Wikipedia:Reference desk/Archives/Science/2016 December 13
| Science desk | ||
|---|---|---|
| < December 12 | << Nov | December | Jan >> | December 14 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
December 13
[edit]What's the chance that a random transit of Mercury?
[edit]Is part of a steak of at least 8 consecutive transits who's years end in 3, 6 or 9? Contemporary transit years usually have a multiple of 3 in the last digit: 1973, 1986, 1993, 1999, 2003, 2006, 2016, 2019, 2032, 2039, 2049. Sagittarian Milky Way (talk) 01:00, 13 December 2016 (UTC)
- It looks like the article explains some of that: the transits are often separated by 13 years. So 73 -> 86 -> 99, 93 -> 06 -> 19 -> 32*, 03 -> 16 fit into this pattern. So it's not random, exactly... Wnt (talk) 04:05, 13 December 2016 (UTC)
- There has recently been a string of them in years ending with a multiple of three - but if you look at the full list of dates and predictions you will note that before this current series there was one in 1953, and then you have to go back to 1769 to find another. Of the next 12 predicted transits, only three meet this criterion. There doesn't seem to be anything to suggest that this isn't just a statistical fluke. Wymspen (talk) 10:05, 13 December 2016 (UTC)
- Considering the time intervals between transits, not so much a fluke; so you have to look at a longer time period. This page contains downloadable data covering all transits, not only of Mercury seen from the Earth, but of many such combinations, over a period of 250,000 years. If you take this data in CSV format and extract just the "Mercury,Earth" lines, you'll find that there are 40,540 of them. The date and time of the start, maximum, and end of each transit are shown in the Julian day system. I extracted the date and time of each maximum and converted them to Gregorian calendar dates by filtering through
perl -ne 'use Astro::Time;
@x = mjd2cal(jd2mjd($_));
printf "%4d-%02d-%02d\n", $x[2], $x[1], $x[0]'
- It turns out that
mjd2calcan't cope with negative Julian dates, and I decided that Sagittarian was probably referring to our current era rather than (say) the year 120,000 anyway, so I stripped out everything but the 4,000-year span from 1 to 4000 AD. Then there were 528 transits in those years:
- It turns out that
- 4, 7, 17, 20, 33, 46, 50, 53, 63, 66, 79, 86, 92, 96, 99, 109, 112, 125, 132, 142, 145, 158, 171, 175, 178, 188, 191, 204, 217, 221, 224, 234, 237, 250, 263, 267, 270, 280, 283, 296, 303, 309, 313, 316, 326, 329, 342, 349, 359, 362, 372, 375, 388, 395, 405, 408, 421, 434, 438, 441, 451, 454, 467, 480, 484, 487, 497, 500, 513, 520, 526, 530, 533, 543, 546, 559, 566, 576, 579, 589, 592, 605, 612, 622, 625, 638, 651, 655, 658, 668, 671, 684, 697, 701, 704, 714, 717, 730, 737, 743, 747, 750, 760, 763, 776, 783, 793, 796, 806, 809, 822, 829, 839, 842, 855, 868, 872, 875, 885, 888, 901, 914, 918, 921, 931, 934, 947, 954, 964, 967, 977, 980, 993, 1000, 1010, 1013, 1023, 1026, 1039, 1046, 1056, 1059, 1072, 1085, 1089, 1092, 1102, 1105, 1118, 1131, 1135, 1138, 1148, 1151, 1164, 1171, 1181, 1184, 1194, 1197, 1210, 1217, 1227, 1230, 1240, 1243, 1256, 1263, 1273, 1276, 1289, 1302, 1306, 1309, 1319, 1322, 1335, 1348, 1352, 1355, 1365, 1368, 1381, 1388, 1398, 1401, 1411, 1414, 1427, 1434, 1444, 1447, 1457, 1460, 1473, 1480, 1490, 1493, 1506, 1519, 1523, 1526, 1536, 1539, 1552, 1559, 1565, 1569, 1572, 1582, 1585, 1598, 1605, 1615, 1618, 1628, 1631, 1644, 1651, 1661, 1664, 1674, 1677, 1690, 1697, 1707, 1710, 1723, 1736, 1740, 1743, 1753, 1756, 1769, 1776, 1782, 1786, 1789, 1799, 1802, 1815, 1822, 1832, 1835, 1845, 1848, 1861, 1868, 1878, 1881, 1891, 1894, 1907, 1914, 1924, 1927, 1940, 1953, 1957, 1960, 1970, 1973, 1986, 1993, 1999, 2003, 2006, 2016, 2019, 2032, 2039, 2049, 2052, 2062, 2065, 2078, 2085, 2095, 2098, 2108, 2111, 2124, 2131, 2141, 2144, 2154, 2157, 2170, 2174, 2177, 2187, 2190, 2203, 2210, 2220, 2223, 2233, 2236, 2249, 2256, 2266, 2269, 2279, 2282, 2295, 2302, 2312, 2315, 2325, 2328, 2341, 2348, 2358, 2361, 2371, 2374, 2387, 2391, 2394, 2404, 2407, 2420, 2427, 