Wikipedia:Reference desk/Archives/Science/2019 August 11
| Science desk | ||
|---|---|---|
| < August 10 | << Jul | August | Sep >> | August 12 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
August 11
[edit]Diamonds
[edit]Considering it takes immense amount of kinetic and heat energy to turn coal into diamonds, I assume large part of that energy is absorbed into the diamond. Following the laws of energy conservation would it then be possible to use diamonds as sort of energy "batteries" and reverse the process to release large amounts of energy again? 91.101.26.175 (talk) 02:23, 11 August 2019 (UTC)
- Your are mistaken. Just because you need "immense" temperature and pressure to turn coal into diamonds doesn't means that the energy intake is anyway commensurate. Diamond does pack slightly more energy than graphite, but no so much: ~1.9 kJ/mole, that it is, ~155 kJ/kg ( https://atct.anl.gov/Thermochemical%20Data/version%201.122/reaction/?&species_number[]=3&species_number[]=1156&c1=-1&c2=1 ) . This is the magnitude (somewhat less) of energy more conveniently stored by a basic battery. Besides, diamond is metastable and does not spontaneously turn into graphite. That is, you have to give some energy first to get more.
- So there is no lack of better options.
- Now, it could be done indeed. Gem fr (talk) 06:35, 11 August 2019 (UTC)
- You can release energy from diamonds by burning them (not recommended). 107.15.157.44 (talk) 07:04, 11 August 2019 (UTC)
- Treating diamonds as charcoal does not seem very cost-efficient. ←Baseball Bugs What's up, Doc? carrots→ 14:54, 11 August 2019 (UTC)
- It would be very economical if you lived on a planet made of diamond such as 55 Cancri e. Or, on Jupiter, you could just stand out in the diamond rain with a bucket – no need to even dig them up. SpinningSpark 17:17, 11 August 2019 (UTC)
- What kinds of vegetables could you grow on Jupiter? ←Baseball Bugs What's up, Doc? carrots→ 17:53, 11 August 2019 (UTC)
- Very expensive ones. Matt Deres (talk) 19:46, 11 August 2019 (UTC)
- What kinds of vegetables could you grow on Jupiter? ←Baseball Bugs What's up, Doc? carrots→ 17:53, 11 August 2019 (UTC)
- It would be very economical if you lived on a planet made of diamond such as 55 Cancri e. Or, on Jupiter, you could just stand out in the diamond rain with a bucket – no need to even dig them up. SpinningSpark 17:17, 11 August 2019 (UTC)
- Treating diamonds as charcoal does not seem very cost-efficient. ←Baseball Bugs What's up, Doc? carrots→ 14:54, 11 August 2019 (UTC)
- You can release energy from diamonds by burning them (not recommended). 107.15.157.44 (talk) 07:04, 11 August 2019 (UTC)
- The OP has made a common mistake in chemistry by confusing chemical thermodynamics with chemical kinetics. Thermodynamics is a state function that is path independent, which is to say the thermodynamics only cares about your two end states (in this case, coal and diamond) without caring one bit about the way in which coal can be turned into diamond, and vice-versa. As noted above, while diamond is at a higher energy state than coal, it is not monumentally so. The reason why coal is so hard to turn into diamonds is that the kinetics of the process (which refers to the specific mechanism it takes to get from coal to a diamond) is slow and requires extreme conditions. --Jayron32 01:58, 12 August 2019 (UTC)
Longwaves and atmospheric opacity
[edit]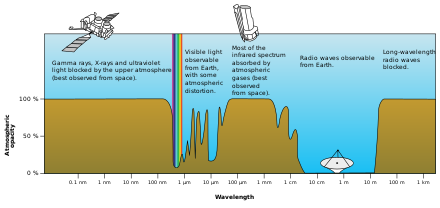
Is this diagram in the Electromagnetic spectrum article anywhere near accurate? BBC Radio 4 Longwave broadcasts on 1500 m which is well inside the band marked as 100% atmospheric opacity, yet the station can be heard half-way round the world. Now I know longwave propagates through this funny ground wave mode, but most of the field is in the air, not in the ground. It is lower loss over higher conductivity terrain (especially ocean) and in the ideal situation with a perfect conductor, all the field is in the air. So surely it can't propagate in an opaque medium by definition? SpinningSpark 17:11, 11 August 2019 (UTC)
