Talk:Radioactive decay/Archive 1
| This page is an archive of past discussions. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
Old comments
Perhaps this page can be folded into the Radioactivity page? It seems a bit redundant having both...
Both are usefull, this one on the mechanisms and pyhsics, the other one for the seconadry effects of decay, on environment, bioloy etc.
Btw. does anyone know if there exists a formula by which, given the number of protons and neutrons, the half-life of a nucleus can be derived?
- The best that I know is Table of Nuclides, which I added to the article. I believe that these are all measured values, I don't think science can calculate these things accurately at present. pstudier 22:55, 7 Oct 2004 (UTC)
- If only nuclear physics were that advanced! Each of the decay mechanisms are very different processes with separate theoretical treatments. I think it's fair to say that most decay rates can be reproduced by theory to within an order of magnitude or thereabouts. All but the lightest nuclei represent extremely complex many-body problems that cannot be solved exactly (not at the moment anyway). You can make general comments, like for a particular isotope (i.e. constant proton number) the beta decay half-life gradually shortens as you move away from the line of stability (increasing or decreasing the number of neutrons).
- There is a formula that given A (total nuber of proton and neutron) and Z (number of proton) give you the approximate mass of the neuclide. But (in my opinion) is an empirical law (it has some parameter that are choose to make it better appriximate the empirical data). From this given a nuclide (A,Z) and a new neuclide (A*,Z*) you cnan know if the transform would be energetically favorible. But you have to take in account also the mass of the particle emitted. Unfourtunatelly it is not so easy, and this is not all of physics.AnyFile 11:38, 22 Nov 2004 (UTC)
- Would someone be able to elaborate on the following: what makes a nuclide unstable? what accounts for differences in radionuclide decay constants? how does radioactive decay relate to the second law of thermodynamics?
Merge
The above argument for maintaining separate articles here and at Radioactivity is no longer valid. We need to decide which article will be kept, and which will be made into a redirect. The case as I see it is thus:
- In favor of Radioactive decay: More precise term. Radioactivity would then be turned into something between a stub and a disambig page, linking perhaps to Radiation, Radioactive decay, and Radioactive contamination.
- In favor of Radioactive decay: Leave redirect at Radioactivity. pstudier 23:00, 2004 Nov 14 (UTC)
- In favor of Radioactivity: More common term, more likely to be searched, easier to link to.
- In favour. At the moment both page are not very scientific. Maybe also a rewriting or an extension are neededAnyFile 11:40, 22 Nov 2004 (UTC)
- Contesting your logic. I've been planning for weeks to rewrite this page, and I plan to proceed as soon as the merge takes place. It just seems foolish to start rewriting an article when I don't yet know what it will be entitled.
- May be I have express myself in a bad way. What I wanted to say is that there a lot of argument not covered and what is already written will need to be changed if I want to enlarge it.AnyFile 15:30, 24 Nov 2004 (UTC)
- Contesting your logic. I've been planning for weeks to rewrite this page, and I plan to proceed as soon as the merge takes place. It just seems foolish to start rewriting an article when I don't yet know what it will be entitled.
--Smack 06:26, 23 Nov 2004 (UTC)
Please add any new arguments to this list. --Smack 22:09, 14 Nov 2004 (UTC)
I can see no problem with keeping both. Wikipedia is not written on paper and data can be held several times over in different places with minimal extra cost. The principal search term will probably be Radioactivity but a more detailed look at Radioactive decay, perhaps detailing the rate of decay of specific elements and isotopes would be very interesting and would be too much detail to hold on the Radioactivity article. Lumos3 09:43, 23 Nov 2004 (UTC)
- While it is often useful to have some minor subtopics discussed in more than one article, it is generally accepted that it is impractical to keep multiple articles on essentially the same topic. Consolidating these two articles in one location will facilitate all manner of maintenance tasks and help prevent inconsistencies from arising. If you wish to continue this discussion further, please do so at Wikipedia talk:Duplicate articles, where it may garner input from Wikipedists more competent to address the issue. --Smack 18:18, 24 Nov 2004 (UTC)
Question of naming
Let's have another vote. Should this article live here, or at Nuclear decay? The present title is favored by a Google test, seven to one. --Smack (talk) 23:56, 24 Mar 2005 (UTC)
- Those in favor of 'Radioactive decay':
Types of radioactive decay
What determines which type of radioactive decay will happen? Thanks. --Eleassar777 12:09, 30 May 2005 (UTC)
- It depends on many things like atomic weight, number of neutrons, number of protons, the relation of the previous values to each other and how much energy is "left over". --metta, The Sunborn 02:20, 31 May 2005 (UTC)
Are there any (simple) equations that roughly describe this? How do these factors interact? Thanks. --Eleassar777 08:38, 31 May 2005 (UTC)
- The decay mode that occurs is the one that releases the greatest amount of energy. As I understand it, if nucleus A can lose energy by emitting particle x, it will. (I'm not sure that this is true, but I don't see any reason why it wouldn't be.) If it can also lose energy by emitting particle y, it can go either way. I thought I'd made this clear in the article. Please tell me what's not clear, so that I can go and fix it.
- I don't think there is an equation that predicts nuclear decays. Everything depends on the strong force, and physicists don't have a good model of that yet. AFAIK, the only analytical tool they have is nuclear binding energies. --Smack (talk) 19:12, 31 May 2005 (UTC)
- Unfortunately things are not as simple as that. It depends on what the nucleus has too much of, protons or neutrons (this tells between beta and alpha decay), then how energetic the atom is (how much gamma emission). Random chance seems to have a large hand in things too. --metta, The Sunborn 22:20, 31 May 2005 (UTC)
- No, no, things really are that simple. All of the considerations you mentioned are covered by the question of what transformation releases the greatest amount of energy. If a nucleus is tremendously proton-rich, it can lose a lot of energy by emitting a proton (and the same for neutrons). If it's in an excited state, it can lose energy by emitting a photon. --Smack (talk) 04:57, 2 Jun 2005 (UTC)
Emission of a Carbon 14 nucleus
One rare decay process that is not mentioned anywhere is the emission of a carbon-14 nucleus. I think it's like spontaneous fission, but acts like alpha decay in that the nucleus that is emitted always has the same mass number (14).
Some isotopes of radium can decay by this method, such as Ra-221, Ra-222, Ra-223, Ra-224 and Ra-226.
Reference: Isotopes of Radium -- B.d.mills (Talk) 03:13, 15 Jun 2005 (UTC)
- The reason they are not mentioned is that they occur so freaking rarely. But you are correct as far as I know. There are other rare decays not mentioned either. They include a Neon nucleus emission, a "double-beta" decay, and a triton decay. For instance the Ra-222, Ra-223, Ra-224 do a C-14 emission decay less than 0.001% of the time, the other two are much less than that. This is wikipedia, add the information if you can. I have much more important things to do, like make sure the actual articles on the elements have the correct data. You would be shocked by the wrong numbers. --metta, The Sunborn 03:39, 15 Jun 2005 (UTC)
- Neon emission, that's one I've not heard of before. Double-beta decay and double-electron capture are relatively common decay modes. They only happen rarely because they are only encountered as decay modes for long-lived isotopes like Calcium-48. I note them here for completeness. I don't plan to edit the articles because my current project is getting the articles for the constellations up to scratch. Good luck with your efforts to make the articles complete and accurate. -- B.d.mills (T, C) 05:44, 22 Jun 2005 (UTC)
Double electron capture is so rare most sources don't even say that it exists. My chart of the nuclides doesn't list it as a decay method, neither does environmentalchemistry.com. I have not heard of it other than here and will ask my professors when I get back to university. However, your quoted source does but it also lists the following types of emissions too:
- Emission of an oxygen-20 nucleus (Th-228)
- Emission of a neon nucleus (U-232)
- Emission of a carbon-14 nucleus (Ac-225)
- Emission of a magnesium-30 nucleus (U-236)
- Emission of a silicon-34 nucleus (Cm-242)
--metta, The Sunborn 15:05, 22 Jun 2005 (UTC)
- Also, as per your request I started an article on all these particle decay emissions under the name, Cluster decay. I figure this is one of the terms used for this class decays. --metta, The Sunborn 19:20, 22 Jun 2005 (UTC)
- I made a minor error, I listed Calcium-48 as an isotope that decays by double elctron capture. It actually decays by double beta emission. For an isotope that decays by double electron capture, see Cadmium-106. Isotopes of Cadmium I get the impression that research into the decay of long-lived isotopes is relatively recent, because older texts still list these isotopes as stable. One test of these references is to inspect their entry for Bismuth. If they state that Bismuth-209 is stable then they may be unreliable sources for decay modes of long-lived isotopes. -- B.d.mills (T, C) 02:15, 23 Jun 2005 (UTC)
- The linked-to article above gives a decay for cadmium 116, while the Wikipedia article lists cadmium 116 as stable. I assume the link is right and Wikipedia is obsolete, is that right? Ken Arromdee 02:04, 18 September 2005 (UTC)
No, it doesn't. --Smack (talk) 04:44, 18 September 2005 (UTC)
- I am afraid environmental chemistry is out of date. The BNL, or the source that we use as the most comprehensive and up-to-date has Cd-116 as a double beta decay with a half-life of 3.1E19 years. [1] --metta, The Sunborn 13:44, 18 September 2005 (UTC)
So does anyone want to fix the cadmium article? Ken Arromdee 21:20, 23 September 2005 (UTC)
I tried fixing it myself. However, the two sources above contradict. One says >1.2E21 years and the other says 3.1E19 years. Which is correct? Ken Arromdee 18:20, 19 October 2005 (UTC)
- The source saying about >1.2E21 years means the neutrinoless double beta decay of Cd-116 whereas 3.1E19 years is the half-life for two-neutrino mode of double beta decay of Cd-116. The first one was never observed (only lower limits on T1/2 are known); the second one is observed by three groups of authors (an the measured values are in agreement). So, the article has to refer to the second value. V1adis1av 18:49, 28 November 2005 (UTC)
what type of radioactivity produces only energy waves
Measurement of radioactive decay
Is this the right page for clarifying the bewildering variety of measurements associated with radioactivity, or has this been done elsewhere? Joffan 01:13, 31 October 2005 (UTC)
- This is probably the right place; if this discussion has been done elsewhere, it probably belongs here. If you want to go looking, start with the disambig on Radiation. --Smack (talk) 22:47, 11 November 2005 (UTC)
Unanswered questions
How common is radioactive decay? Which materials exhibit radioactive decay and why? Where am I likely to encounter them? Rtdrury 05:12, 2 January 2006 (UTC)
- In order: 1) Depends what you mean by "how common" 2)Any materials containing radioactive isotopes as listed on Isotope table (divided) article 3)and depends on your indivudual circumstances. Tompw 12:55, 2 January 2006 (UTC)
- There should be an obvious link to the Isotope table (divided) on this page, something along the lines of 'For a list of elements that Radioactively Decay, click here' or something. Its what I wanted to know when I first came to this page.