2437, 2440, 2450, 2453, 2466, 2473, 2483, 2486, 2496, 2499, 2512, 2519, 2529, 2532, 2542, 2545, 2558, 2565, 2575, 2578, 2588, 2591, 2604, 2611, 2621, 2624, 2637, 2644, 2654, 2657, 2667, 2670, 2683, 2690, 2700, 2703, 2713, 2716, 2729, 2736, 2746, 2749, 2759, 2762, 2775, 2782, 2792, 2795, 2805, 2808, 2815, 2821, 2828, 2838, 2841, 2854, 2861, 2871, 2874, 2884, 2887, 2900, 2907, 2917, 2920, 2930, 2933, 2946, 2953, 2963, 2966, 2976, 2979, 2992, 2999, 3009, 3012, 3022, 3025, 3032, 3038, 3045, 3055, 3058, 3071, 3078, 3088, 3091, 3101, 3104, 3117, 3124, 3134, 3137, 3147, 3150, 3163, 3170, 3180, 3183, 3193, 3196, 3209, 3216, 3226, 3229, 3239, 3242, 3249, 3262, 3272, 3275, 3288, 3295, 3305, 3308, 3318, 3321, 3334, 3341, 3351, 3354, 3364, 3367, 3380, 3387, 3397, 3400, 3410, 3413, 3426, 3433, 3443, 3446, 3456, 3459, 3466, 3479, 3489, 3492, 3505, 3512, 3522, 3525, 3535, 3538, 3551, 3558, 3568, 3571, 3581, 3584, 3597, 3604, 3614, 3617, 3627, 3630, 3643, 3650, 3660, 3663, 3673, 3676, 3683, 3696, 3706, 3709, 3722, 3729, 3739, 3742, 3752, 3755, 3768, 3775, 3785, 3788, 3798, 3801, 3814, 3821, 3831, 3834, 3844, 3847, 3860, 3867, 3877, 3880, 3890, 3893, 3900, 3913, 3923, 3926, 3936, 3939, 3946, 3956, 3959, 3969, 3972, 3985, 3992
- Of these, 67 are within one of the 8 streaks of 8 or more transits in years ending in 3, 6, or 9; so for this time period the probability is 67/528 or about 12.7%. --76.71.5.45 (talk) 10:25, 13 December 2016 (UTC)
- Unfortunately, I've now learned that
mjd2calnot only can't cope with negative Julian dates, it wasn't intended to cope with negative modified Julian dates either. So the years before 1858 in that list must be considered unreliable. My computation may have produced the right result by accident, or it may not. --76.71.5.45 (talk) 07:58, 14 December 2016 (UTC)
- Unfortunately, I've now learned that
- Of these, 67 are within one of the 8 streaks of 8 or more transits in years ending in 3, 6, or 9; so for this time period the probability is 67/528 or about 12.7%. --76.71.5.45 (talk) 10:25, 13 December 2016 (UTC)
- Thanks! I wasn't sure this was going to get answered. Sagittarian Milky Way (talk) 14:08, 13 December 2016 (UTC)
- Neat! What are the odds that of those 8 streaks, each and every one is preceded by a year ending in 0 and followed by one ending in 2 (which also means there are no streaks of 9)? I think you must have happened on some rather precise property of the transits, and yet the 0 and 2 years are sometimes 62 years apart, sometimes 72, sometimes 82! Wnt (talk) 16:39, 13 December 2016 (UTC)
How much does 1 kg of air weigh?
[edit]If I have 1 kg of air in a bag that's, say, 200 g and it's not under pressure, would the bag just weigh 200 g rather than 1.2 kg? Ooh, and what if it was cooled down so it became more dense... would it get heavier then (assuming it was only 200 g before)? --78.148.100.101 (talk) 09:56, 13 December 2016 (UTC)
- 1 kg of air weighs 9.8 newtons, the same as any other substance. --Jayron32 10:52, 13 December 2016 (UTC)
- On the surface of Earth, of course. TigraanClick here to contact me 13:23, 13 December 2016 (UTC)
- In a vacuum of course!
- On the surface of Earth, of course. TigraanClick here to contact me 13:23, 13 December 2016 (UTC)
- Well, if you were to put that bag on a weighing scale, the scale would display "200 g". However, in the process of putting the bag on the scale, you will also have displaced 1 kg of air that was previously sitting over the scale. If you did the experiment in a vacuum (assuming the bag could withstand the pressure), then it would show 1.2 kg. Smurrayinchester 10:58, 13 December 2016 (UTC)
- "1kg of air" is (in many practical cases) effectively weightless. It displaces its own volume in air (standard laboratory conditions assume an atmosphere, not a vacuum) and so it's neutrally buoyant. It would be like trying to weigh an airship: it has mass, it has inertia, but its weight is near-zero. You'd weigh the container, not the air. Although weight isn't the same thing as buoyancy by definition, most scales would measure buoyancy rather than weight.