- The diagram shows the opacity for radiation going from outer space to the earth's surface. Long-wave radio waves are blocked by the ionosphere, but can travel through the lower atmosphere. The diagram is correct. PiusImpavidus (talk) 17:54, 11 August 2019 (UTC)
- The actual wavelength of BBC Radio 4 was 1498.962 m (200 kHz) until 1 Feb 1988 when it changed to 1514.103 m (198 kHz). DroneB (talk) 20:07, 11 August 2019 (UTC)
- Skywave is our article (edit/see below): regarding the effect of ionosphere on radio propagation, although I have no idea if/how this applies to the 1500 m band Gem fr (talk) 21:37, 11 August 2019 (UTC)
- That absolutely is not the right article (although it also uses the same inappropriate diagram). The LW band is only in skywave mode in exceptional circumstances. The normal propagation mode is the ground wave article I linked above. SpinningSpark 22:06, 11 August 2019 (UTC)
- You are right, this was to elaborate on the ionosphere ref just above Gem fr (talk) 22:22, 11 August 2019 (UTC)
- That absolutely is not the right article (although it also uses the same inappropriate diagram). The LW band is only in skywave mode in exceptional circumstances. The normal propagation mode is the ground wave article I linked above. SpinningSpark 22:06, 11 August 2019 (UTC)
- Skywave is our article (edit/see below): regarding the effect of ionosphere on radio propagation, although I have no idea if/how this applies to the 1500 m band Gem fr (talk) 21:37, 11 August 2019 (UTC)
- Actually the diagram looks pretty awful (NOT correct) to me, as it treats absorption (being black-body-like) and reflection (being mirror-like) as if it were the same "blocked" or "opaque". Gem fr (talk) 21:31, 11 August 2019 (UTC)
- Good point. Maybe the diagram should use a different color for long-wavelength radio waves that the lower atmosphere is transparent to, and someone should edit the text caption for that part. --76.69.116.4 (talk) 02:15, 12 August 2019 (UTC)
- But, in terms of opacity, wouldn't absorption and reflection be the same?
- The point is to illustrate what makes it through and what doesn't, not to explain every aspect of the wave's interaction with the atmosphere. ApLundell (talk) 02:37, 12 August 2019 (UTC)
- But is Opacity the relevant property? If it were, the diagram would had answered the OP question, instead of spawning it. Opacity is not even a property of the medium, but a threesome also involving the emission and reception places. Gem fr (talk) 08:04, 12 August 2019 (UTC)
- The bigger point is that this diagram is used in at least two articles that are discussing terrestial communications, not ground observations of space. In those cases only the opacity (or transmittability) of the medium itself is relevant, and for that purpose, the diagram is pretty hopeless. SpinningSpark 12:41, 12 August 2019 (UTC)
- But is Opacity the relevant property? If it were, the diagram would had answered the OP question, instead of spawning it. Opacity is not even a property of the medium, but a threesome also involving the emission and reception places. Gem fr (talk) 08:04, 12 August 2019 (UTC)
- The actual wavelength of BBC Radio 4 was 1498.962 m (200 kHz) until 1 Feb 1988 when it changed to 1514.103 m (198 kHz). DroneB (talk) 20:07, 11 August 2019 (UTC)
- Well, given that this image is shows an illustration of a radio telescope and an orbital telescope, it seems designed to illustrate the value of different wavelengths to astronomy, so I'm pretty sure opacity is the relevant property. Or rather, a useful simplification of the relevant properties.
- It looks like it's mostly used in that context, but as Spinningspark mentions, it's been included as decoration in a few less relevant articles. ApLundell (talk) 17:43, 12 August 2019 (UTC)
- I agree on this. As long as the purpose is "can we study these radiations from outer space standing on the ground or do we need satellites?", the image is just fine. Problems arise from using it
as decoration in a few less relevant articles
. Gem fr (talk) 18:04, 12 August 2019 (UTC)
- I agree on this. As long as the purpose is "can we study these radiations from outer space standing on the ground or do we need satellites?", the image is just fine. Problems arise from using it
Subsidiary question...
