liquid - when not in airtight position, that is lying in open, detonates cause a real explosion practically (like a bomb), or it will just burn with a SWOOSH sound....? Jon Ascton (talk) 10:43, 8 May 2010 (UTC)
- It depends on the speed of the shock wave caused by the suddenly expanding gases released by the explosive. --Chemicalinterest (talk) 11:26, 8 May 2010 (UTC)
- Read the links. Nitroglycerin#Detonation says "... a self-sustained shock wave ... propagates through the explosive medium at some 30 times the speed of sound as a near-instantaneous pressure-induced decomposition of the fuel into a white hot gas. Detonation of nitroglycerin generates gases that would occupy more than 1,200 times the original volume at ordinary room temperature and pressure; moreover, the heat liberated raises the temperature to about 5,000 °C (9,030 °F)". That sounds like a "real explosion" to me. Gandalf61 (talk) 12:07, 8 May 2010 (UTC)
- And 1/10 litre of Nitroglycerin is not "a small quantity". When we made it in school, we detonated microscopic amounts for a quite noticeable bang.--Stephan Schulz (talk) 15:03, 8 May 2010 (UTC)
- For example, consider the iconic image of a stick of dynamite. It's essentially nitroglycerine and sand, wrapped in a cardboard (or paper) tube. The sand reduces the shock-sensitivity, but the paper tube isn't there to confine the blast - only to wrap the material conveniently. That stick of dynamite is still dangerous and can explode. Now, if you're performing road construction and want to remove rock from the highway, you might want to confine the blast to maximize the transfer of energy towards productive, useful purposes - so you might drill a borehole into a rock and use that confined space to harness the blast energy. But the explosive will detonate, confined or unconfined. As far a "plastic", the entire purpose of plasticizer is to reduce shock sensitivity - it has plays very little chemistry role, other than diluting the active ingredients. Nimur (talk) 16:11, 8 May 2010 (UTC)
- Gosh, that's mixed up. It's not sand but diatomaceous earth that is the phlegmatizing agent in nitroglycerine. Second, plasticizer are added to make it 'plastic ' hence plastic explosive!--Aspro (talk) 16:31, 8 May 2010 (UTC)
- It can be anything - sand, silt, mud, sawdust, ground up pieces of paper - it doesn't matter, as long as it's absorbent... The plasticizer replaces the role of this material, and as an added bonus allows the material to be molded. Nimur (talk) 17:02, 8 May 2010 (UTC)
- Gosh, that's mixed up. It's not sand but diatomaceous earth that is the phlegmatizing agent in nitroglycerine. Second, plasticizer are added to make it 'plastic ' hence plastic explosive!--Aspro (talk) 16:31, 8 May 2010 (UTC)
- Think you may be getting mixed up with Gelignite and even that article is inaccurate, as it does sweat – that's what old jelly is renowned for. Although the Dynamite article mentions sawdust, and Nobel used it for his early blasting sticks, Nobel's patent for Dynamite was for the absorption by dichotomous earth (which just happen to be mined close to him in Sweden). The reason why Dynamite was such an improvement on safety was that blasting sticks that used sawdust also 'sweated' - Dynamite didn't. By definition, a stick using anything else is not Dynamite. Just don't believe everything you read on Wikipedia.--Aspro (talk) 17:51, 8 May 2010 (UTC)
- Like Kleenex, dynamite has become a genericized name; but you are absolutely correct. Nimur (talk) 17:54, 8 May 2010 (UTC)
- Think you may be getting mixed up with Gelignite and even that article is inaccurate, as it does sweat – that's what old jelly is renowned for. Although the Dynamite article mentions sawdust, and Nobel used it for his early blasting sticks, Nobel's patent for Dynamite was for the absorption by dichotomous earth (which just happen to be mined close to him in Sweden). The reason why Dynamite was such an improvement on safety was that blasting sticks that used sawdust also 'sweated' - Dynamite didn't. By definition, a stick using anything else is not Dynamite. Just don't believe everything you read on Wikipedia.--Aspro (talk) 17:51, 8 May 2010 (UTC)
- Actually, dynamite is perfectly safe unless detonated. It can be thrown in a fire without exploding. --The High Fin Sperm Whale 22:09, 8 May 2010 (UTC)
- Same with C-4, our guys in 'Nam actually used it as campfire fuel! Nitro, on the other hand, is so sensitive that even a static spark from your pants/skirt could set it off! We didn't do any experiments with nitro in school, and for good reason. 67.170.215.166 (talk) 07:10, 9 May 2010 (UTC)