BlueEVIL42066.20.103.36 16:36, 24 February 2007 (UTC)
Request for Mediation
(template removed - request rejected. Vsmith 04:40, 24 March 2006 (UTC)
I don't agree with the "Random" statements in the article
I am sure all of do not agree on the existence of true randomness.....I for one, do not belive it exists.....evrything is caused by something and also reacts in some way. An atom will not start behaving in a manner that is not triggered by something else. In case of radioactive decay, I am sure you all agree that we do not yet completley understand all the sub-atomic forces working, and their consequences.....so just because we do not know why, it is wrong to assume that something is truly random. In this article, there are several uses of the word random used in a sense implying that true randomness does exist. 'On the premise that radioactive decay is truly random...' etc... I simply wish to change the languge used throughout the article to eliminate this sense, but thought I should bring it out on the discussion board first. Again, my intention is only to slightly modify the language in those parts on the article, not to change or challenege any other factual information provided.
According to the article on randomness on wikipedia.... "The word random is used to express APPARENT lack of purpose, cause, or order." Hence true randomness seems to me as an oxy-moron.
Abhishekbh 04:23, 24 March 2006 (UTC)
- Refusal to believe in randomness, i.e., the believe that everything happens due to a cause or reason, even if we haven't found it, is a philosophical position. It is akin to belief in God, and in fact one favorite argument for God is the percieved need for an uncaused prime cause, or prime mover-- but I fail to see why this solves any problems, and for me, it only creates new ones. In this case, the idea of causality for everything insists that even in cases where things seem to happen for no reason or cause that has been identified, that the unseen cause nevertheless must be presumed to exist anyhow. WHY?
I happen to think this simply introduces an unnecessary assumption, without reason, and should be rejected following Occum's razor. But in any case, I see no reason a priori to reject the very idea of causeless events. If you can't see the cause, you have no good reason to demand that something you can't find or point to, still exists.
And besides, if you reject causeless events, you are left with strict determinism, by excluded middle argument. Are you happy with determinism? I guess you must be <grin>. You have to be <grin>. SBHarris 23:02, 24 February 2007 (UTC)
trefoil
I keep seeing the trefoil upside down in certain publications... is that wrong, or are both directions acceptable?
Measurement of Activity
The measurements of activity I've seen are Bq/gram. I don't know how simple Bq would mean anything significant. In any case, I think its an important relation to have: the relation between half-life and decays/gram/second. Fresheneesz 18:58, 31 May 2006 (UTC)
- IMHO it was the wrong source or you dropped part of the definition. Notice that the dimensional equation corresponding to the definition of A does NOT contain units of MASS. Also, IMHO, when you are close to a radioactive source what fries you is the activity, not the activity (bluntly, the NUMBER OF COUNTS) divided by the mass of the source.
- The activity DIVIDED BY THE MASS is the SPECIFIC ACTIVITY. Please, review your sources before affirming something and always give a reference. I might have been wrong, but I cited the NIST for you and everone else to verify.
- Please clarify the meaning of:
- "I think its an important relation to have: the relation between half-life and decays/gram/second."
- Jclerman 19:21, 31 May 2006 (UTC)
- this source implies that the "radioactivity" is measured in counts per minute per gram. this source indicates 3 different types of "Activity" ratings with units of decays per time per mass. It also says that the total decays per second is called Total decays. this source says that the the "Activity used in calculation" is measured in units of Bq per area OR Bq per mass (other activity ratings are Bq "in ocean" (a volume), Bq "found in the volume of soil"). It also has the title "Total Activity" for flat Bq ratings other than for the ocean and "volume of soil".
- This source, this one, and this one agree with you about specific activity.
- So I think these discrepencies should be noted, I think I'll change the section a bit to reflect that. Fresheneesz 22:01, 31 May 2006 (UTC)
- I thought the meaning of the quote you want me to clarify was pretty clear. Wikipedia didn't have anything about the specific activity, and I think its an important thing to note here. Also, what you do mean by "don't innovate out of context" ? How does one innovate out of context?
- I have given many sources on the ambiguous use of "activity", yet you remove my note about it. Also your change of bolding is misplaced, bolding shouldn't appear in some side note. I'll wait for your comments "tomorrow", but I'm still going to fix the bolding and the defintions that I've given sources for. Fresheneesz 23:50, 31 May 2006 (UTC)
- Also, please if you're going to revert my work, discuss your reversions here before or immediately after. Fresheneesz 00:01, 1 June 2006 (UTC)
- One area in which Bq (or Ci) are used directly without a mass associated is in the disclosure of the amount of radioactivity released by a nuclear weapon test; it is common to say something to the effect that "2.1 kilocuries was released". In this case the additional load of radioactivity on the Earth is the object, not any specific mass of energetic atoms. SkoreKeep (talk) 14:29, 24 March 2011 (UTC)
stimulus info
Stimulus not a term usually found in context of radioactivity descriptions and is not found in the reference given. What are its meaning and its relevance for the article?
...predictions using these constants may be less accurate if the substances are in situtations that provide extra stimulus. [2]
Jclerman 13:06, 20 June 2006 (UTC)
- Note that the stimulus reference concludes, in its last statement:
The radiometric decay rates used in dating are totally reliable. They are one of the safest bets in all of science.
- The article has been edited to reflect the correct statement. Jclerman 00:37, 24 June 2006 (UTC)
nucleus et al
Nuclides are not nuclei. They have been edited. Jclerman 02:08, 24 June 2006 (UTC)
Merger
Modes of Decay has been merged into this page SuperTycoon 16:42, 15 July 2006 (UTC)
Multiple decay chains
A recent edit
The summary for this edit to the "Decay chains and multiple modes" section asks "Is this really true? A given uranium isotope always ends in the same lead isotope, no?". I can't find a reference for uranium having multiple decay modes, but some isotopes of some elements do have multiple decay modes. The section also mentions 212Bi, which is a valid example according to the Decay chain article, so I edited the text to remove mention of uranium. The second part of the summary contains the hidden assumtion that a different decay mode early in a decay chain (or a "branch" to continue the chain analogy) will result in a different stable endpoint. This is not true; according to the Decay chain article, the two decay chains for 212Bi reconverge at 207Pb. DMacks 20:26, 31 July 2006 (UTC)
- Not all the same nuclide will end in the same point. There are branches. At every decays (or at some or most of them) there are a probability that the chain goes in one branch or in an another one). Note that we can not know the path followed by each starting nuclide. We start (as an example) with 1e6 nuclides of U-235 and after some time we look at what we have. We not know which original nuclide became the final one, but we statistically know that a certain fraction would be that nuclide, a certain other fraction that nuclide, etc. This sort of thing is used in analysis in the opposite direction. You can look at what you have now and ask what was before. Only if the proportion among the various nuclide you now have are what are expected by the chains of a particular nuclide you can conclude that in origin there was just that nuclide. On the other hand if the proportions are not the expected one, there have been some contamination or in the original sample there was not just one sort of nuclide. (for such method to be applied you should be sure the sample is completely sealed). -- AnyFile 19:29, 4 October 2007 (UTC)
Decay process- Why?