- To weigh air otherwise, you'd have to enclose it in something and then either increase its pressure by pumping in more air (allowing you to weigh the additional air added), compressing 1kg of air into a smaller container (so displacing less air), weigh it inside a vacuum chamber, or to weigh [sic] its buoyancy when displacing another fluid, such as weighing it in a bathtub of water.
- Its volume, incidentally, is about 850 litres - just less than a cubic metre. Andy Dingley (talk) 11:34, 13 December 2016 (UTC)
- Weight is a force, and Isaac Newton taught us that F = m a. So 1 kg of any substance in any condition (at the Earth's surface) will weigh the same.—PaulTanenbaum (talk) 12:58, 13 December 2016 (UTC)
- ... for actual practical weighing it is helpful to add "in a vacuum" and "at standard gravity". See Weight#Definitions for different definitions. Dbfirs 13:14, 13 December 2016 (UTC)
- The 200g apparent weight assumes that the air in your bag is at the same temperature as the surrounding air. If it is cooler, then the scale will record more than 200g, and if it is warmer than the surrounding air then your bag of air will have an apparent weight of less than 200g. Dbfirs 13:18, 13 December 2016 (UTC)
- This sounds like a variation of the old riddle: "What weighs more? A pound of gold? Or a pound of feathers?" ←Baseball Bugs What's up, Doc? carrots→ 14:59, 13 December 2016 (UTC)
- It's more than that. Feathers are dense (they're light because they're bulky at a macro scale, keratin is still dense) and so they don't float. For both feathers and gold, buoyancy in air isn't an issue. For weighing gases, buoyancy matters. Andy Dingley (talk) 15:06, 13 December 2016 (UTC)
- Andy, I guess you don't actually know the joke. The pound of feathers weigh more. That's because they're a pound avoirdupois, whereas the gold is a pound troy. --Trovatore (talk) 20:25, 13 December 2016 (UTC)
- A given mass of "air" (Nitrogen + Oxygen + etc.) is going to have less buoyancy than an equivalent mass of hydrogen, right? Or not? One traditional answer to the riddle is "a pound of feathers", because gold is measured in Troy weight. ←Baseball Bugs What's up, Doc? carrots→ 15:38, 13 December 2016 (UTC)
- Hm... [1] [2] [3]. (Yes I know what you meant, keratin is denser than water, but I also think it's a bit too complicated to say "feathers don't float" ;) SemanticMantis (talk) 15:54, 13 December 2016 (UTC)
- It's more than that. Feathers are dense (they're light because they're bulky at a macro scale, keratin is still dense) and so they don't float. For both feathers and gold, buoyancy in air isn't an issue. For weighing gases, buoyancy matters. Andy Dingley (talk) 15:06, 13 December 2016 (UTC)
- Weight is just mass times the acceleration due to gravity. This value is not changed when an object (or a parcel of air) is subject to buoyant force. Weight does not necessarily equal total net force. When we study atmospheric science or aerodynamics or any other application in which we care to analyze the physics of an air mass, we definitely don't redefine "weight" to mean something totally different.
- Don't confuse the issue by conflating "weight" with total net force. The total net force accomodates the weight, the buoyant force, and any other factors. The weight really is just the mass times the acceleration due to gravity.
- One kilogram of air, when near Earth's surface, weighs about 2.2 pounds, or 9.81 newtons. It's really that simple. Here's how Wolfram Alpha interprets "weight of 1 kg of air": it performs a unit-conversion assuming standard gravity at Earth's surface.
- Nimur (talk) 17:23, 13 December 2016 (UTC)
- True, but many people think that "weight" is what their mechanical or electronic scales measure, and that seems to be what the questioner was asking about. Dbfirs 17:32, 13 December 2016 (UTC)
- "Weight" has a bunch of meanings, one of which is actually "mass". That's the meaning used in "weights and measures", or in bulk goods sold by weight. --Trovatore (talk) 20:21, 13 December 2016 (UTC)
- Okay: I can concede that many people use the word "weight" to describe something other-than-force-due-to-gravity. If that is the case, we have a problem of linguistic ambiguity; and without additional context, it may be impossible to answer the original question with complete satisfaction.
- In my opinion, the most correct definition of the word "weight" is its pure and unadulterated meaning from elementary physics, which is "force due to gravity," or, "mass times acceleration of standard gravity." This is how weight is defined in many physics textbooks, including my go-to reference, the PHAK: Chapter 4, Principles of Flight; and this definition applies even to parcels of air: "Air is very light, but it has mass and is affected by the attraction of gravity. Therefore, like any other substance, it has weight, and because of its weight, it has force. Since air is a fluid substance, this force is exerted equally in all directions. Its effect on bodies within the air is called pressure."
- Nimur (talk) 21:05, 13 December 2016 (UTC)
- There was a guy on alt.usage.english, I think his name was Gene Nygaard or something similar, who looked into the history of the linguistic issue. If I recall correctly, he came to the conclusion that this insistence on "weight" as force as opposed to mass does not come from the physics community at all, but from the physics education community.
