User:Harshitha sy/sandbox
| Harshitha sy/sandbox |
|---|
Phenylketonuria is an inborn error of metabolism that results in decreased metabolism of the amino acid phenylalanine.[1] Untreated PKU can lead to intellectual disability, seizures, behavioral problems, and mental disorder It may also result in a musty smell and lighter skin. Babies born to mothers who have poorly treated PKU may have heart problems, a microcephaly|small head, and low birth weight.
Phenylketonuria is a genetic disorder Heredity|inherited from a person's parents. It is due to mutations in the PAH gene|PAH gene which results in low levels of the enzyme phenylalanine hydroxylase. This results in the build up of dietary phenylalanine to potentially toxic levels. It is autosomal recessive meaning that both copies of the gene must be mutated for the condition to develop. There are two main types, classic PKU and variant PKU, depending on if any enzyme function remains. Those with one copy of a mutated gene typically do not have symptoms.[2] Many countries have newborn screening programs for the disease.[1]
Treatment is with a diet (nutrition)|diet low in foods that contain phenylalanine and special supplement. Babies should use a special infant formula|formula. The diet should begin as soon as possible after birth and be lifelong. People who are diagnosed early and maintain a strict diet can have normal health and a normal Longevity Effectiveness is monitored through periodic blood tests.Cite error: A <ref> tag is missing the closing </ref> (see the help page).[3]
Pathophysiology[edit]
When Phe cannot be metabolized by the body, a typical diet that would be healthy for people without PKU causes abnormally high levels of Phe to accumulate in the blood, which is toxic to the brain. If left untreated, complications of PKU include severe intellectual disability, brain function abnormalities, microcephaly, mood disorders, irregular motor functioning, and behavioral problems such as attention deficit hyperactivity disorder, as well as physical symptoms such as a "musty" odor, eczema, and unusually light skin and hair coloration.
Classical PKU[edit]
Classical PKU, and its less severe forms "mild PKU" and "mild hyperphenylalaninemia" are caused by a mutated gene for the enzyme phenylalanine hydroxylase (PAH), which converts the amino acid phenylalanine ("Phe") to other essential compounds in the body, in particular tyrosine. Tyrosine is a conditionally essential Amino acid for PKU patients because without PAH it cannot be produced in the body through the breakdown of phenylalanine. Tyrosine is necessary for the production of neurotransmitters like epinephrine, norepinephrine, and dopamine.[4]
PAH deficiency causes a spectrum of disorders, including classic phenylketonuria (PKU) and mild hyperphenylalaninemia (also known as "hyperphe" or "mild HPA"), a less severe accumulation of phenylalanine. Patients with "hyperphe" may have more functional PAH enzyme and be able to tolerate larger amounts of phenylalanine in their diets than those with classic PKU, but unless dietary intake is at least somewhat restricted, their blood Phe levels are still higher than the levels in people with normal PAH activity.[5]
Phenylalanine is a large, neutral amino acid (LNAA). LNAAs compete for transport across the blood–brain barrier (BBB) via the large neutral amino acid transporter (LNAAT). If phenylalanine is in excess in the blood, it will saturate the transporter. Excessive levels of phenylalanine tend to decrease the levels of other LNAAs in the brain. As these amino acids are necessary for protein and neurotransmitter synthesis, Phe buildup hinders the development of the brain, causing intellectual disability.[6]
Recent research suggests that neurocognitive, psychosocial, quality of life, growth, nutrition, bone pathology are slightly suboptimal even for patients who are treated and maintain their Phe levels in the target range, if their diet is not supplemented with other amino acids.[7]
Classic PKU dramatically affects myelination and white matter tracts in untreated infants; this may be one major cause of neurological disorders associated with phenylketonuria. Differences in white matter development are observable with magnetic resonance imaging. Abnormalities in gray matter can also be detected, particularly in the motor and pre-motor cortex, thalamus and the hippocampus.[8]
It was recently suggested that PKU may resemble amyloid diseases, such as Alzheimer's disease and Parkinson's disease, due to the formation of toxic amyloid-like assemblies of phenylalanine.[9]
Other non-PAH mutations can also cause PKU.[citation needed]
Tetrahydrobiopterin-deficient hyperphenylalaninemia[edit]
A rarer form of hyperphenylalaninemia occurs when the PAH enzyme is normal, and a defect is found in the biosynthesis or recycling of the cofactor tetrahydrobiopterin (BH4).[10] BH4 (called biopterin) is necessary for proper activity of the enzyme PAH, and this coenzyme can be supplemented as treatment. Those who suffer from this form of hyperphenylalaninemia may have a deficiency of tyrosine (which is created from phenylalanine by PAH). These patients must be supplemented with tyrosine to account for this deficiency.