Is there a theory about what causes the randomness of decay? I mean why should some atoms go off immediately, and others wait, perhaps, millions of years?--Light current 22:43, 25 September 2006 (UTC)
- Well, think about it. Randomness is just a way of saying the cards or dice behave as if they have no memory. It would be even screwier if there WERE some non-randomness. Think about a really simple random system-- a ball bouncing around inside a large hollow sphere (with some roughness on the inside to change the bounce direction a bit each time) which has a hole in it, just large enough to let the ball out if it hits EXACTLY on target. Over time, such a system will behave as if it has no memory. The ball might take 5 bounces to get out, or a million. But consider a system that DOES seem to have a memory for decay-- like a watch or a car or a human body. Such systems must contain internal parts which store records of the passage of time (like wear-and-tear) as a sort of memory. Which means they need to be MUCH more complicated. So if you see anything that decays by anything other than the standard random type, where no "memory" is involved, THEN is the time to get interested, because those cards DO have a memory. So the system is complicated, shows wear and tear, has internal gizmos that keep track of time and previous events, and so on. I can't even imagine how to do that on an atomic scale, so I'm glad no particle decays behave in any other way than the way they do. SBHarris 23:57, 25 September 2006 (UTC)
- I can see the ball in sphere explanation. That is a mechanism:(but pseudo random because the inside of the sphere cannot have infinitely fine detail). What is the mechanism of radio active decay?--Light current 03:37, 26 September 2006 (UTC)
- Ultimately, at some point, explanations must stop. I don't think the mechanism you seek is known, because it is closely bound up with the mechanism for why things do (or don't) happen in quantum mechanics. One explanation is that actually things really don't happen, yea or nay. Rather, everything happens. For every universe where an atom decays, there is another where it doesn't. That stops the issue of THIS and not THAT. Because then you get BOTH THIS and THAT. But you also get a lot of universes. Not my problem. SBHarris 02:47, 17 February 2007 (UTC)
- Naming a particular quantum mechanical mechanism can still help remove some of this unnecessary "mystery" about the mechanism. For radioactive decay, this explanation is called quantum tunneling. That article offers a brief explanation of what you might be looking for, but it would be a good idea to incorporate some reference to it in the decay article. --68.3.20.93 (talk) 20:45, 7 January 2011 (UTC)
- Ultimately, at some point, explanations must stop. I don't think the mechanism you seek is known, because it is closely bound up with the mechanism for why things do (or don't) happen in quantum mechanics. One explanation is that actually things really don't happen, yea or nay. Rather, everything happens. For every universe where an atom decays, there is another where it doesn't. That stops the issue of THIS and not THAT. Because then you get BOTH THIS and THAT. But you also get a lot of universes. Not my problem. SBHarris 02:47, 17 February 2007 (UTC)
- I can see the ball in sphere explanation. That is a mechanism:(but pseudo random because the inside of the sphere cannot have infinitely fine detail). What is the mechanism of radio active decay?--Light current 03:37, 26 September 2006 (UTC)
Dangers with radioactivity
I think this article should have its own section describing the dangers of radioactivity. Now, it's included in the end of the "Discovery"-section as a historical note only. Kricke 01:27, 17 February 2007 (UTC)
- Agreed, needs an appropriate link to Ionizing radiation where it is discussed at length. 64.148.241.133 (talk) 06:32, 4 February 2008 (UTC)
- No, Dangers of Radioactivity is a different topic from Dangers of Ionizing Radiation. The word "radioactivity" has more than one meaning. In one common usage, it is synonymous with radioactive contamination. Radioactive contamination is dangerous because tiny amounts of it can be deadly (see Alexander Litvinenko poisoning), and also because even if you manage to contain the contaminant, you can never destroy it. 151.201.219.161 (talk) 03:02, 2 December 2008 (UTC)
anons comments inserted in the article
.(Note from curious passerbyer, when it says "all but vanished," doesn't that mean it didnt vanish from the market? I mean it translates to me as anything happened but vanishing from the market. I might be wrong but didn't radioactive treatments vanish from the market? (Touche, my friend. I find your logic rather sensible.))
- Perhaps this definition helps? Regardless, I fixed to use a more straightforward wording. DMacks 17:34, 21 May 2007 (UTC)
Question
Are all matter radioactive? They say that only unstable atoms are radioactive, and that through radioactivity it eventually becomes stable again. So is it then no longer radioactive? Will the world one day be completely stable when everything has become stable? Or...? ► Adriaan90 ( Talk ♥ Contribs ) ♪♫ 18:35, 29 June 2007 (UTC)
- Uhmm .. I actually can not understand your question properly. Anyway I will try to answer. First of all, not all matter is radioactive. For instance 4He and 197Au are stable. About the other point of your question like in thermodynamics, the whole system is loosing energy. Note however that are in nature event that create height weight nuclides that are radiactive. In stars such nuclides are product and during supernova explosions very weight nuclides are produced and expelled. -- AnyFile 19:41, 4 October 2007 (UTC)
- It depends on the timescale. If you are thinking of the normal "chemical" timescale of up to a few billion years, there are many stable isotopes. But if you are thinking about really long timescales, such as 10100 years, all ordinary matter can decay, according to our article on the heat death of the universe. :) --Itub (talk) 14:20, 25 February 2008 (UTC)
NOTE
I think a graph showing an exponential decline curve would be useful in this article. —Preceding unsigned comment added by 212.219.123.32 (talk) 12:03, 4 October 2007 (UTC)
Negatron Emission
I know of the existence of a type of decay called negatron emission, however I do not see it listed on this page. Perhaps someone should add it. Nschoem 01:03, 21 February 2008 (UTC)
- It seems that negatron is just an electron, so it sounds like the Beta-Negative decay entry. DMacks (talk) 01:10, 21 February 2008 (UTC)
- The beta-negative link just leads to the Beta decay page. And I am pretty certain it is a different type of decay. 68.142.137.202 (talk) 02:37, 22 February 2008 (UTC)
Time variant decay
It has been discovered that nuclear deacy vares over time, or more accurately: with the Earth's distance from the sun. Can someone make a section of this please?
http://science.slashdot.org/science/08/08/29/1227239.shtml --J-Star (talk) 23:31, 29 August 2008 (UTC)
- It's a very interesting paper that's gotten a lot of attention, but it is still only a single speculative, non-peer-reviewed pre-print. In other words it's one idea among many, without confirmation or general acceptance (so far), and we shouldn't give it undue weight. --Amble (talk) 18:43, 21 November 2008 (UTC)
Q.: Shouldn't relativity be applied to the integral transformation points of a decay series for a better description of the passage of time? ie thinking about the clocks experiment and our static location in spacetime when an observation is made; the assumption of linear temporal decay from thenthere to herenow must be innacurate? (not only given a differential for time passed but also vector location changes as the earth spins etc). DGreenhill 151209 —Preceding unsigned comment added by 82.111.134.82 (talk) 12:03, 15 December 2009 (UTC)
Question on field of study
Is Radioactivity a physics or a chemistry related subject? —Preceding unsigned comment added by NoPity2 (talk • contribs) 16:52, 14 October 2008 (UTC)
- Both. Chemistry is a branch of physics, after all. Even chemists admit that. It's only when you call chemistry JUST a branch of physics, that their hackles rise. SBHarris 17:42, 14 October 2008 (UTC)
- Radioactivity is physics. I never heard of anyone claiming that radioactivity was in the branches of chemistry, other than the Nobel committee who gave Rutherford his prized (and he was sorta pissed about it being a chemistry prize). Well, and Sbharris now :P.Headbomb {ταλκκοντριβς – WP Physics} 08:56, 17 November 2008 (UTC)
- It's a bit arbitrary, of course, but the existence of fields of study such as radiochemistry, nuclear chemistry, and radiation chemistry suggests that some aspects of radioactivity could be considered "branches of chemistry". --Itub (talk) 12:07, 18 November 2008 (UTC)
- Radioactivity is physics. I never heard of anyone claiming that radioactivity was in the branches of chemistry, other than the Nobel committee who gave Rutherford his prized (and he was sorta pissed about it being a chemistry prize). Well, and Sbharris now :P.Headbomb {ταλκκοντριβς – WP Physics} 08:56, 17 November 2008 (UTC)
- Well I for one don't consider radiochemistry/nuclear chemistry/etc. to be chemistry (study of chemical reactions). When anyone speaks of chemistry, they speak of things like 2H + 1O → H20. What happened is when nuclear reactions became of interest, they've copied the name because they, like in chemistry, were studying reactions, albeit this time nuclear ones. Chemistry is a branch of physics, but while nuclear chemistry is also a branch of physics, its not a branch of chemistry. And while part of radiochemical phenomena indeed are under the wing of chemistry, we should also remember that a part of electrical phenomena are also under the wing of chemistry. But no one would call electricity to be a branch of chemistry. Anyway, this is not very important, and in the end somewhat dependant on how you organize things. Headbomb {ταλκκοντριβς – WP Physics} 22:47, 21 November 2008 (UTC)
Originally, research on radioactivity was a branch of chemistry, because the first problem was to chemically isolate and identify the substance emitting the radiation. Nowadays I think things like "radio-chemistry" denotes the chemistry of compounds of radioactive elements, much as "organic chemistry" denotes the chemistry of compounds of carbon. For example, in medicine, you want to use compounds that can efficiently deliver the radioactive elements to the tissue to be radiated. Finding, making, and using such compounds is chemistry, not physics. WmMBoyce (talk) 00:07, 29 September 2009 (UTC)
Contradiction between Radioactivity and Marie Curie articles
From Radioactivity:
- Curie later died from aplastic anemia assumed due to her work with radium, but later examination of her bones showed that she had been a careful laboratory worker and had a low burden of radium.