- But one manual about making nitroglycrine I read says that it burns with a clear blue flame. In fact this is the test for real nitro... Jon Ascton (talk) 13:41, 9 May 2010 (UTC)
- Yeah, that's what it does, 'less it blows up in your face. Care to try it some time? 67.170.215.166 (talk) 07:16, 10 May 2010 (UTC)
irradiating an egg
[edit]What happens to an egg when it is irradiated? 71.100.0.29 (talk) 12:45, 8 May 2010 (UTC)
- The bacteria are killed in it, but I don't think the egg itself is harmed. --Chemicalinterest (talk) 12:47, 8 May 2010 (UTC)
- It depends mainly on how much irradiation they are subjected to In-shell irradiation of eggs . Here in Europe, health grounds tend to be considered before profit, therefore, this process is not permitted for most food stuffs -including eggs. I think this link covers most of the objections The Irradiation of Eggs: The Details.--Aspro (talk) 13:22, 8 May 2010 (UTC)
- Let's be clear -- if there is a living embryo in the egg, it will be killed by irradiation. Most scientists think that the edibility of the egg would not be impaired. Looie496 (talk) 18:24, 8 May 2010 (UTC)
- FWIW I've found a document that give the European position, which also gives examples of which food stuffs are sometimes irradiated over here. Commission adopts first EU report on irradiated food--Aspro (talk) 19:21, 8 May 2010 (UTC)
- If those EU blockheads really considered health grounds rather than all that ultra-left-wing neo-Luddite green hype, they would've allowed most foods to be radiated -- it's one of the most effective ways to kill any critters living in the food, as well as being a lot better for the consumer's health than salting it or boiling it or putting in a bunch of antiseptic additives that could affect human metabolism. Too bad that they fall so easily for all those lies from the green movement who want us all to start living like the Apache Indians or something... 67.170.215.166 (talk) 07:18, 9 May 2010 (UTC)
- Isn't it better to simply eat fresh or frozen food unprocessed food? I don't know where you live but as Europe adopts more and more American style food processing techniques, so it discovers that it becomes ever more burdened with American style health problems. Currently we are getting very alarmed with childhood obesity and childhood diabetes, which is racing up to USA levels fast. These days you don't have to eat salt beef and things -so those arguments are something of a non sequitur. As I'm not against irradiation per se you can't call me a Green either. Remember, it took years of big fines and bad publicity to reach the current level compliance in the food industry. Letting the fox guard the chick shed, (i.e., letting lobbyist tell US politician what to allow in the food industry) is I would say - being the blockhead. --Aspro (talk) 12:55, 9 May 2010 (UTC)
- Aspro, I wasn't talking about lobbyist influence on the FDA (which I do not condone in any way -- the FDA should be free to do the right thing about food safety without political pressure), but specifically about the Green blockheads opposing irradiation, which is a perfectly safe food-preservation procedure that has no known effects on the food's nutritional value as opposed to salting or canning, which could in some cases introduce unhealthy substances into the food (e.g. excessive sodium in the case of salting). My beef (no pun intended) is with the statement that [because of health concerns], irradiation is not permitted in the EU, and the blockheads I refer to are members of that (mostly Green) community which objects to irradiation because of some imaginary "health concerns" (even though there are none) and thus by their influence consign many tons of fresh food to the landfill through spoilage that irradiation could have easily prevented. In short, this is not a debate about food safety in general, but specifically about irradiation and the raving lunatics and pandering politicians who oppose it despite its merits. In other words, as bad as it is to let the food industry set food safety standards, it's just as bad to leave this task to the madmen in the Green movement who have a vested interest in taking our way of life back to pre-industrial levels. 67.170.215.166 (talk) 07:39, 10 May 2010 (UTC)