[edit]...which could potentially lead to article improvement. Does anybody have a source of data that I could turn into an svg that is actually relevant to terrestial radio transmission? SpinningSpark 17:57, 12 August 2019 (UTC)
- some radio-related org? maybe even some national agency of regulation? If none found, https://commons.wikimedia.org/wiki/Commons:Graphic_Lab/Illustration_workshop could be of help Gem fr (talk) 09:17, 14 August 2019 (UTC)
- The Graphics Workshop can help draw something. They can't magic up the data. SpinningSpark 09:41, 14 August 2019 (UTC)
- Note the day/night (corrected link) difference in terrestrial radio propagation. DroneB (talk) 21:32, 14 August 2019 (UTC)
- You are referring to skywave propagation I think. I don't think that that makes a substantial difference to my question. I may be wrong, but I don't believe that there is a great deal of difference in the opacity of the troposphere between day and night. The fact that some transmitters bounce the signal off the ionosphere to get greater range certainly has a nighttime difference, but it doesn't change the loss experienced travelling through the air. SpinningSpark 16:15, 15 August 2019 (UTC)
- Note the day/night (corrected link) difference in terrestrial radio propagation. DroneB (talk) 21:32, 14 August 2019 (UTC)
- The Graphics Workshop can help draw something. They can't magic up the data. SpinningSpark 09:41, 14 August 2019 (UTC)
- some radio-related org? maybe even some national agency of regulation? If none found, https://commons.wikimedia.org/wiki/Commons:Graphic_Lab/Illustration_workshop could be of help Gem fr (talk) 09:17, 14 August 2019 (UTC)
How many moons with Luna's apparent Diameter?
[edit]In regards to various Fantasy worlds...
- )With long term stability (>100,000 years) how many moons could a Earthlike planet have whose apparent diameter from the planet is equal to Luna?
- )Similarly, with long terms stability, how many "suns" could an Earthlike planet have whose apparently diameter from the planet is equal to Earth's sun?
Naraht (talk) 19:12, 11 August 2019 (UTC)
| obsolete answer, better one below |
|---|
| The following discussion has been closed. Please do not modify it. |
|
- Close binary suns can be stable. Slightly oranger and it can be most Earthlike, you might not even notice the color difference. Sagittarian Milky Way (talk) 23:25, 11 August 2019 (UTC)
- of course they can be stable, it is not the question. The question is: can one of them have a planet which will see both suns with the same apparent diameter?Gem fr (talk) 07:31, 12 August 2019 (UTC)
- That includes the planet. This is the system mentioned in the answer below, taking advantage of luminosity decreasing faster than diameter as you look at colder spectral types in the main sequence. Sagittarian Milky Way (talk) 13:19, 12 August 2019 (UTC)
- of course they can be stable, it is not the question. The question is: can one of them have a planet which will see both suns with the same apparent diameter?Gem fr (talk) 07:31, 12 August 2019 (UTC)
- First of all, in astronomical terms 100 thousand years is a blink of an eye. Long term is more like 100 million years.
- Lagrange points are not going to help you. L1, L2 and L3 are unstable, L4 and L5 are stable only with a sufficiently large mass ratio. Using L4 or L5, all objects are at the same distance from each other and two objects will only have the same apparent diameter if they have the same actual diameter. Without ridiculous differences in density (ice versus lead), they will have similar masses, making L4 and L5 unstable.
- You can have more than one moon orbiting your planet. To give all the same apparent diameter, assuming similar densities and using Kepler's third law, their masses must be proportional to the square of their orbital periods. That would be a remarkable coincidence, but not impossible. Furthermore, the Moon has a mass of 1/81 of the Earth's mass. That's tiny compared to Earth, but huge for a moon, which is typically not more than about 1/10000 of its host planet's mass. Such massive moons will disturb each other's orbit. If you put them in resonant orbits, like Jupiter's Io, Europa and Ganimedes, it may work. Small moons in tight orbits will work better than large moons in wide orbits. My hunch (and a detailed calculation is not trivial): Put a Moon-like moon in the Moon's orbit (period about 28 days), a smaller moon in a 14 day orbit, another one in a 7 day orbit, that should work. Three moons is doable, five plausible, eight out of the question. Don't give any moons an orbital period shorter than about 8 hours, as they will get inside the Roche limit. These large moons are probably formed by giant impacts. That's a formation method that's not likely to work multiple times without destroying the moon formed in the previous giant impact. So formation of such a system of moons is very unlikely, but if formed, it may be reasonably stable.
- The second question is a bit harder to answer. There are multiple configurations for multiple suns. You could have two orange dwarf stars of spectral type K2 in a tight orbit around each other and your planet in a 0.75 astronomical unit orbit around them both. The stars will have the same apparent size as the sun and because of their lower temperature (4850K), the temperature of the planet will be like Earth's (data from Carroll and Ostlie). Or you could put your planet in a tight orbit around a small star and have that star in a wide orbit around a larger star, but radii of main sequence stars don't vary by a very large ratio, so that's tricky. You can't put an Earth-like planet in a tight orbit around a white dwarf, as the red giant progenitor of that white dwarf would have destroyed the planet. A planet in orbit around binary M-dwarfs, in turn orbiting around binary O-stars gives four and should dynamically be reasonably stable, but your planet will be vaporised. You could have a planet orbiting a tight binary of two K dwarfs, all together in a wide orbit around a red giant. That gives 3 stars, but red giants don't last very long.