- I have not examined his evidence in detail, but this seems plausible to me. Weight-as-force is not a very fundamental quantity for physics; it's relative to the local gravitational field rather than being a property of the object. It's fundamental for certain branches of engineering, but engineers are not so picky about this sort of thing. But for physics teachers, it's very convenient to have a separate word, even somewhat arbitrarily chosen, because getting students to understand that two concepts are different is already challenging without having to use the same word for them.
- I think we see the same effect in the shibboleth that "velocity is a vector; speed is a scalar". There is no etymological reason whatsoever to make this distinction — you just have one Latinate and one Germanic word, and you arbitrarily pick the Latinate one as meaning the vector. How you would do this in, say, Italian, I have no idea. And a real physicist is unlikely to be bothered if you mention the "velocity of light". But, again, for teaching purposes, it's convenient. --Trovatore (talk) 22:15, 13 December 2016 (UTC)
- "Weight" has a bunch of meanings, one of which is actually "mass". That's the meaning used in "weights and measures", or in bulk goods sold by weight. --Trovatore (talk) 20:21, 13 December 2016 (UTC)
- True, but many people think that "weight" is what their mechanical or electronic scales measure, and that seems to be what the questioner was asking about. Dbfirs 17:32, 13 December 2016 (UTC)
- The best way to describe the difference between mass and weight is this. Imagine a car transported to the Moon, where gravity is one-sixth that on Earth. The car weighs one-sixth of its weight on Earth, so it would take far less effort to jack it up to change a flat tyre. However, the car's mass is the same as on Earth, so it would take the same effort to push start it (neglecting tyre rolling resistance and wind speed). Akld guy (talk) 21:23, 13 December 2016 (UTC)
- There's a simpler way: mass is measured by inertia. Weight is measured by gravity. --Jayron32 04:22, 14 December 2016 (UTC)
- It's a contingent fact that inertial mass and gravitational mass are the same, not a logical necessity. "Mass" is really three different concepts, actually: Inertial mass, gravitational mass, and energy/quantity-of-matter (actually I suppose the last two are not logically necessarily the same either, so maybe four). Experimentally, they are all equal within the limits of measurement. Mach's principle was supposed to explain the identity of inertial and gravitational mass, but in spite of the fact that it was a major conceptual heuristic for Einstein when developing GR, I gather that it is not really believed anymore, so it may be that there is still something to be explained even for those two. --Trovatore (talk) 04:39, 14 December 2016 (UTC)
- I don't think that's what Jayron was getting at. I think what he meant was, mass is a measurement of the "m" in F=ma, while weight is a measurement of mg, whatever local g happens to be. Which is, of course, the definition of both things. Weight != gravitational mass. Someguy1221 (talk) 05:13, 14 December 2016 (UTC)
- Well, again, depends on the sense of "weight" you have in mind. See above remarks about Gene Nygaard's findings. --Trovatore (talk) 05:53, 14 December 2016 (UTC)
- I don't think that's what Jayron was getting at. I think what he meant was, mass is a measurement of the "m" in F=ma, while weight is a measurement of mg, whatever local g happens to be. Which is, of course, the definition of both things. Weight != gravitational mass. Someguy1221 (talk) 05:13, 14 December 2016 (UTC)
- It's a contingent fact that inertial mass and gravitational mass are the same, not a logical necessity. "Mass" is really three different concepts, actually: Inertial mass, gravitational mass, and energy/quantity-of-matter (actually I suppose the last two are not logically necessarily the same either, so maybe four). Experimentally, they are all equal within the limits of measurement. Mach's principle was supposed to explain the identity of inertial and gravitational mass, but in spite of the fact that it was a major conceptual heuristic for Einstein when developing GR, I gather that it is not really believed anymore, so it may be that there is still something to be explained even for those two. --Trovatore (talk) 04:39, 14 December 2016 (UTC)
- There's a simpler way: mass is measured by inertia. Weight is measured by gravity. --Jayron32 04:22, 14 December 2016 (UTC)
- This is sort of a diversion - it isn't related directly to the weight/mass distinction. There are two definitions of weight here: a) as a measurement - what the scale says, assuming certain conditions like weighing in air. Or b) as a true force, deduced from your knowledge which includes the buoyancy of surrounding air and/or the pressure and composition of air, or by any other means you can think of, including being told how many kg of air you're weighing. The experimental measurement is quite predictable: an analytical balance with miles of air on the pan will register zero, at least if it's been tared correctly. It doesn't really matter if it's "wrong" so much as that you know the procedure by which it was obtained. The theoretical measurement is also easy, if you multiply the kg by the force of gravity to get a force, which may be reported by re-dividing that ratio to give a kg equivalent weight.