From Marie Curie:
- Her death ..., was from aplastic anemia, almost certainly contracted from exposure to radiation.
I feel that the introduction should acknowledge the monograph by Rutherford, Chadwick and Ellis first published 1930, latest edition 'Radiation from Radioactive Substances' Cambridge Univ.Press 1951 —Preceding unsigned comment added by Michael Nettleton (talk • contribs) 15:19, 8 January 2010 (UTC)
Symbols
PLEASE FIX THE SYMBOLS ---- They are showing as red error messages (could not parse.... etc) - scroll to bottom half of page. The section after the decay chains is riddled with them! 122.49.138.138, 13:27, 21 February 2009
"Spontaneously" loses energy and "currently" impossible
I question the changes made to the intro paragraph on 20 May 2010 by Androstachys. First the word "spontaneously" was removed as "misleading" in the phrase "... an unstable atomic nucleus *spontaneously* loses energy by emitting ...". Why is this word misleading? Perhaps its meaning could be made clearer. I propose inserting the sentence "The emission is spontaneous in that the nucleus decays without collision with another particle."
In the same edit the word "currently" was inserted in the phrase "... it is currently impossible to predict when a given atom will decay ...", with a reference that does not justify the word "currently". I think a more accurate formulation would be "According to quantum mechanics, it is impossible to predict when a given atom will decay". This would clarify that not only do we currently have no theory to predict the decay of a given atom, but we also have a very general theory (accepted by the majority of physicists) which says that we can never predict the decay of a given atom.
I will wait a few days for comments before making these changes. Dirac66 (talk) 16:27, 26 May 2010 (UTC)
- Please, go right ahead and make the changes. It is an improvement. Dauto (talk) 05:18, 29 May 2010 (UTC)
The reference given for "according to quantum mechanics", however, does not mention quantum mechanics or how QM explains or predicts (no pun intended) that such a prediction is impossible. —Preceding unsigned comment added by 173.11.1.218 (talk) 23:16, 24 November 2010 (UTC)
- True. This reference was actually in the article prior to the edit of 29 May 2010 mentioned above, as support for the unpredictability of decay. We should have another reference which explicitly refers to the probabilistic nature of a radioactive nucleus as a quantum system. It could be inserted either in the intro or in the Explanation section. Dirac66 (talk) 01:56, 25 November 2010 (UTC)
The history of Radioactivity
I'm not sure that the history section is correct. Pages like [3] [4] [5] [6] [7] [8] does point to that Abel Niepce de Saint-Victor might have discovered radioactivity before Becquerel. Reko (talk) 18:15, 22 June 2010 (UTC)
Should this article contain a summary of the nuclides that undergo radioactive decay?
- Moved from User talk:Headbomb
Which types of atoms actually decay? There isn't even a summary in this Wiki article. I put a pretty good one in, and you removed it, because it's duplicated somewhere else. Okay, quick, people: without looking at Headbomb's edit summary, WHERE would you find such a thing? SBHarris 18:56, 13 September 2010 (UTC)
- Hey, Headbomb, the section on exponential decay is completely duplicated in that article. Suggest you remove it, as well. Wikipedia, after all, should be made as short as possible. Do you not agree? SBHarris 18:59, 13 September 2010 (UTC)
- Please knock off the sarcasm. The place for such a table is in nuclide not on radioactive decay, where it's tangential at best. I'll also point out that that table will need referencing, because right now it reeks of WP:SYNTH. Headbomb {talk / contribs / physics / books} 19:06, 13 September 2010 (UTC)
- It "reeks" of nothing, O disliker of sarcasm, as this is a derogatory term for odors, not a proper wikipedian description for characteristics of somebody else's writing. It is in fact a very simple numerical count per WP:CALC, because I made it by counting the types of nuclides in list of nuclides, which in turn has an external reference. This is the way most new tables in Wikipedia are constructed, in fact (If they were copied from somewhere else, they would constitute plagarism; doing your own table by means other than plagarism always requires some measure of synthesis).
On the larger issue, whether a summary of the various types of atoms that are radioactive actually belongs in, or has "a place" in, the article on radioactive decay, is not for you to say-- it's a matter for consensus decision. Which I've now asked for, on the TALK page. As also, is the question of whether the mathematics of the solution of the first-order exponential differential equation has a place in this article, rather than the article that actually deals fully with the mathematics of this, which is exponential decay. Don't forget that much "sarcasm" is merely a serious logical suggestion that somebody doesn't like, because it exposes their biases. SBHarris 19:26, 13 September 2010 (UTC)
- It "reeks" of nothing, O disliker of sarcasm, as this is a derogatory term for odors, not a proper wikipedian description for characteristics of somebody else's writing. It is in fact a very simple numerical count per WP:CALC, because I made it by counting the types of nuclides in list of nuclides, which in turn has an external reference. This is the way most new tables in Wikipedia are constructed, in fact (If they were copied from somewhere else, they would constitute plagarism; doing your own table by means other than plagarism always requires some measure of synthesis).
- Please knock off the sarcasm. The place for such a table is in nuclide not on radioactive decay, where it's tangential at best. I'll also point out that that table will need referencing, because right now it reeks of WP:SYNTH. Headbomb {talk / contribs / physics / books} 19:06, 13 September 2010 (UTC)
This discussion is becoming heated. May I suggest as a possible compromise that the article include a brief statement, that the summary of how many nuclides decay by each mode can be found in the article on Nuclide. This would make the information more accessible to interested readers of this article, while keeping the article from becoming unnecessarily long. Perhaps in the See also section, but with an explanatory comment describing the table, or else just prior to the See also section. Dirac66 (talk) 00:59, 14 September 2010 (UTC)
- Fine. SOFIXIT. Personally I think that any editor who deletes another's contribution attempting to do a job, in a case where something is clearly needed, and nothing is presently in place, has the obligation to write something else as an alternative, to do the job. In this case, that would be Headbomb. IOW, Headbomb, if you don't like what I've got, let's see what YOU'VE got. You write WP content and I'll criticize-- how's that? SBHarris 02:09, 14 September 2010 (UTC)
Can someone include the actual dangers of different types of radiation
It would be very nice for this article to indicate in some way, not only the different types of radiation, but also which types of radiation dangerous and what is considered a dangerous dosage level.
My understanding was that basically it's only Gamma radiation that can be extremely dangerous, but I'm not an expert.
Zuchinni one (talk) 21:31, 17 October 2010 (UTC)
- Most of that information is in the article on ionizing radiation. See also the "see also" articles in that article, at the end. In response to your request, I've included the mentioned article as the "main" article in the "hazards" section here in radioactive decay. Thanks for pointing out that it's not immediately obvious where to go if you want to follow this more deeply. This article is more about the process than the products of decay, and there is more info on the hazards in the articles for the products. For example, on each type of radiation listed in the article on radiation. SBHarris 22:08, 17 October 2010 (UTC)
Curie
The "Danger of radioactive substances" section claims Marie Curie had a low exposure to radium and had been careful with it; but our Marie Curie article mentions that her papers and even cookbook are radioactive and dangerous to this day. Which is it? Comet Tuttle (talk) 05:12, 3 November 2010 (UTC)
History of discovering the danger
Could a knowledgeable editor add some content on the history of how the dangers of ionizing radiation were discovered? Comet Tuttle (talk) 05:14, 3 November 2010 (UTC)
Beta precedes gamma - ambiguous wording.
Re the following sentence added today by SBHarris: "The relationship of types of decays also began to be examined: for example it was found that beta decay almost invariably preceded gamma decay, and not the reverse."