- PiusImpavidus (talk) 10:34, 12 August 2019 (UTC)
- Regarding the planet orbiting a white dwarf, could it have been captured later ? Maybe from a passing star with that planet in a distant orbit ? For example, if Sedna passed close to a white dwarf, couldn't it be captured ? SinisterLefty (talk) 14:55, 13 August 2019 (UTC)
- White dwarf § Debris disks and planets: Yes, or a planet can inspiral after the star leaves the red giant phase, though the planet will be quite toasty, because the orbital energy gets transformed to heat by tidal interactions. --47.146.63.87 (talk) 01:17, 19 August 2019 (UTC)
- Regarding the planet orbiting a white dwarf, could it have been captured later ? Maybe from a passing star with that planet in a distant orbit ? For example, if Sedna passed close to a white dwarf, couldn't it be captured ? SinisterLefty (talk) 14:55, 13 August 2019 (UTC)
The International Space Station can reach London latitude and no more. Coincidence?
[edit]Well up to 10 miles north of Parliament, sometimes it's only a few miles. This is the latitude to move to if you like seeing it overhead. Sagittarian Milky Way (talk) 23:07, 11 August 2019 (UTC)
- Yes, it is a coincidence. What is your evidence to believe that it isn't before we go off on another wild goose chase researching another one of your unanswerable suppositions? --Jayron32 01:54, 12 August 2019 (UTC)
- It only even qualifies as a coincidence if there is something special about London. I mean, the extreme latitude that the thing reaches is equal to its orbital inclination, which happens to be 51.64°. If the orbital inclination was 43.7° then you could make similar remarks about Toronto. If it was 39.9° you'd say it about Beijing. If it was 70°, then Tromsø. You name a possible inclination within a reasonable range and look at a map, and you'll find a city at that latitude north or south.
- But all the same, there is a science point here. The easiest orbital inclination to put a satellite into is one that's the same as the latitude of its launch point. According to International Space Station, some of the components of the station were launched from Baikonur Cosmodrome and others from Kennedy Space Center. Baikonur is at about 46° latitude and Kennedy is quite a bit lower. According to International Space Station#Orbit, the actual orbital inclination of the station was chosen so that it can be easily reached from Baikonur. But then why 51-plus degrees and not 46? My guess is that the more highly inclined orbit gives a longer launch time window when a spacecraft can be launched from Baikonur to reach the station, since the the station will spend more time close to 46° north latitude—but is that the only reason, or even the main reason, for the choice? --76.69.116.4 (talk) 02:34, 12 August 2019 (UTC)
- Launches from Baikonur don't go due east to avoid dropping stages on China. 51 degrees is lowest inclination which avoids overflight of China during launch. Fgf10 (talk) 07:27, 12 August 2019 (UTC)
- Ooh, thanks! --76.69.116.4 (talk) 08:32, 12 August 2019 (UTC)
- The 51.64° inclination is to prevent hardware dropping on China, but it's very convenient too. It's only slightly more than the latitude of Baikonur, so that Baikonur passes very slowly through the ISS' orbital plane, giving a long launch window. This allows them to choose the launch time right when the ISS passes through the zenith, allowing 4 orbit rendezvous and docking. You rarely get that opportunity from Kennedy/Canaveral or Wallops, where 2 day rendezvous is the norm.