- Isn't it better to simply eat fresh or frozen food unprocessed food? I don't know where you live but as Europe adopts more and more American style food processing techniques, so it discovers that it becomes ever more burdened with American style health problems. Currently we are getting very alarmed with childhood obesity and childhood diabetes, which is racing up to USA levels fast. These days you don't have to eat salt beef and things -so those arguments are something of a non sequitur. As I'm not against irradiation per se you can't call me a Green either. Remember, it took years of big fines and bad publicity to reach the current level compliance in the food industry. Letting the fox guard the chick shed, (i.e., letting lobbyist tell US politician what to allow in the food industry) is I would say - being the blockhead. --Aspro (talk) 12:55, 9 May 2010 (UTC)
- Food irradiation is generally used on fresh foods. As 67.170 alludes to, processed foods usually have other means of being kept bacteria free (i.e. cooking, drying, salting, canning, or using other preservatives), and so irradiation is not needed or used. For what it's worth, I always assume that my eggs are contaminated, and make sure to cook them thoroughly. Buddy431 (talk) 16:08, 9 May 2010 (UTC)
Insoluble chromate
[edit]Is there any insoluble hexavalent chromium compound? I reacted sodium hypochlorite with the product of the reaction of Nichrome with hydrochloric acid. It formed a dark orange-brown precipitate. What is that precipitate? It lightens when hydrogen peroxide is added to it, and dissolves in hydrochloric acid to turn yellow. --Chemicalinterest (talk) 14:43, 8 May 2010 (UTC)
- Remember the nickel? Sodium hypochlorite is alkaline and will neutralise the acid precipitating hydroxides. Take a look at nickel chromate and nickel hydroxide. Are either of these possible? Graeme Bartlett (talk) 03:36, 9 May 2010 (UTC)
- It probably is nickel chromate, but I don't know why it is insoluble. --Chemicalinterest (talk) 19:15, 9 May 2010 (UTC)
- Whoops, it is insoluble. --Chemicalinterest (talk) 19:25, 9 May 2010 (UTC)
- 2 NiCl2 + 2 CrCl3 + 10 NaOH + 3 NaClO → 2 NiCrO4 + 10 NaCl + 5 H2O This only happens when there is no residual acid in the reaction between Nichrome and hydrochloric acid. If there is, it forms Cl2 and yellow solution. --Chemicalinterest (talk) 19:40, 9 May 2010 (UTC)
- The yellow solution will probably be the result of the dissolution of NiCrO4. --Chemicalinterest (talk) 19:44, 9 May 2010 (UTC)
- What does NiCrO4 produce when it dissolves in acid? (Question in lower section) --Chemicalinterest (talk) 11:07, 10 May 2010 (UTC)
- The yellow solution will probably be the result of the dissolution of NiCrO4. --Chemicalinterest (talk) 19:44, 9 May 2010 (UTC)
- 2 NiCl2 + 2 CrCl3 + 10 NaOH + 3 NaClO → 2 NiCrO4 + 10 NaCl + 5 H2O This only happens when there is no residual acid in the reaction between Nichrome and hydrochloric acid. If there is, it forms Cl2 and yellow solution. --Chemicalinterest (talk) 19:40, 9 May 2010 (UTC)
- Whoops, it is insoluble. --Chemicalinterest (talk) 19:25, 9 May 2010 (UTC)
Chromium and acid
[edit]Does chromium react with acids to form chromium(II) chloride or chromium(III) chloride? I have some information that may support the former. --Chemicalinterest (talk) 18:44, 8 May 2010 (UTC)
- I think CrCl2, because 2CrCl3 + H2 → 2CrCl2 + 2HCl. However, I think in high concentrations of acids, CrCl3 will from. --The High Fin Sperm Whale 20:45, 8 May 2010 (UTC)
Because the resulting solution reacts with ammonia to form an easily oxidized green precipitate. --Chemicalinterest (talk) 22:16, 8 May 2010 (UTC)
- In that case, I think that your getting CrCl3. I think the reaction goes like this:
3HCl + Cr → CrCl3 + 1½H2
then:
CrCl3 + NH3 → CrN + 3HCl
--The High Fin Sperm Whale 04:44, 9 May 2010 (UTC)
- My first question is how you know that the reaction product with ammonia is easily oxidized green precipitate. Chromium(II) compounds are instable and difficult to make, so you and always with chromium(III). Chromium(III) chloride with ammonium you get a complex which will change colour to dark blue or violet on heating and go back to green after some time. The problem you encounter could also be that you add to much base and you precipitate chromium(III) hydroxides which at the end will be transformed to chromium(III) oxide. But if you like that kind of old chemistry get a Gmelin Handbook of chemistry from early 19th century, there most of your questions will be answered. --Stone (talk) 08:36, 9 May 2010 (UTC)
- Why would it be CrN? CrCl2 + 2 NH4OH → Cr(OH)2 + 2 NH4Cl
- Also, why are the links to chromium(II) chloride when the formula is CrCl3?