- Now one thing I should add, just in case the OP is confused on the basic basics, is that of course a kg of air will give a zero reading on the balance only if it is at the same density as the surrounding air, so that the buoyancy makes up for it. Compress that air by 10%, and 10% of the buoyancy is lost, so 10% of the true weight is recorded. Wnt (talk) 16:33, 14 December 2016 (UTC)
- Aye, when divining the actual truth value of the true force the significance of a person's empirical weight compared with their body mass shouldn't be overlooked entirely either. As this medieval balance showed a: she weighed the same as a duck. And b) "she's a witch"; a dark force to be reckoned with justly, as it was deduced from an enlightened knowledge as to why witches burn. -Modocc (talk) 20:51, 14 December 2016 (UTC)
Power Splitter
[edit]Hi, I know that a power splitter is a component that splits the power into two flows with equal powers. Now, my question is:
Assume that the current flows from A to a power splitter that splits it to two points B and C, and after C there's a resistor. Let us analyse this situation:
- The powers in B and C are equal as a power splitter splits the power equally.
- The current in B is greater than in C, as there's a resistor after.
However, the power is proportional to the current, so this does not make any sense to me...? עברית (talk) 12:55, 13 December 2016 (UTC)
- Please specify what kind of "power splitter" you have in mind. Said bluntly, there is no electronic device that can split power, or voltage, or current (three different things) by a ratio that does not depend on what is plugged on the output(s). What is usually done is that such a device will present a large electrical impedance on its output, so that the electrical load located down the line has a small effect. (There is a short paragraph at Voltage_divider#Loading_effect.) TigraanClick here to contact me 13:16, 13 December 2016 (UTC)
- In electric utilities, phase shifting transformers are able to regulate the flow of true AC power as well as the flow of reactive power or VARs by adjusting the phase angle by several degrees, using tapchangers which can operate while under load. They might have ratings of up to 300 mega-voltamperes at 138 kilovolts. If a bus at a substation has to outgoing lines to remote busses, phase shifters could be used to adjust the power flow or var flow to the two remote busses up to the design limits of the phase shifters. But I expect the OPs "power splitter" is a more ideal device which one cannot go out and purchase. Edison (talk) 13:40, 13 December 2016 (UTC)
- Power dividers are components that are engineered to share power in a specified ratio, which may be 50%:50% or another, between output loads whose impedances are both known and typically 50 ohm. The article Power dividers and directional couplers gives a comprehensive overview of the technologies used for various frequency ranges. Power dividers are sometimes called power splitters but the term power splitter is also used in marketing simple parallel connector cables. The latter merely supply equal voltages, are not designed for radio frequencies, and the power division is 50%:50% only if the loads are equal resistance. If not, the powers are proportional to the currents, or equivalently, inversely proportional to the load resistances. Blooteuth (talk) 17:52, 13 December 2016 (UTC)
- In electric utilities, phase shifting transformers are able to regulate the flow of true AC power as well as the flow of reactive power or VARs by adjusting the phase angle by several degrees, using tapchangers which can operate while under load. They might have ratings of up to 300 mega-voltamperes at 138 kilovolts. If a bus at a substation has to outgoing lines to remote busses, phase shifters could be used to adjust the power flow or var flow to the two remote busses up to the design limits of the phase shifters. But I expect the OPs "power splitter" is a more ideal device which one cannot go out and purchase. Edison (talk) 13:40, 13 December 2016 (UTC)
- The OP seems to have made a typo. He says "the current in B is greater than in C, as there's a resistor after." but he just told us that the resistor is at C, not B. He needs to sort this out before an explanation can be attempted. Akld guy (talk) 21:14, 13 December 2016 (UTC)
- →Power factor. Some load on AC needs to be corrctes. Some devices use a PFC.
- If You are operating a power grid, You need to load balance the wires and keep the phase synchronized. This would be possible to do it by increasing of decrasing a transformers ratio or shift, when unsychroniszed. When connecting unsynchronized power, worse will happen. It is the same as change + and - of DC. It is like connecting power supplies in series and then shortcut the whole circuit. --Hans Haase (有问题吗) 17:34, 14 December 2016 (UTC)
Storing data in ice Ih
[edit]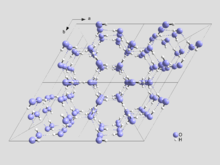
Any six water molecules can form a ring in which hydrogen bonds point from one to the next in a loop:
O-H ~ O-H ~ O ~ H H ~ O ~ H-O ~ H-O
I would suggest this ring could (in theory) be reversed, without changing any of the rest of the ice crystal in any way:
O ~ H-O ~ H-O H ~ ~ H O-H ~ O-H ~ O
This should store one bit of information. I think I've actually seen some publication in which water molecules in a single molecule layer were manipulated this way.
Looking at the structure of Ice Ih, above, it seems well designed for such storage. A single set of rings at the center is (apparently) unusable because we see H-O-H ~ O ~ H-O-H bonding that we can't alter without making larger changes to the structure. The other eight seem open for business, as best as I can tell. However, changing one ring necessarily involves a shift of one hydrogen in its neighbor. It doesn't change the whole neighboring ring because the change is made up elsewhere, but that neighbor might not be possible to shift around in a loop any more. Each usable ring neighbors on three normal and three unusable rings, I think, so I'm guessing you get 2 rings per unit cell to shift without restriction (depending on what data is in them some other bits might still be hidden in the connecting rings). Each water is in one usable, one unusable, one interstitial cell, so that's 2 bits (plus...) in 32 waters. I haven't thought about the z axis in this at all...