- Further, ESA is an important partner in ISS and I'm quite sure they (and their taxpayers) are happy they can see the ISS passing overhead and watch the pictures taken by astronauts of their home countries. PiusImpavidus (talk) 10:56, 12 August 2019 (UTC)
- Do you have any source for the assertion that launch windows are longer if your latitude is close to the target inclination, or can you unpack that a bit? It doesn't make sense to me. You seem to imply that that target spacecraft has longer dwell times near the northern (or southern) most point of its orbit. However, for (near-)circular orbits like the ISS, orbital speeds are constant, therefore latitude should be irrelevant (for launch windows, different for performance). IE a spacecraft in a 51 degree orbit is going to spend as much time overhead at 46 degrees latitude as it is on the equator. Are you perhaps thinking of a Molniya or tundra type orbits? The increased dwell times of those orbits are a function of their eccentricity. Fgf10 (talk) 11:07, 12 August 2019 (UTC)
- Launches from Baikonur don't go due east to avoid dropping stages on China. 51 degrees is lowest inclination which avoids overflight of China during launch. Fgf10 (talk) 07:27, 12 August 2019 (UTC)
- But all the same, there is a science point here. The easiest orbital inclination to put a satellite into is one that's the same as the latitude of its launch point. According to International Space Station, some of the components of the station were launched from Baikonur Cosmodrome and others from Kennedy Space Center. Baikonur is at about 46° latitude and Kennedy is quite a bit lower. According to International Space Station#Orbit, the actual orbital inclination of the station was chosen so that it can be easily reached from Baikonur. But then why 51-plus degrees and not 46? My guess is that the more highly inclined orbit gives a longer launch time window when a spacecraft can be launched from Baikonur to reach the station, since the the station will spend more time close to 46° north latitude—but is that the only reason, or even the main reason, for the choice? --76.69.116.4 (talk) 02:34, 12 August 2019 (UTC)
Chemical species
[edit]What is a chemical species? I don't understand chemically identical molecular entities that can explore the same set of molecular energy levels on a characteristic or delineated time scale. I first encountered Reactive oxygen species and figured that different compounds were different species (O2 and H2O2 are both reactive species of oxygen), but they're definitely not chemically identical to each other. Nyttend (talk) 23:26, 11 August 2019 (UTC)
- O2 and H2O2 are NOT both reactive species of oxygen. They are each different species of their own. see below. Gem fr (talk) 08:19, 12 August 2019 (UTC)
- Well... they kind-of are. It sort of depends on what is meant by "species", and in this case the idea of "reactive species of oxygen" is just that the substance produces the equivalent of singlet oxygen in situ. When peroxides react, they react in the way that singlet oxygen reacts, doing singlet oxygen type things, so peroxides are included in that category. Language is messy. --Jayron32 18:06, 12 August 2019 (UTC)
- O2 and H2O2 are NOT both reactive species of oxygen. They are each different species of their own. see below. Gem fr (talk) 08:19, 12 August 2019 (UTC)
- A species in chemistry is a substance whose constituent particles are of identical properties. In some contexts, it's just a way of saying "atoms and/or ions and/or molecules" without having to type all of that out, but in a more strict context, it also differentiates between, for example, the different types of O2 molecules; strictly speaking singlet oxygen is a different species than triplet oxygen even though they are both O2, because each species has its own unique set of energy levels distinct from the other; thus they do not have identical properties. In the context of the phrase "reactive oxygen species", that just means that the concept excludes triplet oxygen, but includes singlet oxygen, as well as other oxygen containing compounds that behave like singlet oxygen in situ. --Jayron32 01:52, 12 August 2019 (UTC)
- Huh, this is all new to me. I'm aware of the concept of isomers (although I don't understand why the shape of a molecule matters), but aside from isomer-related differences, I figured that every molecule with a specific chemical formula was identical to every other molecule with the same formula. Sorry to ask another question, but in a way I'm more confused: are octane and butane both species of hydrocarbons? Their constituent particles are of identical qualities (carbon and hydrogen atoms), so it sounds like they meet your definition, but since the two molecules are not chemically identical, I suspect that I'm misunderstanding something. Nyttend (talk) 02:01, 12 August 2019 (UTC)
- octane and butane are each different species, "hydrocarbon" is NOT a species.
- I provided a few examples in the article. Wait a few days for some specialist, which I am not, to validate, but I am pretty sure it will help you right now Gem fr (talk) 06:40, 12 August 2019 (UTC)
I don't understand why the shape of a molecule matters
A (very simplified) explanation is that molecular reactions depend on stripping part of one molecule away, so it's easier if that part is protruding, waiting to be snatched by another molecule with better "affinity". There are also questions of bond energy differences (which cause differences in physical properties such as boiling point etc.). Even isobutane and n-butane are not chemically equivalent despite having the same atoms, unlike your octane/butane example (8 vs 4 carbon atoms). TigraanClick here to contact me 08:24, 12 August 2019 (UTC)- Regarding the shape of a molecule: Chemistry is the study of how various chemical entities interact with each other. That is, how one molecule interacts with another molecule. This can include things like how a group of the same molecules interact among themselves (intermolecular forces) and how one molecule interacts with a different molecule to undergo chemical reactions. Since all of these interactions are governed by the ways in which the molecules collide and interface in 3 dimensions, it is ALL an exercise in geometry: the shape of the molecule itself is the key piece of information in deciding how it will interact. Consider two isotopes, propene and cyclopropane. The fact that one of them is a straight chain with a double bond and the other is a ring, even though they are both C3H6, has a tremendous effect on how each molecule behaves, both internally and externally.