- After the ammonia is reacted with the chromium chloride, it forms the hydroxide precipitate. The hydroxide precipitate is green, then after about 5 minutes of exposure to air, it turns brown, whether wet or dry. It is the precipitate of the hydroxide when I react with ammonia. Note: I only use ammonia because I don't have much of any other base. I only have about 1 g of KOH, and no NaOH. Is the chromium(II) hydroxide more green than the chromium(III) hydroxide, which is why the brown color comes out. --Chemicalinterest (talk) 19:24, 9 May 2010 (UTC)
- To Stone: I think that the reaction of chromium with hydrochloric acid forms chromium(II) chloride; it becomes oxidized over time to form chromium(III) oxide. --Chemicalinterest (talk) 19:30, 9 May 2010 (UTC)
- The chromium(II) is very reactive and without proper Schlenk-methods you will not be able to obtain it. Even with them the light blue Chromium(II) reduces the water and with production of hydrogen you get the Chromium(III). --Stone (talk) 21:21, 9 May 2010 (UTC)
- With ammonia you get the very dark blue green chromium(III) hydroxide.
- The colour change in chromium(III) salt solutions from green to violett is no oxidation or reduction, but a simple change in the ligands of the chromium. --Stone (talk) 21:21, 9 May 2010 (UTC)
My first question is how you know that the reaction product with ammonia is a easily oxidized?--Stone (talk) 21:22, 9 May 2010 (UTC)
- Because of the color change; that's why I think it is oxidized. It did not change from green to violet, which would be dehydration, but from green to brown, like the oxidation of ferrous hydroxide to ferrous oxide(which turns from green to brown). The chromium(II) wouldn't reduce the water: at least not at room temperature. The reduction potential for Cr3+ (or the oxidation of Cr2+) is -0.42. Only an oxidizing agent which has a number above (less negative) it can oxidize it.The potential for the oxidation of water (2 H2O + 2 e- → H2 + 2 OH-) is -0.8277. But if the water is a little acidic, the potential for the reduction of acid is (2 H+ + 2 e- → H2) +0.00, which it can easily oxidize. If it is alkaline, it can precipitate the chromium(II). That is why it is only stable in very pure solution.
- So chromium(II) cannot form in acid then. The reactions would be: Cr + 2 HCl → CrCl2 + H2 2 CrCl2 + 2 HCl → 2 CrCl3 + H2 --Chemicalinterest (talk) 11:05, 10 May 2010 (UTC)
Idea! The easily oxidized green precipitate is just chromium and nickel oxides. They are easily oxidized when alkaline, such as in the reaction with ammonia. They react to form nickel chromate. 4 NiO + 2 Cr2O3 + 3 O2 → 4 NiCrO4 Then NiCrO4 + 2 NaClO → Na2CrO4 + NiCl2 + O2 If it is green, i.e. still nickel and chromium oxides, when it is reacted with NaClO it turns brown, (nickel chromate) then forms sodium chromate. --Chemicalinterest (talk) 12:57, 10 May 2010 (UTC)
is it possible to have a defective sense of rhythm?
