Wikipedia:Reference desk/Archives/Science/2010 February 15
| Science desk | ||
|---|---|---|
| < February 14 | << Jan | February | Mar >> | February 16 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
February 15[edit]
Cooking rice under pressure[edit]


The two pictures used by the article rice cooker provide an interesting contrast:
- The more expensive one on the left has a valve in the lid. It is a pressure cooker.
- The cheaper one on the right, as you can see, has a small opening in its thick glass lid. It cooks under normal air pressure and steam is not retained in the cooker.
I think people who eat sticky rice mainly use pressure cookers. Even if you are not using these advanced computerized models, the less expensive cookers use a very heavy lid as a pressure valve. I have seen some traditional wood-burning rice cookers in eastern Asian countries. They use over-sized heavy wooden lids. Some high-end sushi restaurants still use these traditional cookers because they are simply GREAT!
I guess the one on the right is generally used to cook non-sticky long grain rice (e.g., Basmati rice). I am not really sure about it. If steam can easily escape the cooker, how do they cook the rice? -- Toytoy (talk) 02:11, 15 February 2010 (UTC)
- The difference is rice being cooked at around 100 degrees C and 1 atmospheres pressure; and rice being cooked at say 125 degress C and 1.5-ish atmospheres pressure. In both cases the rice is cooked; in the latter case, more quickly. See Pressure cooking. --Tagishsimon (talk) 02:25, 15 February 2010 (UTC)
- How would a heavy lid act as a pressure valve? I have an old Panasonic rice cooker from the 80s, and there's no pressure cooking involved. It just has a small hole in the big plastic hinged lid. The steam escapes, yes, but at a much slower rate than if there were no lid at all. By the time the rice is finished cooking, there's still plenty of steam left in the pot.
- AFAIK, it doesn't take any kind of fancy rice-cooker to make sticky rice. You just need some glutinous rice. Indeterminate (talk) 03:38, 15 February 2010 (UTC)
- Certainly you can cook using a pot and a light-weight lid. The rice shall be edible but it may not be tasty. I have been told not to open the lid while cooking and "resting" countless times. That's why I thought keeping steam inside the cooker must be a way to make rice taste good. Failure to do so may compromise the taste. The heavy lid does not increase the pressure as much as a pressure cooker. However, the heavy lid without a hole may still retain steam much more than the lid-with-a-hole cookers. -- Toytoy (talk) 04:53, 15 February 2010 (UTC)
Is this girl lying to me?[edit]
I have been talking to this girl for quite some time, and she has been telling me she weighs 125-130 lbs. A month and a half ago she went into to hospital for an emergency appendectomy and was released the same day. 3 weeks ago she had to go to the ER twice because of bleeding in her esophagus and had to have stitches put in to stop bleeding. She said she was given some pills to gain weight as well as some other medicine, at this time she tells me she weighed 140 lbs. This weekend I saw her for the first time and she was FAT. She tells me she gained 40 lbs in 3 weeks (to 180 lbs) and that it is a result of her medical treatment and largely "just water" whatever the hell that means...
1) Is this at all plausible--can someone gain 40 lbs in 3 weeks?
2) what is "water weight"
3) Will she revert back to her previous size (if she is being truthful) and if so how long will it take and will her skin be all stretched and stuff?
4) Are there any ways I can tell if she is being truthful or lying?
XM (talk) 02:20, 15 February 2010 (UTC)
- I deleted this as a medical question. The OP disagrees, and has reposted it. Another opinion, please. -- Flyguy649 talk 03:56, 15 February 2010 (UTC)
- He's not technically asking for medical advice. Well, he technically is asking for medical advice. I don't see the problem with this one though, he isn't seeking treatment or anything like that. Beach drifter (talk) 03:59, 15 February 2010 (UTC)
- What I am asking is not medical advice. No medical decisions will be made based on whatever information I get here. I am asking for advice on whether or not a situation is medically possible and if so, how long it will take to resolve back to the previous state. XM (talk) 04:04, 15 February 2010 (UTC)
- This girl is not going to become skinny, if that is want you want to hear. Beach drifter (talk) 04:05, 15 February 2010 (UTC)
- What I am asking is not medical advice. No medical decisions will be made based on whatever information I get here. I am asking for advice on whether or not a situation is medically possible and if so, how long it will take to resolve back to the previous state. XM (talk) 04:04, 15 February 2010 (UTC)
AFTER EDIT CONFLICT:
- Well I was hoping for a bit more depth in an answer. Do you think she actually gained the weight rapidly, or was just fat all along and lying to me? If it is the former, could she lose it once she resumes her normal lifestyle along with some aggressive exersize? XM (talk) 04:11, 15 February 2010 (UTC)
- But it seems it would be pretty impossible to squarely address the question without entering the realm of analyzing a medical condition, and the big problem with that is all the missing pieces that we know nothing about. In my opinion it is an unanswerable question, and any attempt to respond to it entails giving out medical opinions in a reckless fashion. Bus stop (talk) 04:08, 15 February 2010 (UTC)
- Again, nothing said here is going to effect (or is it affect) any medical decisions at all. I just want to know if something is medically possible. Medical opinions in this situation would not be reckless as they arent going to have any medical affect whatsoever. XM (talk) 04:18, 15 February 2010 (UTC)
- yes, it's possible, depending on the kind of medication she's been given
- 'water weight' usually means that the body is retaining excess water rather than excreting it. This can happen because of hormonal imbalances, kidney problems, certain medications, or other causes. 40 lbs of water weight gain strikes me as an awful lot, but I'm not a doctor.