So...
- a) Has anyone professional actually evaluated this system in terms of information storage, and come up with the actual upper limit? I bet I fall far short of it, though I'd be surprised if they can do more than one bit per six waters.
- b) In the Ih structure shown, the ice is stacked up layer on layer with rings in opposite directions. I assume that is slightly lower energy. Is it true? And how much lower? If some moron melts an alien civilization's priceless data archive in a beaker, can he potentially recognize after the fact (due to the lower amount of heat absorbed) that he just did a dumb thing?
- c) *Is* an Ice Ih structure tweaked extensively in the way I suggest still Ice Ih by definition?
- d) Is it conceivable to measure which way a single ring of Ice Ih is oriented in a crystal using one of the known advanced microscopic techniques?
- e) Is it conceivable to shift the entire ring of hydrogens in some kind of concerted process without utterly destroying the surrounding crystal lattice?
- f) Could another form of ice work better?
Wnt (talk) 20:19, 13 December 2016 (UTC)
- I'm not aware of any water-as-information-storage research, nor did I find any with a few minutes of searching. I do know of some research on storing information in molecules: this paper [4] makes a case for synthesized DNA, this one [5] uses more exotic self-assmembled constructs. A while back, single-molecule magnets [6] had some buzz, but I haven't seen much of that recently. Interesting idea though. SemanticMantis (talk) 21:19, 13 December 2016 (UTC)
- If you reversed the O-H...O order it would affect the adjacent ring with O...H-O, so by reversing the OH it would be a higher energy ring repelled by the adjacent rings. I don't think there would be much to prevent it rotating back into the most stable state. Probably there would be an activation energy of zero and the altered HO order would have an energy at a local maximum. So it would decay on the order of picoseconds back to the stable ice structure. Perhaps you could detect the changed orientation by its effect on infrared spectroscopy or the nuclear magnetic resonance of the protons in the altered position. Using neutrons to determine where the hydrogen is, will likely destroy the crystal. The answer to (c) is that it would be a kind of defect. Amorphous ice would contain more defects and thus more potenital information. Graeme Bartlett (talk) 01:20, 14 December 2016 (UTC)
- @Graeme Bartlett: I'm not sure if you thought that through right... The shift affects two rings, but the other one doesn't need to shift any further to accommodate it. For example, in the diagram below the left water has a hydrogen out of plane and the right hydrogen does not. But shifting the bonds in the lower ring only does not change how many hydrogens each water is bound to, nor does it interfere with the hydrogen bonds shown in the upper ring.
O O
\ ~
H H
~ /
O-H ~ O
~ \
H H
/ ~
O O
- Now yes, I think there must be some kind of difference between having two hydrogen bonds stacked side by side that are the same vs. opposite orientations, but is that really something that would decay in picoseconds or rotate freely? Wnt (talk) 15:06, 14 December 2016 (UTC)
- This sounds vaguely similar to various pseudoscientific theories of structured water. See also; List_of_topics_characterized_as_pseudoscience#Idiosyncratic_ideas, Hexagonal water, Penta Water and Polywater. μηδείς (talk) 03:42, 14 December 2016 (UTC)
- Using the directionality of the O–H bonds to store information requires that the direction is static. Once one sets it one way or the other--either at the time of crystallization, or changeable by some action after crystallization--it needs to stay that way. But could the O–H actual bond stretch enough (with concomitant compressoin of the H···O hydrogen-bond) that the H winds up on the "other" O, which would result in the reversal of ring direction? Also consider proton tunneling that would have the same effect even without such vibration doi:10.1103/PhysRevLett.112.148302 DMacks (talk) 04:03, 14 December 2016 (UTC)
- I also wonder if the H-O-H-OH (etc.) bonds in ice crystals are distinctly isolatable to individual water molecules. In liquid water, individual hydrogens are highly labile (see autoionization of water) which makes me wonder if the bonding pattern in ice allows one to identify individual water molecules, or if the inter- and -intra molecular OH bonds are not essentially equivalent, or highly labile, which would make the orientation unimportant. --Jayron32 04:09, 14 December 2016 (UTC)
- I'm assuming that if you cool this ice enough, the bonds become non-labile (which makes them some sort of example of "zero point energy", perhaps, that can't be extracted with simple cooling?). I don't have any idea if they are labile in relatively warm ice, though clearly if the ice is near equilibrium with liquid the data should start getting erased.