Dihydrobiopterin reductase activity is needed to replenish quinonoid-dihydrobiopterin back into its tetrahydrobiopterin form, which is an important cofactor in many reactions in amino acid metabolism. Those with this deficiency may produce sufficient levels of the enzyme phenylalanine hydroxylase (PAH) but, since tetrahydrobiopterin is a cofactor for PAH activity, deficient dihydrobiopterin reductase renders any PAH produced unable to use phenylalanine to produce tyrosine. Tetrahydrobiopterin is a cofactor in the production of L-DOPA from tyrosine and 5-hydroxy-L-tryptophan from tryptophan, which must be supplemented as treatment in addition to the supplements for classical PKU.
Levels of dopamine can be used to distinguish between these two types. Tetrahydrobiopterin is required to convert Phe to Tyr and is required to convert Tyr to L-DOPA via the enzyme tyrosine hydroxylase. L-DOPA, in turn, is converted to dopamine. Low levels of dopamine lead to high levels of prolactin. By contrast, in classical PKU (without dihydrobiopterin involvement), prolactin levels would be relatively normal.
Tetrahydrobiopterin deficiency can be caused by defects in four genes. They are known as HPABH4A, HPABH4B, HPABH4C, and HPABH4D.[11]
Metabolic pathways[edit]

The enzyme phenylalanine hydroxylase normally converts the amino acid phenylalanine into the amino acid tyrosine. If this reaction does not take place, phenylalanine accumulates and tyrosine is deficient. Excessive phenylalanine can be metabolized into phenylketones through the minor route, a transaminase pathway with glutamate. Metabolites include phenylacetate, phenylpyruvate and phenethylamine.[12] Elevated levels of phenylalanine in the blood and detection of phenylketones in the urine is diagnostic, however most patients are diagnosed via newborn screening.
Screening[edit]
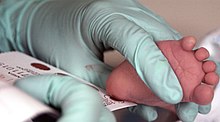
PKU is commonly included in the newborn screening panel of many countries, with varied detection techniques. Most babies in developed countries are screened for PKU soon after birth.[13] Screening for PKU is done with bacterial inhibition assay (Guthrie test), immunoassays using fluorometric or photometric detection, or amino acid measurement using tandem mass spectrometry (MS/MS). Measurements done using MS/MS determine the concentration of Phe and the ratio of Phe to tyrosine, the ratio will be elevated in PKU.[14]
Treatment[edit]
PKU is not curable. However, if PKU is diagnosed early enough, an affected newborn can grow up with normal brain development by managing and controlling phenylalanine ("Phe") levels through diet, or a combination of diet and medication.
Diet[edit]
People who follow the prescribed dietary treatment from birth may have no symptoms. Their PKU would be detectable only by a blood test. People must adhere to a special diet low in Phe for optimal brain development. Since Phe is necessary for the synthesis of many proteins, it is required for appropriate growth, but levels must be strictly controlled.
Optimal health ranges (or "target ranges") are between 120 and 360 µmol/L or equivalently 2 to 6 mg/dL, and aimed to be achieved during at least the first 10 years,[15] to allow the brain to develop normally.
In the past, PKU-affected people were allowed to go off diet after approximately eight, then 18 years of age. Today, most physicians recommend low Phe levels throughout life. For adults, somewhat higher levels of Phe may be tolerable, but restriction is still advised to prevent mood disorders and difficulty concentrating, among other neurological problems.[16]
The diet requires restricting or eliminating foods high in Phe, such as soybeans, egg whites, shrimp, chicken breast, spirulina, watercress, fish, nuts, crayfish, lobster, tuna, turkey, legumes, and lowfat cottage cheese.[17] Starchy foods, such as potatoes and corn are generally acceptable in controlled amounts, but the quantity of Phe consumed from these foods must be monitored. A food diary is usually kept to record the amount of Phe consumed with each meal, snack, or drink. An "exchange" system can be used to calculate the amount of Phe in a food from the protein content identified on a nutritional information label. Lower-protein "medical food" substitutes are often used in place of normal bread, pasta, and other grain-based foods, which contain a significant amount of Phe. Many fruits and vegetables are lower in Phe and can be eaten in larger quantities. Infants may still be breastfed to provide all of the benefits of breastmilk, but the quantity must also be monitored and supplementation for missing nutrients will be required. The sweetener aspartame, present in many diet foods and soft drinks, must also be avoided, as aspartame contains phenylalanine.
Different people can tolerate different amounts of Phe in their diet. Regular blood tests are used to determine the effects of dietary Phe intake on blood Phe level.