Does this mean: 1) If a given nucleus emits a beta and a gamma, then the beta is emitted first? Or 2) If a given nucleus emits a gamma, then a beta is emitted first? Or 3) If a given nucleus emits a beta, then a gamma is emitted subsequently? Please clarify which is meant. Dirac66 (talk) 21:35, 8 November 2010 (UTC)
- It means all those things, so long as we're not talking 100%. There are ways to create excited nuclei which gamma decay other than beta decay, but (at least on Earth) beta decay is by far the most common mechanism that produces produce gamma-decaying isotopes. I'll see what I can do. SBHarris 22:39, 8 November 2010 (UTC)
- OK, thanks. The article is clearer now. I wasn't sure if it was 1) or 2), but I see that it is 1) and 2) (usually). However "all those things" would include 3) which I added for logical completeness, and which surprises me more as I thought that many beta decays are to the nuclide ground state. So I'm just checking that the omission of 3) in the article edit was deliberate - if so I agree and we can leave it there. Dirac66 (talk) 23:07, 8 November 2010 (UTC)
- You bring up an issue that makes me realize it has to be rewriten again. Though most beta decays aren't to the ground state, the gamma emission that happens when they do go to ground after that, is so fast (10-12 sec) that it might as well be simultaneous. The metastable states that are slow enough to measure a half-life for the pure gamma decay, are indeed a rarity, simply because metastable states are relatively rare. SBHarris 04:06, 9 November 2010 (UTC)
- OK, thanks. The article is clearer now. I wasn't sure if it was 1) or 2), but I see that it is 1) and 2) (usually). However "all those things" would include 3) which I added for logical completeness, and which surprises me more as I thought that many beta decays are to the nuclide ground state. So I'm just checking that the omission of 3) in the article edit was deliberate - if so I agree and we can leave it there. Dirac66 (talk) 23:07, 8 November 2010 (UTC)
Links to bound state beta decay
We now have 3 red links for "bound state beta decay". Before anyone adds a new article on this subject, I would like to suggest that we instead add a new section to the existing article on beta decay. We can then point the links from this article to that section. Dirac66 (talk) 00:56, 15 November 2010 (UTC)
- Looks like you did that, and created the section there in the beta decay wiki. Good solution. You know, this article is more complete than your usual encyclopedia article, by far. It's really come a long way. SBHarris 04:43, 25 November 2010 (UTC)
Universal decay law?
There is no obvious mention that for the decay process A → B the equation
is the universal decay law for a nuclide A, nor is there the more generalized A → B → C
with solution
where specifically NA0 = initial number of nuclides of type A. All symbols with usual meanings. I will slightly tweak context for what it is, and try to add referances while at it.
Maschen (talk) 22:07, 27 October 2011 (UTC)
- You might also link the two-step equations to the similar derivation at Rate equation#Consecutive reactions. That article is concerned with chemical reactions, but for first-order chemical reactions the math is the same as for nuclear reactions. Dirac66 (talk) 01:29, 28 October 2011 (UTC)
Finished with my reformulation. You have a point Dirac66, - i'll add a link of analogy.
Furthermore I have a niggling feeling that people will complain about the overlap between parts of the exponential decay article and this one. For those that will: I don't care about arguments. For this article, the equations are explained in detail and in specific context of radioactivity, in that article there is not much explaination for the chain decay law as a differential equation itself - the whole point of what I just did is to outline the maths behind the law of radioactive decay...
Maschen (talk) 18:00, 28 October 2011 (UTC)
uses
how about a section on uses of radioactivity — Preceding unsigned comment added by 109.148.122.30 (talk) 20:29, 17 December 2011 (UTC)
Speed limit of the universe
Section Explanation, second paragraph, last sentence: "The limits of these timescales are set by the sensitivity of instrumentation only, and there are no known natural limits to how brief or long a decay half life for radioactive decay of a radionuclide may be."
Actually the universe does have a speed limit of c, so that nuclear fragments cannot separate by a nuclear distance of 10-15m in a time shorter than 10-15m / c = 3 x 10-24 s. This is why there are several radionuclides with half-lives between 10-24 and 10-20 s, but none shorter. Except that the article now includes a value of 3 x 10-27 s for He-2, implying that the two protons separate 1000 times faster than the speed of light. This value is unsourced and I do not believe it, so I have marked it as Citation needed. See also my further comments at Talk:Isotopes of helium. Dirac66 (talk) 14:58, 19 December 2011 (UTC)
- I have now checked the NUBASE evaluation of nuclear and decay properties. He-2 is not listed. The shortest-lived isotope which is listed is H-7 with a half-life of 23 ys = 2.3 x 10-23 s. The longest-lived is Te-128 as in this article now, but the half-life is a little shorter: 2.2 Yy = 2.2 x 1024 year = 6.9 x 1031 s instead of 2.4 x 1032 s. Dirac66 (talk) 22:00, 24 December 2011 (UTC)
- Also the Nudat 2.6 interactive data base says that He-2 is unknown. For H-7 it gives 2.9 x 10-23 s and for Te-128 it gives 2.41 x 1024 year = 7.6 x 1031 s. I don't know which of the two databases for the nuclides is more accurate, but the agreement is pretty good. I haven't verified that Nudat has no nuclide beyond these two extremes - Nubase is in list format so much better adapted for that job than Nudat which is interactive. Dirac66 (talk) 22:31, 24 December 2011 (UTC)
| This page is an archive of past discussions. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
Introduction has grown too long
The introductory paragraph is entirely too detailed and much of the material therein needs to be moved to later paragraphs and trimmed out of the intro. — Preceding unsigned comment added by Zedshort (talk • contribs) 20:38, 1 April 2012 (UTC)
- Says you, after adding positron capture back into the lede, when it is a hypothetic process never yet seen, and only possible in complex-nuclei antimatter (maybe Captain Kirk and Spock see it in their engines). That's pretty hypocritical. There are NOT "five" processes of radioactive decay, but as many or more as appear in the table, and these don't count hypothetical ones. Just because you count five in a set of college class notes (your reference) [9] doesn't mean five is the number. Moreover, Internal conversion is a far more common process in nature than spontaneous fission, so if you're going to mention these in the lede in order of importance, try following your own rules and suggestions. SBHarris 20:48, 1 April 2012 (UTC)
- My attempts are only constructive and not to insult. The illustration in the body of the text suggests and most any reference would mention five methods. While positron capture has not yet been witnessed experimentally that does not make it of no interest. As far as anti-matter reactions go, I am sure you know there is positron emission from nuclei. Which process is most common is interesting and I suppose the order in which they are listed might reflect that. Once again the lede is entirely too long. Please try to stay on subject and to not try to smear me by using pejorative references to Star Trek it sounds adolescent. No one of us owns this article.
- Please sign your edits. Use four tildes like this: ~~~~. You've been here since October and it's high time you learned the basics of editing.
- I'm sure your edits were good faith and meant as constructive. However, they were not, as you are adding false information and promoting things of little interest (positron capature) to the lede.
- This lede is indeed too long, but the way to cut a lede is to avoid mentioning trivia. A process that has never been detected (positron capture) is trivia. You can mention it farther down in the article.
- Positron capture is not positron emission. The latter happens commonly, and might be mentioned in the lede as a type of beta decay (technically it is beta-plus decay).
- There are no illustrations "in the body of the text" that show five types of radioactive decay and it would not matter even if there were. There are not 5 types and anybody illustating five types would just show themselves as an ignorant illustator. No, "most any reference" at the college or grad or research level would mention far more than 5 types types of decay, including internal conversion and emission of protons and neutrons.
- References to Star Trek are to point out that science fiction processes like positron capture should be discussed as hypotheticals (since SF does come true, as in the moon landing). We have seen antimatter, but never the complex higher atomic number type we would need to see, in order to see positron capture. One would need at least anti-beryllium-7 (complete with at least one positron in an atomic orbital) to see it. However, such hypotheticals are not lede material, in a lede that is already too long.