- Now you have me wondering if there is a "phase transition" between Ice Ih that can freely exchange protons and Ice Ih that cannot... Wnt (talk) 15:11, 14 December 2016 (UTC)
- My greater question is if the squiggles and the dashes in your diagram are identifiably distinct, or if they are really just the same bond. All models are wrong and I'm reminded of cases like the Kekule structure of benzene and other issues related to Resonance, where the issue is not the actual behavior of electrons, but of our inadequate models in describing them. That is, in ice is the O-H bond distance the same or different for so-called "intermolecular" and "intramolecular" O-H bonds, especially over time scales where one would be making measurements. If so, then there really isn't a difference between said bonds, and your directionality breaks down entirely. --Jayron32 15:33, 14 December 2016 (UTC)
- I have the impression that hydrogen bond fundamentals are clearer than that. But yes, it would be interesting to know the deterioration as a function of time and temperature. Wnt (talk) 16:11, 14 December 2016 (UTC)
- Ultimately it's a question of order of magnitude: Is it milliseconds or millenia? --Jayron32 20:16, 14 December 2016 (UTC)
- I haven't read it yet, but I suspect this may answer your question. It is titled "The Hydrogen Bond in the Solid State." --Jayron32 20:19, 14 December 2016 (UTC)
- @Jayron32: Good find! Still have much to see there. It does say that there are symmetrical hydrogen bonds in which donor and acceptor are the same; but water is as classical an H-bond as is to be found. Anyway, I'm going to start with one basic datum I noticed: H-bonds in water have an energy of 4.7-5 kcal/mol. At 273 K, given that the Boltzmann constant is 1.987 cal/mol K, the Boltzmann factor is e^(1000*4.85/(1.987*273)) = 7644. I think. So more than one time out of 10000 that we look at an ice crystal, the H bond could be broken... maybe. By contrast the O-H bond strength is 458 kJ/mol, or 109 kcal/mol, to keep with where I was, so the fraction of the time it is found intact is 2e+87, according to my copy of R. Caveat being that I have no idea what determines how many times the system gets to "look" per second when deciding if the bond gets broken or not! But clearly the O-H covalent bonds rule the roost on how often any such shifting takes place, and the compensation from an H-bond is going to be a minor factor by comparison. Wnt (talk) 21:01, 14 December 2016 (UTC)
- I haven't read it yet, but I suspect this may answer your question. It is titled "The Hydrogen Bond in the Solid State." --Jayron32 20:19, 14 December 2016 (UTC)
- Ultimately it's a question of order of magnitude: Is it milliseconds or millenia? --Jayron32 20:16, 14 December 2016 (UTC)
- I have the impression that hydrogen bond fundamentals are clearer than that. But yes, it would be interesting to know the deterioration as a function of time and temperature. Wnt (talk) 16:11, 14 December 2016 (UTC)
- My greater question is if the squiggles and the dashes in your diagram are identifiably distinct, or if they are really just the same bond. All models are wrong and I'm reminded of cases like the Kekule structure of benzene and other issues related to Resonance, where the issue is not the actual behavior of electrons, but of our inadequate models in describing them. That is, in ice is the O-H bond distance the same or different for so-called "intermolecular" and "intramolecular" O-H bonds, especially over time scales where one would be making measurements. If so, then there really isn't a difference between said bonds, and your directionality breaks down entirely. --Jayron32 15:33, 14 December 2016 (UTC)
- Even if it worked, it seems impractical:
- 1) You're using 12 atoms to store a single bit. Can't we get that lower with a crystal where 2 interchangeable atoms can be used at each node ? Or perhaps more than 2 interchangeable atoms, to go beyond one bit per atom ?
- 2) Water ice isn't very stable under normal condition here on Earth. Obviously it must be kept frozen, which would require an incredibly reliable cooling system and electricity supply, but even then there's still sublimation (phase transition), so you'd need to cover the ice with something that wouldn't react with it or allow sublimation. Better yet, use something more stable to store your data. StuRat (talk) 15:20, 14 December 2016 (UTC)
- I wasn't really picturing finding this on Earth, but during some exploration. Alternatively some silly alien might set up a Fortress of Solitude we have to reverse engineer to keep up on weapons technologies. Wnt (talk) 16:06, 14 December 2016 (UTC)
- Even on other worlds, water is still too reactive. You'd want something more inert. StuRat (talk) 20:37, 14 December 2016 (UTC)
- If data is stored in ice instead of current conventional methods, you wouldn't need an electromagnet to corrupt the data. All you'd need is a blow dryer. ←Baseball Bugs What's up, Doc? carrots→ 21:53, 14 December 2016 (UTC)
- That's a valid point. There are times when we want data to be easy to destroy, such as code books that were in lead cases so they could be tossed overboard if the ship was taken. Codes in ice could be quickly destroyed. StuRat (talk) 16:17, 15 December 2016 (UTC)
- I don't see these as serious objections (you could say the server rooms at Wikipedia would catch on fire without careful climate control). But if you're worried, chemists being chemists, they've come up with various ways of making ice at room temperature. [7][8] (that second is an article I was thinking of at the beginning, but they didn't actually manipulate the ice to store information, I'm afraid I projected that onto the result; also there was some skepticism [9] and I didn't keep track who won out) [10] Maybe we can work out some kind of scaffolding that fits into unused parts of the ice Ih crystal lattice and makes it more stable, containing some useful molecular circuitry for reading and writing bits. And try to make it green, to match the color of that crystal Superman kept fiddling with in the old movie series... Wnt (talk) 18:12, 17 December 2016 (UTC)