Supplements[edit]
Supplementary "protein substitute" formulas are typically prescribed for people PKU (starting in infancy) to provide the amino acids and other necessary nutrients that would otherwise be lacking in a low-phenylalanine diet. Tyrosine, which is normally derived from phenylalanine and which is necessary for normal brain function, is usually supplemented. Consumption of the protein substitute formulas can actually reduce phenylalanine levels, probably because it stops the process of protein catabolism from releasing Phe stored in the muscles and other tissues into the blood. Many PKU patients have their highest Phe levels after a period of fasting (such as overnight), because fasting triggers catabolism.[18] A diet that is low in phenylalanine but does not include protein substitutes may also fail to lower blood Phe levels, since a nutritionally insufficient diet may also trigger catabolism. For all these reasons, the prescription formula is an important part of the treatment for patients with classic PKU.
The oral administration of tetrahydrobiopterin (or BH4) (a cofactor for the oxidation of phenylalanine) can reduce blood levels of this amino acid in some people.[19][20] Most people; however, have little or no benefit.[21]
Tentative evidence supports dietary supplementation with large neutral amino acids (LNAAs).[22] The LNAAs (e.g. leu, tyr, trp, met, his, ile, val, thr) may compete with phe for specific carrier proteins that transport LNAAs across the intestinal mucosa into the blood and across the blood brain barrier into the brain. It use is really only indicated in adults who will not follow an appropriate diet.[1]
Another interesting treatment strategy for is casein glycomacropeptide (CGMP), which is a milk peptide naturally free of Phe in its pure form[23] CGMP can substitute the main part of the free amino acids in the PKU diet and provides several beneficial nutritional effects compared to free amino acids. The fact that CGMP is a peptide ensures that the absorption rate of its amino acids is prolonged compared to free amino acids and thereby results in improved protein retention[24] and increased satiety[25] compared to free amino acids. Another important benefit of CGMP is that the taste is significantly improved[24] when CGMP substitutes part of the free amino acids and this may help ensure improved compliance to the PKU diet.
Furthermore, CGMP contains a high amount of the phe lowering LNAAs, which constitutes about 41 g per 100 g protein[23] and will therefore help maintain plasma phe levels in the target range.
Women[edit]
For women with phenylketonuria, it is important for the health of their children to maintain low Phe levels before and during pregnancy.[26] Though the developing fetus may only be a carrier of the PKU gene, the intrauterine environment can have very high levels of phenylalanine, which can cross the placenta. The child may develop congenital heart disease, growth retardation, microcephaly and intellectual disability as a result.[27] PKU-affected women themselves are not at risk of additional complications during pregnancy.
In most countries, women with PKU who wish to have children are advised to lower their blood Phe levels (typically to between 2 and 6 mg/dL) before they become pregnant, and carefully control their levels throughout the pregnancy. This is achieved by performing regular blood tests and adhering very strictly to a diet, in general monitored on a day-to-day basis by a specialist metabolic dietitian. In many cases, as the fetus' liver begins to develop and produce PAH normally, the mother's blood Phe levels will drop, requiring an increased intake to remain within the safe range of 2–6 mg/dL. The mother's daily Phe intake may double or even triple by the end of the pregnancy, as a result. When maternal blood Phe levels fall below 2 mg/dL, anecdotal reports indicate that the mothers may suffer adverse effects, including headaches, nausea, hair loss, and general malaise. When low phenylalanine levels are maintained for the duration of pregnancy, there are no elevated levels of risk of birth defects compared with a baby born to a non-PKU mother.[28]
See also[edit]
- Hyperphenylalanemia
- Lofenalac
- Tetrahydrobiopterin deficiency
- Flowers for Algernon, which features a character who has phenylketonuria
References[edit]
- ^ a b c Al Hafid, N; Christodoulou, J (October 2015). "Phenylketonuria: a review of current and future treatments". Translational pediatrics. 4 (4): 304–17. doi:10.3978/j.issn.2224-4336.2015.10.07. PMC 4728993. PMID 26835392.
- ^ Cite error: The named reference
NIH2016was invoked but never defined (see the help page). - ^ "Videographer with Rare Disease Turns the Lens on Himself". everydayhealth.com.
- ^ "Phenylketonuria". Healthline. 20 August 2012.
- ^ http://www.genenames.org Phenylalanine hydroxylase (PAH) gene summary, retrieved September 8, 2006
- ^ Pietz J, Kreis R, Rupp A, Mayatepek E, Rating D, Boesch C, Bremer HJ (1999). "Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria". Journal of Clinical Investigation. 103 (8): 1169–1178. doi:10.1172/JCI5017. PMC 408272. PMID 10207169.
- ^ Enns GM, Koch R, Brumm V, Blakely E, Suter R, Jurecki E (1 October 2010). "Suboptimal outcomes in patients with PKU treated early with diet alone: Revisiting the evidence". Molecular Genetics and Metabolism. 101 (2–3): 99–109. doi:10.1016/j.ymgme.2010.05.017. PMID 20678948.
- ^ "Neurowiki2012 - Phenylketonuria". wikispaces.com.[self-published source?]