[edit]is it possible to have a defective sense of rhythm or no sense of rhythm? Thank youl. 84.153.199.22 (talk) 18:51, 8 May 2010 (UTC)
- Yeah. I know quite a few drummers like that! —Preceding unsigned comment added by RampantFairy (talk • contribs) 19:04, 8 May 2010 (UTC)
- See Amusia#Temporal relations. Qwfp (talk) 19:51, 8 May 2010 (UTC)
- Comment from Roger Taylor on a BBC programme about guitar players last night. "How do you know if there's a drummer at the door?" "Because they knock three times then come in late." An alternative. "Who hangs around with musicians?" "Drummers". --Phil Holmes (talk) 09:50, 9 May 2010 (UTC)
- Sorry I can't resist my fav drummer joke: What's the difference between a drummer and a drum machine? You only have to punch the song into a drum machine once. :D Vespine (talk) 23:11, 10 May 2010 (UTC)
gas vent
[edit]can some1 explain how the monoxide vent to gas central heating works? its been cold in my basment and i hear wind gushing thru my ducts. i suspects the cap on the monoxide vent is missing or something. —Preceding unsigned comment added by Tom12350 (talk • contribs) 19:41, 8 May 2010 (UTC)
- Get a technician. You'll want to find the carbon monoxide leak if there is one, it's very toxic and very unnoticeable by smell, sight or taste. Regards, --—Cyclonenim | Chat 00:34, 9 May 2010 (UTC)
- Get a qualified engineer to look at it. But at the earliest opportunity, I suggest you try to find the outlet yourself and check that it is not obstructed.--Shantavira|feed me 08:20, 9 May 2010 (UTC)
- You can also buy carbon-monoxide detectors - if you have that kind of a system, I'd strongly recommend getting one. SteveBaker (talk) 16:22, 9 May 2010 (UTC)
omg thers no monoxide leaking. thats not my question i was asking how they work. can some1 give me a diagram. —Preceding unsigned comment added by Tom12350 (talk • contribs) 18:28, 9 May 2010 (UTC)
- I've never heard of a monoxide vent. Where did you hear that term? There most definitely is not carbon monoxide flowing through your heating ducts unless you have a very very severe failure of your system. Ariel. (talk) 22:04, 9 May 2010 (UTC)
- Googling suggests that some people refer to the ordinary exhaust vent as a "monoxide vent", but you're right that its primary purpose is to vent ordinary exhaust gases & vapours, of which carbon monoxide should be a very small component, though there will always be some present: my own gas water heater typically tests out at an acceptable 12 parts per million, for example.
- I suggest to Tom12350 that you contact the manufacturer to ask for a handbook for your particular appliance/system, as there is doubtless a great deal of variation between makes and models. I'd also endorse Shantavira's suggestion to get a professional gas appliance engineer to look at it: this stuff is too potentially dangerous for anyone unqualified to mess with, and in the UK it's illegal - see Gas safe register. 87.81.230.195 (talk) 00:43, 10 May 2010 (UTC)
Yellowjackets
[edit]Hoe many body lengths do they fly per minute? This just a curiosity question. —Preceding unsigned comment added by Unambiguous13 (talk • contribs) 21:01, 8 May 2010 (UTC) Find out how many miles per hour they fly. Divide it by sixty to get miles per minute. Multiply that by 5280 to get how many feet per minute. Find out how long a wasp's body is. Divide 1 foot into the length of the body in feet. Multiply that by the number of feet per minute it flies. That will give you the body lengths. PS: Sorry if you don't live in the US. --Chemicalinterest (talk) 23:18, 8 May 2010 (UTC)
- (Edit Conflict) That would depend very much on whether they (generally called 'wasps' outside North America, by the way) were foraging amongst food sources, covering distance purposefully, escaping from danger, or attacking. Our article Yellow jacket gives a typical (worker) body length of ½"/12mm. This site quotes a (non-foraging) wasp flight speed of 0.094mph (in contrast to a foraging flight speed of 0.008mph), while this one gives a wasp flight speed of 2.5m/s (= 5.59mph). (Both sites found in the first half dozen Google hits from searching 'wasp flight speed'.) If my maths are correct, these two figures give body length per minute figures of about 198 and 11,806 (or more realistically 200 and 12,000) respectively. 87.81.230.195 (talk) 23:38, 8 May 2010 (UTC)
- "Generally called wasps outside North America"? What are you talking about? They're called wasps in North America, too, because they are wasps. But not all wasps are yellowjackets. It's a specific kind of wasp. --Trovatore (talk) 06:54, 9 May 2010 (UTC)
- The word "yellowjacket" isn't used outside North America, though. They are just called "wasps". If you like you can talk about specific species of wasps, but most people never would. --Tango (talk) 15:39, 9 May 2010 (UTC)
- "Generally called wasps outside North America"? What are you talking about? They're called wasps in North America, too, because they are wasps. But not all wasps are yellowjackets. It's a specific kind of wasp. --Trovatore (talk) 06:54, 9 May 2010 (UTC)
- That second site is linked to as the source for the first site. The first site is just a random idiot on the internet and should be ignored. --Tango (talk) 02:49, 9 May 2010 (UTC)
- Good spot, Tango. It's not entirely clear if the first site got those specific figures from the second, but in any case different sites cite widely varying figures for speeds of various types of flight, so a definitive single answer is uncalculable. 87.81.230.195 (talk) 00:26, 10 May 2010 (UTC)
did people really have to stay quiet on submarines?