How do F-35s and other stealth aircraft avoid collisions with each other in mid-air? --Llaanngg (talk) 21:38, 13 December 2016 (UTC)
- In peacetime or during training, stealth aircraft have radio transponders that indicate the airplane's position to radar systems on the ground and in other aircraft. In war, while flying over a combat zone where anti-aircraft fire is expected, this transponder will obviously be turned off, along with the aircraft's active radar system. I would imagine stealth aircraft formations would stay safe by maintaining discipline and eye contact, but I'm just speculating. I'm not sure you'll even be able to find official documents regarding wartime stealth combat doctrine, though there may be some good sources out there speculating, or first-hand accounts from pilots. Someguy1221 (talk) 21:45, 13 December 2016 (UTC)
- It is a mistake to think of combat radar units in terms of video game "radar" that shows an omniscient all-around view. The radar in the F-35 is derived from the AN/APG-77, which is restricted to a 120° field of view, and that likely compromises range and directed power when operating at maximum aperture. Instead, basic fighter maneuvers, the portion of combat where vigorous manuevering is required, has always relied on visual identification of other aircraft, both friendly and enemy. The F-35 is not particularly unique in this regard (it does have a new 360° situational awareness system, but I suspect that relying on that in a dogfighting situation would be contraindicated). Pilots avoid hitting other airplanes by looking around. — Lomn 22:04, 13 December 2016 (UTC)
- ...then how do we conduct aerial combat operations in IMC? I think the answer is much more complicated than we are permitted to know... and probably has a lot to do with secretive electronics, AWACS, and special forces ground combat controllers... Nimur (talk) 22:12, 13 December 2016 (UTC)
- (ec) Yes, I was coming back to add that a discussion of modern fighter doctrine needs to consider the presence of AEW&C, dedicated high-power radar platforms that are used to detect enemy aircraft and vector friendly aircraft. So, first, your stealth aircraft isn't the primary search platform. Second, much like submarines and active sonar, a fighter (particularly a stealthy fighter) trying to use its radar as a search platform is effectively screaming "WHERE ARE YOU? WHERE ARE YOU?" into the void and hoping to hear something echo off a distant target. It might work, but it will definitely alert opposing forces to the presence and general location of the stealth aircraft. A platform like an F-35 will generally not use its radar in an air-to-air engagement until it is ready to fire at something it already knows is there. It will not use radar for something so mundane as keeping track of its squadron in a threat environment. — Lomn 22:13, 13 December 2016 (UTC)
- ...then how do we conduct aerial combat operations in IMC? I think the answer is much more complicated than we are permitted to know... and probably has a lot to do with secretive electronics, AWACS, and special forces ground combat controllers... Nimur (talk) 22:12, 13 December 2016 (UTC)
- [11] has the answer (kind of): Fly single-ship rather than in a formation, segregate airspace by altitude or other division, use a pre-programmed route, keep visual connection and avoid IMC flight, and use an optical datalink (existence is dubious).
- The pre-programmed route bit is how Serbia shot down a F-117 stealth fighter, which was flying every day the same route. The Serbs just shot at a location it would most probably be.
- I also wonder whether they could turn the stealth mode on/off for a short period of time and send or receive a burst of information. After all, when they enter stealth mode again, they will need just some seconds to be several miles away.
Hofhof (talk) 23:52, 13 December 2016 (UTC)
- That's not quite how our 1999 F-117A shootdown article tells it - although it mentions deciphering NATO transmissions, it claims the main factor was that the Serbs were able to change the frequency of their radar, and found a frequency band that scattered off the bomb doors and wheel covers when they were open. If you've got a better source, it would be good to add that to the article. Smurrayinchester 09:37, 15 December 2016 (UTC)
- Monitoring and managing aircraft is the job of ground units. There are mobile air control squadrons who specifically are tasked with moving to the theater of operations and managing air operations. 209.149.113.5 (talk) 20:01, 14 December 2016 (UTC)
- When the Serbs claimed the very first ever stealth aircraft "kill", it did no doubt cause a re-think about vulnerabilities, that stealth aircraft might not be the "apex predators" once thought. If what the poster says here is true, it shows a lack of basic strategic doctrine on the part of control. Sending a plane down exactly the same route many days in a row? Recent developments of Passive radar have further amplified this concern, to which even stealth aircraft are vulnerable. And Iran bringing down a stealth drone almost fully intact, basically, simply by jamming or hijacking the link to control, shows another angle of attack, at least when one does not have a human pilot on board. Unrelated vulnerability, but I think SkyGrabber is shareware or freeware, freely available from the developer's website for anyone, and could read military drone video feeds live. No encryption??? Eliyohub (talk) 04:50, 15 December 2016 (UTC)
- When I was in the military, flight routes changed with each fly-over. The IFF was changed out daily. Radar deployment was moved during breaks in flights. Therefore, a claim that the entire flight policy changed is hard for be to believe when the only source is some guy posting on Wikipedia. 209.149.113.5 (talk) 13:04, 15 December 2016 (UTC)