- ^ Adler-Abramovich, Lihi; Vaks, Lilach; Carny, Ohad; Trudler, Dorit; Magno, Andrea; Caflisch, Amedeo; Frenkel, Dan; Gazit, Ehud (2012). "Phenylalanine assembly into toxic fibrils suggests amyloid etiology in phenylketonuria". Nature Chemical Biology. 8 (8): 701–6. doi:10.1038/nchembio.1002. PMID 22706200.
- ^ Surtees R, Blau N (2000). "The neurochemistry of phenylketonuria". European Journal of Pediatrics. 169: S109–S113. doi:10.1007/PL00014370. PMID 11043156.
- ^ Online Mendelian Inheritance in Man (OMIM): 261640
- ^ Michals K, Matalon R (1985). "Phenylalanine metabolites, attention span and hyperactivity". American Journal of Clinical Nutrition. 42 (2): 361–5. PMID 4025205.
- ^ Mayo Clinic Staff (2007-12-20). "Phenylketonuria (PKU)". Mayo Clinic. Retrieved 2008-03-13.
- ^ Sarafoglou, Kyriakie; Hoffmann, Georg F.; Roth, Karl S. (eds.). Pediatric Endocrinology and Inborn Errors of Metabolism. New York: McGraw Hill Medical. p. 26.
- ^ Chapter 55, page 255 in:Behrman, Richard E.; Kliegman, Robert; Nelson, Waldo E.; Karen Marcdante; Jenson, Hal B. (2006). Nelson essentials of pediatrics. Elsevier/Saunders. ISBN 1-4160-0159-X.
- ^ "National PKU News: Adults". pkunews.org.
- ^ "Foods highest in Phenylalanine". self.com.
- ^ MacDonald A, Rylance GW, Asplin D, Hall SK, Booth IW (1998). "Does a single plasma phenylalanine predict quality of control in phenylketonuria?". Archives of Disease in Childhood. 78 (2): 122–6. doi:10.1136/adc.78.2.122. PMC 1717471. PMID 9579152.
- ^ Burton, Barbara K.; Kar, Santwana; Kirkpatrick, Peter (2008). "Sapropterin". Nature Reviews Drug Discovery. 7 (3): 199–200. doi:10.1038/nrd2540.
- ^ Michals-Matalon K (2008). "Sapropterin dihydrochloride, 6-R-L-erythro-5,6,7,8-tetrahydrobiopterin, in the treatment of phenylketonuria". Expert Opin Investig Drugs. 17 (2): 245–251. doi:10.1517/13543784.17.2.245. PMID 18230057.
- ^ Cite error: The named reference
Camp2014was invoked but never defined (see the help page). - ^ van Spronsen, FJ; de Groot, MJ; Hoeksma, M; Reijngoud, DJ; van Rijn, M (December 2010). "Large neutral amino acids in the treatment of PKU: from theory to practice". Journal of Inherited Metabolic Disease. 33 (6): 671–6. doi:10.1007/s10545-010-9216-1. PMC 2992655. PMID 20976625.
- ^ a b Etzel MR (Apr 2004). "Manufacture and use of dairy protein fractions". The Journal of Nutrition. 134 (4): 996S–1002S. PMID 15051860.
- ^ a b van Calcar SC, MacLeod EL, Gleason ST, Etzel MR, Clayton MK, Wolff JA, Ney DM (Apr 2009). "Improved nutritional management of phenylketonuria by using a diet containing glycomacropeptide compared with amino acids". The American Journal of Clinical Nutrition. 89 (4): 1068–77. doi:10.3945/ajcn.2008.27280. PMC 2667457. PMID 19244369.
- ^ MacLeod EL, Clayton MK, van Calcar SC, Ney DM (Aug 2010). "Breakfast with glycomacropeptide compared with amino acids suppresses plasma ghrelin levels in individuals with phenylketonuria". Molecular genetics and metabolism. 100 (4): 303–8. doi:10.1016/j.ymgme.2010.04.003. PMC 2906609. PMID 20466571.
- ^ Lee PJ, Ridout D, Walter JH, Cockburn F (2005). "Maternal phenylketonuria: report from the United Kingdom Registry 1978–97". Archives of Disease in Childhood. 90 (2): 143–146. doi:10.1136/adc.2003.037762. PMC 1720245. PMID 15665165.
- ^ Rouse B, Azen C, Koch R, Matalon R, Hanley W, de la Cruz F, Trefz F, Friedman E, Shifrin H (1997). "Maternal phenylketonuria collaborative study (MPKUCS) offspring: Facial anomalies, malformations, and early neurological sequelae". American Journal of Medical Genetics. 69 (1): 89–95. doi:10.1002/(SICI)1096-8628(19970303)69:1<89::AID-AJMG17>3.0.CO;2-K. PMID 9066890.
- ^ lsuhsc.edu Genetics and Louisiana Families