- Of course nobody WP:OWNs this article, and I welcome comments from people who actually know enough about radioactive decay to know that there are not "five types" of it, as you keep insisting. That makes me think you haven't even read the article you're editing. Could you please do that? SBHarris 23:37, 1 April 2012 (UTC)
>Actually, I have read the entire article and like most articles on wikipedia it is cluttered, poorly phrased and needs reorganizing, hence my attempts. I am sorry if I gave offense by adding the hypothetical process of positron capture but I find the potential of that process interesting and like to include it just for the sake of completeness. If there are more than five processes of radioactive decay I think they should all be added. Perhaps a table of those dozen or more methods would be the best format with all the products included. But from most of the sources I have read typically only three or five are listed. I wonder, have we had a dispute somewhere in the past as you seem to have taken offense at my being here and making these changes? I think I remember you adding to the article on the Energy-Catalyzer. Sincerely — Preceding unsigned comment added by Zedshort (talk • contribs) 00:40, 2 April 2012 (UTC)
- You say you've read the article? So how is it that you somehow missed seeing the very table that you ask for? I guess you didn't read it very carefully, eh? And you're still not signing your messages; I suppose you prefer not to learn? And no, I've never edited Energy Catalyzer. SBHarris 00:52, 2 April 2012 (UTC)
The general solution to the recursive problem are given by Bateman's equations
Hi,
the general solution to the recursive problem by Bateman's equations does not seem to be correct. The correct equation is shown at http://www.physicsforums.com/showthread.php?t=452474
may be someone can change this, with best wishes Wolfgang — Preceding unsigned comment added by 212.95.7.181 (talk) 12:58, 12 April 2012 (UTC)
- I see that Bateman's equations for the general case have now been placed in this article. Should we also note that Bateman's equation for the two-decay case is in the article on Transient equilibrium? I am not certain whether it is the same Bateman's equation. Dirac66 (talk) 16:28, 12 April 2012 (UTC)
Hello all. The formula for Bateman's equations given in the physics forum link above and now on the wikipedia page does not agree with that given in Kenneth S Krane's Introductory Nuclear Physics (Unit II Nuclear Decay and Radioactivity, Chapter 6 Radioactive Decay, Page 173 in my copy). Krane doesn't have the decay constant after the sum term in the Nd formula. If someone could confirm and change I'd be much obliged. [This comment by 86.177.136.35, 24 June 2012]
- Strange. If we take the equation now in the article and omit the λi after the sum as you suggest, then the units come out wrong. There will be a λD with units of reciprocal time in the denominator which does not cancel, so ND will have units of time. I don't have Krane's book, but it would seem that either he has erred or you have read him wrong. Perhaps someone can check Ref.8, the article by Cetnar. Dirac66 (talk) 23:04, 24 June 2012 (UTC)
- Ah, that may be because of a disagreement between this article and Krane for the Ci equation. In this article there is only one product symbol. Krane has separate product symbols for the numerator and denominator and only the denominator is neglected for i=j. I think this would lead to dimensions T^D on the numerator and T^D-1 on the denominator? Edit: A possible test presents itself: the Bateman equation for the case where D=2 is widely quoted. Derive the Bateman equation for D=2 using the method given here and the method in Krane and compare against another reference source? [This comment also by 86.177.136.35, 24 June 2012]
- Aha. The extra factor in Krane's numerator is the λi which the formula now in the article (which cites Cetnar's paper) places before the one product symbol. So the two formulas are equivalent, and the only question is which is the clearer presentation for this article. I vote for the one now in the article since it shows the λi explicitly. (And to sign your posts, click on the four ~ below, 4 lines under the Save page button on the right.) Dirac66 (talk) 01:40, 25 June 2012 (UTC)
- Of course! My apologies for being so dense. Thank you for clearing that up. 86.148.86.94 (talk) 22:25, 25 June 2012 (UTC)
- Aha. The extra factor in Krane's numerator is the λi which the formula now in the article (which cites Cetnar's paper) places before the one product symbol. So the two formulas are equivalent, and the only question is which is the clearer presentation for this article. I vote for the one now in the article since it shows the λi explicitly. (And to sign your posts, click on the four ~ below, 4 lines under the Save page button on the right.) Dirac66 (talk) 01:40, 25 June 2012 (UTC)
Prediction of very long decay rates.
For example, the article on Cadmium states that: "The two natural radioactive isotopes are 113Cd (beta decay, half-life is 7.7 × 10^15 years) and 116Cd (two-neutrino double beta decay, half-life is 2.9 × 10^19 years)." How is such an extraordinarily long decay rate calculated/predicted? 124.184.13.246 (talk) 09:01, 31 August 2012 (UTC)
Try asking the reference desk. Wikipedia:Reference desk/Science --Klausok (talk) 11:20, 31 August 2012 (UTC)
- The values are calculated for exponential decay using the measured decay rate -dN/dt = λN, where λ = 1/τ = decay constant. Then λ = -(1/N)dN/dt, and the half-life t1/2 = τ ln 2 = (ln 2)/λ = (N ln 2)/(dN/dt). Dirac66 (talk) 20:08, 15 September 2012 (UTC)
Please spell check
Look for 'isomeric' instead of 'isometric' and if doing this myself is not illegal please reply telling me that i should have done then and thereKhpatil (talk) 14:54, 7 December 2012 (UTC)
- Isomeric occurs 3 times in the article, and it is the correct spelling in the context of radioactivity. An isomeric transition in nuclear physics involves the decay of a nuclear isomer with emission of a gamma-ray. Isometric on the other hand has no meaning in radioactivity or nuclear physics which I know of. Dirac66 (talk) 19:15, 7 December 2012 (UTC)
Are you CRAZY??
Quote: "Primordial nuclides found in the earth are residues from ancient supernova explosions which occurred before the formation of the solar system."
EVIDENCE for this? ANY? EVIDENCE? AT ALL? — Preceding unsigned comment added by 78.156.126.230 (talk) 15:36, 20 December 2012 (UTC)
- The Nucleosynthesis article seems reasonably cited. DMacks (talk) 16:08, 20 December 2012 (UTC)
- Wikipedia articles should not cite each other (see WP:CIRCULAR), so I will remove the cite of nucleosynthesis. Also, the statement is inaccurate. The lighter nuclides come mostly from big bang synthesis and ordinary steller synthesis. RockMagnetist (talk) 17:02, 20 December 2012 (UTC)
- Keep panties on. This is an article on radioactive decay and the statement above clearly was meant to refer to radioactive primordial nuclides, of which there are 34 (Te-130 was erroneously reported radioactive but this was retracted). The lightest of these is K-40 so (with that possible exception, and we don't know the ratio) all are comfortably supernova products not AGB or Big Bang material. That was the point of the initial statement above, which I've now qualified so there can be no misunderstanding. I can't tell if our incredulous IP objector is a red giant star fan or a creationist or what. Finally, referring to another article which is well-referenced from without is hardly a violation of WP:circular. Somebody needs to read WP:SS. The conclusion here isn't central to this article subject and we don't need the burden of refs for it. If you must have one or two, fine, but it's not controversial and is a waste of space. SBHarris 20:41, 20 December 2012 (UTC)
- The numbered editor was certainly overexcited, but s/he did point out that this article gave insufficient guidance as to where to find evidence for the statement about supernovae. In my teaching experience, one student who asks a question (even rudely) usually represents a number of others who wonder the same thing. So I thought it best to include a pointer to the more detailed discussion which DMacks had suggested above, in order to help future readers who may ask themselves the same question (hopefully more politely). I thought of a footnote first, but I accept RockMagnetist's correction to a Main article link, which is Wiki style for pointing to more detailed discussion of a (sub)section topic.
- As for the restriction to radioactive primordial nuclei, we could say instead (or also) that all primordial nuclei heavier than iron (or nickel?) are from supernova explosions. This would go slightly outside the article topic, but also give more information very concisely.
- Sorry, forgot to sign previous comment. Dirac66 (talk) 21:54, 20 December 2012 (UTC)
- Keep panties on. This is an article on radioactive decay and the statement above clearly was meant to refer to radioactive primordial nuclides, of which there are 34 (Te-130 was erroneously reported radioactive but this was retracted). The lightest of these is K-40 so (with that possible exception, and we don't know the ratio) all are comfortably supernova products not AGB or Big Bang material. That was the point of the initial statement above, which I've now qualified so there can be no misunderstanding. I can't tell if our incredulous IP objector is a red giant star fan or a creationist or what. Finally, referring to another article which is well-referenced from without is hardly a violation of WP:circular. Somebody needs to read WP:SS. The conclusion here isn't central to this article subject and we don't need the burden of refs for it. If you must have one or two, fine, but it's not controversial and is a waste of space. SBHarris 20:41, 20 December 2012 (UTC)
- Wikipedia articles should not cite each other (see WP:CIRCULAR), so I will remove the cite of nucleosynthesis. Also, the statement is inaccurate. The lighter nuclides come mostly from big bang synthesis and ordinary steller synthesis. RockMagnetist (talk) 17:02, 20 December 2012 (UTC)
- Thanks for the clarification. It never hurts to be clear. Sbharris, it's ironic you point to WP:SS because in Wikipedia:SS#References it says "Each article on Wikipedia must be able to stand alone as a self-contained unit (exceptions noted herein)." (I don't know what exceptions they are referring to.) It makes sense for each article to have its own citations because other articles can change - and actually Nucleosynthesis isn't particularly well provided with citations. I have never seen an inline citation described as a "waste of space" before. RockMagnetist (talk) 22:54, 20 December 2012 (UTC)
- Come to think of it, an external citation doesn't even take up more space than the internal citation that I removed. RockMagnetist (talk) 23:00, 20 December 2012 (UTC)
- I was thinking of internal space (what you see in edit mode) but never the less I'll admit you're right that cites are needed everyplace. Even summary sections with main articles need a cite or two. In an article like the United States (summarized by section as heavily as any you'll see) the standard seems to be about one cite per paragraph. So knock yourself out here, but it shouldn't take more than one or two on this particular point.
As to the more interesting question of where heavier-than-iron nuclides come from, it's complicated. About half of the nuclei heavier than iron are s-process nuclei that required some slow cooking somewhere (like AGB stars where free neutrons come from alpha reactions on C-13 and Ne-22) and the rest are r-process nuclides that require a truly titantic neutron flux from some fusion bomb event (like the first H-bombs making Es and Cf out of U and Pu). The sites for this are probably high mass supernovae that aren't quite large enough to make black holes, but have as many free neutrons at the end as possible. Certainly U and Th were made that way, and I'll have to go through the tables to see about the other 30-odd primordial radioactives. Some may be s-process nuclides that haven't been (or at least were not REQUIRED to have been) put through a genuine supernova or even nova, so we'd be liars if we said that all such nuclides heavier than iron had. Apparently red giants make some really heavy nuclides by the s-process, dreging them up and saute-ing them, then eventually puffing them gently out into space to make later generation solar systems like ours. SBHarris 06:02, 21 December 2012 (UTC)
- I was thinking of internal space (what you see in edit mode) but never the less I'll admit you're right that cites are needed everyplace. Even summary sections with main articles need a cite or two. In an article like the United States (summarized by section as heavily as any you'll see) the standard seems to be about one cite per paragraph. So knock yourself out here, but it shouldn't take more than one or two on this particular point.