[edit]did people really have to stay quiet on submarines or is that just a movie thing? 85.181.146.182 (talk) 22:06, 8 May 2010 (UTC)
also: can you literally hear a high pitch "ping" if another ship or sub is acti ely sonarinf your position?
also, why in films do subs try to go very deep to become impervious cant ships see them there too or drop charges? 85.181.146.182 (talk) 22:49, 8 May 2010 (UTC)
- Well if you did not want to be detected on the submarine it is a good idea to be quiet. The ship could have a microphone in the water to listen to submarine engines or other sources of noise. A submarine near the surface may be visible through the water and mat disturb the surface with a wake, ALso deep down it will be harder to hit, and harder to detect on a sonar, due to increased range, and particularly hard if it was close to the sea floor as the reflection of the sea bed will mask the vessel. Graeme Bartlett (talk) 03:24, 9 May 2010 (UTC)
- Plus, in deep water it's possible for the submarine to go below a thermocline layer, which would tend to reflect sound and make it harder for a surface ship to detect the sub. Fair winds to you 67.170.215.166 (talk) 07:23, 9 May 2010 (UTC)
- I have a work colleague who spent many years on submarines during the 'cold war' period and he relates that absolute silence was paramount to avoid detection. Crew used slippers not shoes to avoid detectable footfall. Remember that water is a hugely more efficient transmitter of sound than air, ask the whales (no, not that Whales!) Caesar's Daddy (talk) 07:56, 9 May 2010 (UTC)
- Plus, in deep water it's possible for the submarine to go below a thermocline layer, which would tend to reflect sound and make it harder for a surface ship to detect the sub. Fair winds to you 67.170.215.166 (talk) 07:23, 9 May 2010 (UTC)
- We have an article on sonar. The ping or chirp given out by the other vessel would not be audible to the human ear because the frequency is just a bit too high for most people to hear, and it wont re-radiated into the sub through the pressure hull anyway. However, the sonar operator can hear it, because the frequency is lowered into the audible range via a heterodyne circuit. See also Counter Sonar measures.--Aspro (talk) 11:18, 9 May 2010 (UTC)
- Modern SONAR is far outside of the range of frequencies that humans can hear - but since many submarine movies are set in World War II, we're not really talking about modern SONAR. I think the technology of the time would have made using audible frequencies quite attractive since you wouldn't need any fancy electronics to decode it - just a guy with sharp hearing. If the guy on the anti-submarine ship could hear the return echo (albeit with sensitive directional microphones) then the ping that was emitted would have had to be pretty loud. The guys inside the submarine would be hearing the sound after it had travelled only half the distance that the sonar guy would be hearing it at - so for them, it would be at least four times as loud - more than that since some energy would be lost in the reflection off of the submarine's hull. Since they are all trying to be super-quiet, I think it's possible that they could hear the 'pings'. SteveBaker (talk) 16:20, 9 May 2010 (UTC)
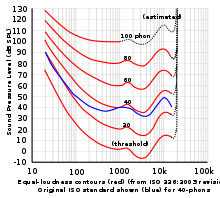
- If they could have done that without the ambient noise swamping the signal they may have adopted (or stayed with) audible frequencies. Also, higher frequencies are more directional and it is important to not only obtain the range but the direction of the returning echo too. Therefore, they used a range of about 20kHz to 25 kHz. The article on Hearing_range reads: “Specifically in humans, we have a maximum aural range of 12 Hz under ideal laboratory conditions[1] to 20,000 Hz in some individuals, but the range shrinks during our lifetime, usually beginning at around the age of 8 with the higher frequencies fading. If you look at the equal-loudness contours diagram on the right you will see that sensitivity falls off dramatical with increasing pitch. The background noises in the ocean would swamp the echo out preventing (any children they would have to employ) from detecting the sub. Also, heterodynes are hardly fancy electronics – the common domestic radios of the era used one. --Aspro (talk) 18:16, 9 May 2010 (UTC)