- Regarding the difference between various chemical species with the same formula (like singlet oxygen and triplet oxygen), WHEN molecules interact the interact through their electrons. Electrons around a molecule are organized into various electron energy levels, sometimes called molecular orbitals. When a particular molecule like oxygen has two states (singlet and triplet) that refers to two different ways those orbitals are organized; since there are two different ways they are organized, WHICH specific organization matters with regards to how each of the two states of O2 will react with other substances. I hope that helps. --Jayron32 12:11, 12 August 2019 (UTC)
…I figured that every molecule with a specific chemical formula was identical to every other molecule with the same formula.
That depends on what chemical formula you mean. A molecular formula, such as C
2H
6O, just tells you the building blocks for that molecule. Just as you can make more than one thing with a set of Lego or similar toys, you can arrange atoms in different ways. Chemical nomenclature exists to describe an exact molecule, such that if you know the rules you can build that molecule yourself knowing only the systematic name. These include those long names you've probably seen, such as (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid. --47.146.63.87 (talk) 22:34, 12 August 2019 (UTC)
- Huh, this is all new to me. I'm aware of the concept of isomers (although I don't understand why the shape of a molecule matters), but aside from isomer-related differences, I figured that every molecule with a specific chemical formula was identical to every other molecule with the same formula. Sorry to ask another question, but in a way I'm more confused: are octane and butane both species of hydrocarbons? Their constituent particles are of identical qualities (carbon and hydrogen atoms), so it sounds like they meet your definition, but since the two molecules are not chemically identical, I suspect that I'm misunderstanding something. Nyttend (talk) 02:01, 12 August 2019 (UTC)
Quick question after reading all of this — if X is a species of Y, does that mean that they're chemically identical molecular entities etc.? In other words, if X is a species of Y, is Y necessarily a species of X? I had used it in the biological sense (if X is a species of Y, Y is a broader concept that includes X), so that's why I spoke of octane and butane being species of hydrocarbons. The responses I've been given don't seem to make sense with that (particularly "hydrocarbon is not a species"), so I'm imagined this idea as a way of harmonising what I'm seeing. Nyttend (talk) 16:52, 13 August 2019 (UTC)
- No, different species are explicitly not chemically identical. For example, singlet oxygen and triplet oxygen undergo different reactions. Therefore, they are, by definition, NOT chemically identical. --Jayron32 18:16, 13 August 2019 (UTC)
- Note the ellipsis; the point is that I'm quoting the whole definition, not saying that they're identical. Nyttend (talk) 01:52, 14 August 2019 (UTC)
- To use your biological metaphor, hydrocarbon would be a class or order, alkane would be the family, and butane and octane species within those. Now, the chemical classification doesn't work the same way (chemical categories do not just branch out in a tree, they overlap; molecules can be, say, saturated or not, cyclic or not, organic or not...) , so take this metaphorically. Gem fr (talk) 18:21, 13 August 2019 (UTC)
- Ammospiza maritima and Ploceus velatus are both in order Passeriformes, so we say they're both species of passerines; that's the setting in which I'd initially referred to butane and octane maybe both being species of hydrocarbons, as they're examples of hydrocarbons. Now to reword the question (hopefully this is the end of this thread) — if X is a species of Y, is the opposite always true, or can we validly say "X is a species of Y" to indicate that X is a species that falls under a more general classification called Y? Nyttend (talk) 01:59, 14 August 2019 (UTC)
- Chemists don't do that, AFAIK. So why would you? I never heard "X is a species of Y" from a chemist teacher or practitioner. They will simply say that, fi, cyclohexane is an hydrocarbon, is cyclic, but not aromatic, is saturated, etc. Remember, the biological classification is a tree, the chemical classification is not. Gem fr (talk) 09:12, 14 August 2019 (UTC)
- Ammospiza maritima and Ploceus velatus are both in order Passeriformes, so we say they're both species of passerines; that's the setting in which I'd initially referred to butane and octane maybe both being species of hydrocarbons, as they're examples of hydrocarbons. Now to reword the question (hopefully this is the end of this thread) — if X is a species of Y, is the opposite always true, or can we validly say "X is a species of Y" to indicate that X is a species that falls under a more general classification called Y? Nyttend (talk) 01:59, 14 August 2019 (UTC)