Exact probability distribution of N(t) when it is not assumed continuous
The article says:
"[...] Although the parent decay distribution follows an exponential, observations of decay times will be limited by a finite integer number of N atoms and follow Poisson statistics as a consequence of the random nature of the process. [...]"
I'm pretty sure that the exact distribution is not Poisson's; it is Binomial with parameters and . I have done the math derivation myself.
I added the "citation needed" template as a warning for readers.
GNU/Octave code to simulate problem: http://pastebin.com/UHsrfXu0 (comments in Spanish)
Generated image: http://postimg.org/image/dqe92tzkn/
(In the background you can see a 3D plot (with color) of the Binomial probability function (of N(t)) evolving with time)
— Preceding unsigned comment added by Francisco Albani (talk • contribs) 08:02, 19 June 2013 (UTC)
- The discussion of Poisson statistics was confused. I have corrected it and added a citation. I think your calculation is reproducing the usual derivation of the exponential decay law by considering finite time intervals. RockMagnetist (talk) 14:23, 19 June 2013 (UTC)
Chain of two decays: 'the equation is not"
From the article:
- adding the increasing (and correcting) term obtains the law for a decay chain for two nuclides:
- The equation is not
- since this implies the number of atoms of B is only decreasing as time increases, which is not the case.
I don't think having incorrect equations in an article is a good idea: when people are looking for the formula, they won't pay much attention to the text, and even when they know the last one is wrong, they may accidentally copy it (easy mistake to make: you look up from your paper, see a line that begins with what you just wrote, so you assume that's the one you were copying). I doubt that people who know differential equations would need to be told explicitly why the two terms don't have the same sign, and you certainly don't need an incorrect formula to explain the correct one. Ssscienccce (talk) 19:14, 20 January 2014 (UTC)
- I agree that incorrect equations are undesirable and can lead to confusion, so I will now remove the first of the above three equations (with one term only) and the third (with the wrong sign). The correct second equation is still explained very adequately. Dirac66 (talk) 23:58, 20 January 2014 (UTC)
Radioactive Dating Filed Under Wrong Heading
This text, which appears under the "Discovery and History" heading, should be moved to the "Occurrence and applications" section:
- The discovery of radioactive elements in the 1890s opened the way for new dating techniques that suggested an age for Earth of several billion years.
That is, assuming it belongs in the article at all. There are numerous applications of radioactivity that are not listed anywhere in this article; Food irradiation, cancer treatment, tracing underground pipes, labeling chemicals for biological experiments, smoke detectors, radio-thermal generators,... That's just scratching the surface. There's no scientific reason why dating rocks and fossils is any more special than any of those. 129.42.208.183 (talk) 23:10, 30 January 2014 (UTC)
Concerning all the changes I made to the article
You are welcome. Zedshort (talk) 01:37, 30 September 2014 (UTC)
Changing decay rates
Otto Reifenschweiler also observed changes in decay rates in Tritium Reduced radioactivity of tritium in small titanium particles (published in Physics Letters A.)
Claus Rolfs has performed experiments in which he accelerated radioactive decay Half-life heresy: Accelerating radioactive decay
Should these two also be mentioned in the section ? --POVbrigand (talk) 14:13, 7 May 2012 (UTC)
Evidence against correlations... NOT
Looking to the 3 figures on the reference nr. 29, "Evidence against correlations..." one has to conclude that their claim is not substantiated because a synchronous variation will not show on the graphs. On what basis they assume very unlikely ? quoting: "If the Jenkins proposal were correct, it is very unlikely that the alpha, beta-minus, beta-plus, and electron-capture decays of all radioactive isotopes would be affected in quantitatively the same way. Thus the ratios of counts observed from two different isotopes would also be expected to show annual variations."
79.168.86.244 (talk) 18:14, 10 May 2015 (UTC)
wikidata
This article just links to a few items on wikidata, I don't know why. Please make attention.--171.232.148.168 (talk) 15:09, 15 June 2015 (UTC)
"inaccurate constants"
"Although these are constants, they are associated with statistically random behaviour of populations of atoms. In consequence, predictions using these constants are less accurate for small number of atoms."
I changed this to be somewhat *less* misleading (current edit):
"Although these are constants, they are associated with the statistical behavior of populations of atoms. In consequence, predictions using these constants are less accurate for individual atoms."
- Because deleting it yielded a reversion from another user. The original statement was patently misleading. The problem with small numbers of atoms is not with constants or statistical behavior, but rather with analytical techniques. Except in a few cases where half lives are too long to be very well constrained. So...it really is wrong/misleading. My best guess is that it was put in by a creationist type to cast doubt on the veracity of radioisotopic dating. Geochem. PhD here. I apologize if the format of this talk page edit is not perfect; I do not edit wikipedia with any regularity. Saw this error while perusing the page. Can someone with more clout on here delete the phrase and keep it that way? — Preceding unsigned comment added by Meteoritekid (talk • contribs) 09:21, 12 June 2015 (UTC)
- It's a bit clumsily worded, but all the original statement is trying to say is that smaller samples give rise to greater uncertainties and why this is (= the mean of a stochastic process). I don't see the connection to creationist twaddle. As for "individual atoms", that makes it plain wrong - you shouldn't ever use half-lives for individual atoms. Kolbasz (talk) 19:46, 18 June 2015 (UTC)
Tough LaTeX
The last part of the "half-life" subsection contains a bit of LaTeX {{math|''t'' = ''T''{{sub|1/''n''}}}} that comes out nonsense (as {{{1}}} on all my browsers), and which needs to be fixed. But I don't see anything obviously wrong with it. Can somebody figure out what the second expression needs to be?
Here it is:
Mathematically, the nth life for the above situation would be found in the same way as above—by setting N = N0/n, {{{1}}} and substituting into the decay solution to obtain
Thanks! SBHarris 00:29, 30 September 2015 (UTC)
- Is this what you're after? t = T1/n It is HTML, not LaTeX. RockMagnetist(talk) 00:36, 30 September 2015 (UTC)
- I think that's what I'm after indeed, but can you change it the same form as the previous equation N = N0/n? Yes I recognize wiki-markup and HTML whenever I see chevron brackets <>,</>, but the previous expression doesn't use any. So what is it, then? SBHarris 00:45, 30 September 2015 (UTC)
- Good catch! The problem was the equality sign. Kolbasz (talk) 07:44, 30 September 2015 (UTC)
- Aha, it's a wiki math template thing (the curly brackets suggested a template thing). In this case a wiki-template for texhtml. Bizarre. You had to add a 1= ? Why does WP keep fooling with other systems that work fine? Do they think physicists and mathematicians use wiki-templates? SBHarris 19:45, 30 September 2015 (UTC)
- There are good technical reasons to do stuff like embedded equations via templates. But because of the way templates work, certain characters are going to be interpreted as control characters by the template parser - this is pretty much unavoidable. Unfortunately for mathematics though, the syntax for named arguments in MediaWiki happens to be |[name]=[value]. So in the original markup, {{math|''t'' = ''T''{{sub|1/''n''}}}}, the {{math}} template sees that as "do my template magic with the argument ''t'' set to ''T''{{sub|1/''n''}}" because of the equality sign. Since the template doesn't have an argument ''t'', you get a null result of {{{1}}} instead. Adding the 1= makes it interpret it as "do my template magic with argument number 1 set to ''t'' = ''T''{{sub|1/''n''}}" instead, which is what we want ({{foo|bar}} and {{foo|1=bar}} mean the same thing - invoke the template "foo" with the first argument as "bar"). The alternative fix is to escape the offending characters with {{}} instead. Kolbasz (talk) 21:25, 30 September 2015 (UTC)
- Aha, it's a wiki math template thing (the curly brackets suggested a template thing). In this case a wiki-template for texhtml. Bizarre. You had to add a 1= ? Why does WP keep fooling with other systems that work fine? Do they think physicists and mathematicians use wiki-templates? SBHarris 19:45, 30 September 2015 (UTC)
- Good catch! The problem was the equality sign. Kolbasz (talk) 07:44, 30 September 2015 (UTC)
- I think that's what I'm after indeed, but can you change it the same form as the previous equation N = N0/n? Yes I recognize wiki-markup and HTML whenever I see chevron brackets <>,</>, but the previous expression doesn't use any. So what is it, then? SBHarris 00:45, 30 September 2015 (UTC)
Vector version available on Periodic Table Stability & Radioactivity image
Just thought I'd let you know so you can see if it works for you. If there's something that can be improved, please let me know. Thanks, Morgan Phoenix (talk) 04:33, 19 January 2016 (UTC)
Orders of magnitude
Hello all. There is a quote:
and range over 55 orders of magnitude in time
In the interest of engaging and exciting those who may not understand what even 7 orders of magnitude of seconds means (human lifetime), perhaps some sort of comparison would drive home the magnitude (ha!) of the 55 orders of magnitude quote? Buzzm (talk) 00:48, 25 January 2016 (UTC)
- Perhaps we could compare to the known range of distances. The radius of an atomic nucleus is about 10-13 m, and the radius of the observable universe is about 1011 light-years x 1016 m / light-year = 1027 m, or 40 orders of magnitude larger.Dirac66 (talk) 01:05, 25 January 2016 (UTC)
- Well, there is the article Time (Orders of magnitude), which I took the liberty of wikilinking in the lead. You might be able to pull some copy out of that. Kolbasz (talk) 08:32, 25 January 2016 (UTC)
I would like to propose that this article be renamed to Nuclear decay. There is already an article for Particle decay, which can be "radioactive" as well, so I don't feel the title is very accurate, or at least is not very clear as to the distinction between Radioactive decay and Particle decay. I feel it would be cleaner to name this article Nuclear decay, and have Radioactive decay link to it, as that is usually what is meant. — Preceding unsigned comment added by 70.247.169.219 (talk) 18:46, 26 March 2016 (UTC)
- Since there is a redirect for it, there doesn't seem to be a big reason for the change. As well as I know it, "radioactive" normally describes atoms, and is also better known to the general public. (That is, muons decay but aren't considered radioactive.) The name could be changed, and the redirect reversed, but that seems to me to be more work than it is worth. Gah4 (talk) 15:51, 3 June 2016 (UTC)
Diagram Problem
The diagram that shows all the isotopes possible plotted on N vs Z is cool. The diagram just below it that shows how various decay modes transition within the former diagram is cool. what's not cool is that they have contradictory axes; the top diagram has N vertically while the bottom one has Z vertically. The two could make beautiful harmony, if only they could agree. Also, in the result transition diagram, there is a small epsilon associated with beta+, a positron. I've never seen that convention before. Am I just behind the nuclear physics joke curve? :) SkoreKeep (talk) 06:31, 3 January 2013 (UTC)
Oops, pardon me. The transition diagram is just above, not below, the N/Z plot. — Preceding unsigned comment added by SkoreKeep (talk • contribs) 06:35, 3 January 2013 (UTC)
- The lower complicated one is standard. It would be nice if somebody would make a version of the upper one showing transmutations, that had its axes flipped in the same direction. Perhaps you could write a note to the original maker?