- A modern nuclear submarine in operation underwater makes about as much noise as a motorcycle or a brass band, due to propeller noise, fluid flow through pipes, and pumps.. Slippers versus normal shoes would seem to be the least of their worries. In the old WW2 movies, when a diesel electric sub was operating on batteries and the sonar was crude, shutting down pumps and fans and generally making no sound would have made more of a difference. Copied from my earlier posting on the topic: The Russian Sierra class had a reported 120 decibels and the U.S. Los Angeles class produced 110 decibels. By comparison, a loud rock concert is reported to be about 115 decibels, a power saw at 3 feet 110 db, and a motorcycle 100 db. There is a lot of heavy equipment operating on a submarine while it is in motion. Another source on the noise levels of various nations' subs, both diesel, battery and nuke, is at [2]. AWW2 diesel/electric sub submerged did not have to operate pumps to cool a reactor core, and could basically shut down all motors and drift. Edison (talk) 00:06, 10 May 2010 (UTC)
- Wouldn't the important factor be how much louder the sub is than the oceans' background noise at the particular frequencies (no doubt a wide range) the sub makes? (added) Perhaps Ambient noise level is closer to what I was thinking of. --220.101.28.25 (talk) 05:14, 10 May 2010 (UTC)
- A modern nuclear submarine in operation underwater, Edison, can make use of natural convection in the reactor core while operating at slow speeds (which is what they do most of the time, precisely to reduce noise), thus allowing the reactor pumps to be shut down and cutting the noise down to a whisper. The figures you gave are only valid when the sub is racing at full speed, and this is because of propeller cavitation (which is a factor on all subs at high speed, regardless of whether they're diesel or nuclear); the powerplant noise (except on some kinds of subs like the Alfa) is generally a much smaller component of total noise (though still significant at high speeds). Also, modern subs have rubber tiles on the hull to absorb engine noise. The Sierra class submarines were indeed noisy, because they were designed mostly for high speed without regard for noise -- just like the earlier Alfas, they were mainly designed for inshore defense and for chasing down carrier groups. High-frequency sonar is absorbed much more by the water, so there's a tradeoff between better resolution and maximum range. The Los Angeles sub used 2 different sonars, an audible sonar for long-range detection and an ultrasonic sonar for more precise location at shorter ranges (don't know about the new Virginia class submarines, though). FWiW 67.170.215.166 (talk) 08:03, 10 May 2010 (UTC)
Bird/crocodile grouping
[edit]Is there a taxonomic group that includes birds and crocodiles but excludes mammals? I remember reading that birds and crocodiles have similar lung structures, which suggests that they are more closely related than ether are to mammals, but the last common ancestors of the two I can find are Amniote and Tetrapod, which each include mammals. —Arctic Gnome (talk • contribs) 22:28, 8 May 2010 (UTC)
- You are thinking of the clade Sauropsida (Goodrich, 1916), if I am not mistaken. Intelligentsium 22:45, 8 May 2010 (UTC)
- The minimal group that includes birds and crocodiles is archosauria, I believe. The split between mammals and the others goes back to the very early split between diapsids (birds, crocodiles, reptiles) and synapsids (mammals and many extinct groups), which diverged well over 300 million years ago. Looie496 (talk) 23:56, 8 May 2010 (UTC)
- Thanks. So is Archosaur a subgroup of Sauropsida? The relationship between the two groups isn't fully explained in either article. Also, the article on crocodilia seems to have merged the terms into Archosauromorpha. —Arctic Gnome (talk • contribs) 01:33, 9 May 2010 (UTC)
- The tree diagrams in Sauropsida and archosauromorpha show the relationships -- archosauria is a subset of archosauromorpha; everything outside that subset is extinct, though. Looie496 (talk) 01:50, 9 May 2010 (UTC)
- Thanks. So is Archosaur a subgroup of Sauropsida? The relationship between the two groups isn't fully explained in either article. Also, the article on crocodilia seems to have merged the terms into Archosauromorpha. —Arctic Gnome (talk • contribs) 01:33, 9 May 2010 (UTC)
- The minimal group that includes birds and crocodiles is archosauria, I believe. The split between mammals and the others goes back to the very early split between diapsids (birds, crocodiles, reptiles) and synapsids (mammals and many extinct groups), which diverged well over 300 million years ago. Looie496 (talk) 23:56, 8 May 2010 (UTC)