The epsilon stands for a a separate process called electron capture that always competes with positron decay, requires less energy, and produces the same transmutation. SBHarris 08:35, 3 January 2013 (UTC)
It was in the article and you only had to refresh, but here it is anyway:

M∧Ŝc2ħεИτlk 09:26, 3 January 2013 (UTC)
- Wow I cannot get my browser to refresh. Do you know how without logging out? I even see the old version here on the talk page. I DO see the new one when clicking the photo itself and (yes) that's exactly what was needed. Thanks! SBHarris 19:25, 3 January 2013 (UTC)
Obscure symbol
Is the symbol ε standard? I have never seen it used to mean electron capture before and am more familiar with EC. Anyway ε is not defined in this article, neither in the caption to the figure discussed above, nor in the accompanying text, nor in the tables of different types of decay. Nor is it defined in the article on Electron capture. If we are going to use this symbol in the figure we must define it, but I think it would be simpler to just write out electron capture in the figure itself. Dirac66 (talk) 20:03, 3 January 2013 (UTC)
- If it's ok I tweaked the caption of the image, I've never come across the epsilon notation for electron capture either... M∧Ŝc2ħεИτlk 20:14, 3 January 2013 (UTC)
- I went ahead and changed "ε" to "EC" for short. Better? M∧Ŝc2ħεИτlk 20:25, 3 January 2013 (UTC)
- Yes, EC is better, thank you. The only trouble is that like SBHarris, I only see the new image by clicking on the photo itself to go to the image file. In the article itself I still see the 2009 version with Z on the vertical axis and N horizontal, and refreshing my browser (IE8) does not seem to help. And I also tried Mozilla Firefox and still saw the 2009 version. Can there be a bug in the Wikipedia system for this image?? Dirac66 (talk) 21:58, 3 January 2013 (UTC)
- I've notified the issue here, comments are welcome. Apologies, M∧Ŝc2ħεИτlk 22:23, 3 January 2013 (UTC)
- Actually it depends where I look. I do see the new 2013 version at the top of the image file page File:Radioactive decay modes.svg, but on the article page Radioactive decay, I see the old 2009 version. And I have a PC, but I just got the same results on a Mac belonging to someone who would not have this image cached (because she never looks at physics articles). Dirac66 (talk) 22:45, 3 January 2013 (UTC)
- I've notified the issue here, comments are welcome. Apologies, M∧Ŝc2ħεИτlk 22:23, 3 January 2013 (UTC)

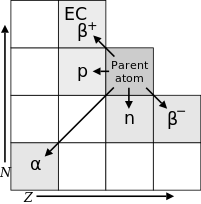
- LOL. Dirac66 has applied ye olde scientific method by using another computer and discovering that image cache-ing is indeed not the problem. I found that the image shows the newer version when simply displayed without the thumb box. From then on, it was a matter of applying controls to isolate the variables one-at-a-time. So, all you science dudes, what is the difference between the two images left and right? Don't look below, it's a puzzle.
- An old name for K-capture is epsilon decay (which redirects to electron capture on WP), and if you google "electron capture epsilon" you get some interesting cases where the epsilon is used as a symbol for this decay. The German WP article on electron capture mentions this, but the English one does not.
- Interactive Chart of Nuclides uses epsilon, and that is the way I always knew it.Gah4 (talk) 16:45, 3 June 2016 (UTC)
- Well, as long as its visible in the main article to everyone there's no problem. Thanks for clearing that up! M∧Ŝc2ħεИτlk 08:58, 4 January 2013 (UTC)
- Yes, it works for me too now. Very good, and I will remember your hypothesis if I ever see the problem in another article. As for epsilon decay, I would guess this name was proposed after Thomson's delta rays, and that physicists later abandoned both letters for the more descriptive modern terms. Dirac66 (talk) 12:27, 4 January 2013 (UTC)
- Well, as long as its visible in the main article to everyone there's no problem. Thanks for clearing that up! M∧Ŝc2ħεИτlk 08:58, 4 January 2013 (UTC)
- All the table of the nuclides that I know of have Z on the y-axis, and N on the x-axis. That conveniently allows different isotopes of an element to be read in the natural (for most of us) horizontal direction. Both diagrams in 'types of decay' don't work this way. It seems from the description above that this should have been fixed by now. Gah4 (talk) 15:57, 3 June 2016 (UTC)
6He and 8He
Look at Isotopes of helium #Table: these isotopes appear to be able simultaneously emit a β− and split to two nuclei: 6
2He
→ 4
2He
+ 2
1D
+
e−
+
ν
e. This article does not know such type of decay. Incnis Mrsi (talk) 18:21, 1 February 2013 (UTC)
- Could be, but many unusual things happen at the low N, Z end of the table. Gah4 (talk) 00:26, 13 October 2016 (UTC)
lifetime units
but the mean life and half-life t1/2 have been adopted as standard times associated with exponential decay. Just about everywhere except nuclear decay, 1/e life is used. One exception is optics, where optical absorption is exponential base 10. (Or log base 10 if you do it the other way around.) Gah4 (talk) 00:32, 13 October 2016 (UTC)
Rate of radioactive decay influenced by "the Sun".
The theory as I read it was that the rate at which neutrinos pass through a body can influence the rate of radioactive decay of that body. Since the overwhelming proportion of neutrinos passing through the earth (or indeed our easily accessive solar system) is from the sun (an "almost" point source of neutrinos at planetary distances), then obviously any change in the neutrino-producing component of solar activity will change the neutrino flux and hence the radioactive decay rate, as will the distance from the Sun. But to repeat, I have no reference for it, so I'm only including it here as an anecdote.
The other significant part of this is that IF it is true, then with neutrinos passing through solid matter so easily, their effect on radioactive decay will be felt equally on the surface of the earth as it would be inside the earth, and at any depth. 124.184.13.246 (talk) 08:56, 31 August 2012 (UTC)
- If you search manganese + radioactive + neutrino on your favorite search engine, you will find many results. There is no theory here. The physicists (Fischbach and Jenkins) who published that took a guess that it might be because of neutrinos. They also took into consideration 30 year old data and considered equipment related anomalies, but how can you do that if you don't have the equipment. The isotope they talk about is Mn 56. They also talk about a solar flare in Dec 13, 2006. Does a solar flare emit neutrinos? I though you get neutrinos when you have beta decay. Anyway, what is so special about a solar flare? Vmelkon (talk) 07:00, 13 October 2013 (UTC)
law of small numbers
I tried a link to statistical significance for Although these are constants, they are associated with the statistical behavior of populations of atoms. In consequence, predictions using these constants are less accurate for minuscule samples of atoms. but it was reverted. I had thought about law of small numbers, but that is a disambiguation page, and none of the choices are close at all. There are other ways to lose statistical significance, but not having enough data points is the usual way. Any other suggestions? Gah4 (talk) 06:08, 13 October 2016 (UTC)