- She may or may not lose the excess weight - without knowing the cause, the medicines she's on, what her natural weight is, or other information there is no way to tell
- why is it important? she's probably struggling with her own self image (whether the weight is natural or recently gained makes no difference); you're struggling with your own self image (you're apparently more worried about what she'll look like on your arm than what she's like as a person). If you're going to be a jerk and reject her because she's heavy, then go ahead and be an honest jerk; don't try to put the blame on her by making her into a liar. --Ludwigs2 04:12, 15 February 2010 (UTC)
- Ludwigs, which is more likely, that she will lose the weight, or that she wont? And to answer your forth question there are two possible explainations for this situation, in one the girl is honest and her weight is temporary in the other the girl isn't and she is going to be permanently fat. I am okay with one of those explainations, not with the other. But I do need to find out which it is, or at least which is most likely XM (talk) 04:23, 15 February 2010 (UTC)
- That question is literally impossible for us to answer. Ludwigs is speaking in purely general terms. It is possible for a person to gain weight due to water retention; it is possible for a person to gain weight due to medical treatments, and it is possible for a person to gain a large amount of weight in a short amount of time. Those are all possibilities based on biology, and without any real comment on the specifics of any one particular case, especially on the one you cite. Wikipedia's reference desk contributors are unable to make specific comments on a specific case as that would constitute a form of medical advice. No one here, or indeed anywhere on the internet, can make any comments as to the specifics of your friends case, as to why she may have gained weight, whether she is telling the truth or not, how she may lose it, etc. etc. All of that is between her and her doctors, we can only answer the general biological questions, not on her case. It will be up to you to decide what it all means in her case. --Jayron32 04:52, 15 February 2010 (UTC)
- Jayron, So it is possible to gain that much in such a short time? If what she is telling me is medically possible, then I am inclined to believe her until other evidence presents itself. But 40 lbs in 3 weeks seems like a lot to me. XM (talk) 04:59, 15 February 2010 (UTC)
- I have no idea if that specific amount in that specific time is actually possible. What you should do is read the Wikipedia article weight gain and follow any links from that article, including external links, and arrive at your own conclusions. This is not an endorsement of any information contained in either that article or especially in any of those links. I have no idea if the information is valid or reliable, but if you want some information, its a better start than asking for medical advice from random strangers on teh intrewebz. If you want a poorly informed and unverifiable medical opinion, at least make it your own poorly informed and unverifiable opinion, and don't rely on someone elses. --Jayron32 05:14, 15 February 2010 (UTC)
- @ jayron: This isn't a medical question, it's an emotional one. XM is trying to find some excuse to be pissed off at this girl because she's violated his heartfelt expectations. He has all the medical information he needs to decide whether he can realistically work himself up to an effective level of self-righteous indignation; I suggest we leave him to explore his inner self on his own.
 --Ludwigs2 07:22, 15 February 2010 (UTC)
--Ludwigs2 07:22, 15 February 2010 (UTC)
- @ jayron: This isn't a medical question, it's an emotional one. XM is trying to find some excuse to be pissed off at this girl because she's violated his heartfelt expectations. He has all the medical information he needs to decide whether he can realistically work himself up to an effective level of self-righteous indignation; I suggest we leave him to explore his inner self on his own.
- I have no idea if that specific amount in that specific time is actually possible. What you should do is read the Wikipedia article weight gain and follow any links from that article, including external links, and arrive at your own conclusions. This is not an endorsement of any information contained in either that article or especially in any of those links. I have no idea if the information is valid or reliable, but if you want some information, its a better start than asking for medical advice from random strangers on teh intrewebz. If you want a poorly informed and unverifiable medical opinion, at least make it your own poorly informed and unverifiable opinion, and don't rely on someone elses. --Jayron32 05:14, 15 February 2010 (UTC)
- Jayron, So it is possible to gain that much in such a short time? If what she is telling me is medically possible, then I am inclined to believe her until other evidence presents itself. But 40 lbs in 3 weeks seems like a lot to me. XM (talk) 04:59, 15 February 2010 (UTC)
- You know, Ludwigs, your condescending comments aren't really appericiated by me or probably anyone else on the Ref desk. I find your comments extremely rude, emotionally charged, and without basis. I asked if the claim of someone was within the realm of possibility, because if they aren't then that means I have been lied to. Additionally, in the event that she is being truthful, I want to know the likelihood that she will revert to an attractive state. Evidently this is what you have a problem with causing you refer to me as self-righteous. I'm sorry if someone decided they didn't want to be with you because of your physical appearance, but comments like yours are simply uncalled for and most unwelcome. XM (talk) 07:51, 15 February 2010 (UTC)
- They're appreciated by me, and I suspect by most other people on the desk. If you're going to be a douche, be a douche, but don't try to dress it up as morally righteous. 86.176.48.57 (talk) 13:13, 15 February 2010 (UTC)
- XM - If you don't trust the explanation given by the girl, ultimately what difference do the explanations given by complete strangers make? Your statement "I want to know the likelihood that she will revert to an attractive state" says pretty much everything anybody needs to know. The truth makes no difference in either matter - you appear not to trust her, and you appaer not to care for her sufficiently to disregard her current weight. 194.221.133.226 (talk) 12:30, 15 February 2010 (UTC)
- Is the girl in question an infallible vector of information on weight gain and weight loss and related questions? Or is she capable of slipping up in some minor detail concerning the dialogue of self analysis concerning the recent body changes? It is possible that the biomass in question is inconsistently distributed and the dialogue being reported is not as accurate as it should be. Therefore the referred to weight gain should perhaps be monitored to allow for the perceptual adjustments that sometimes occur over time. Bus stop (talk) 13:11, 15 February 2010 (UTC)
- Presumably the hidden question here is that you'd like to know if you should carry on the friendship/relationship, based on (regardless of whether or not she was/is lying) whether or not she can lose the weight sufficiently to attract you. I think if you have to ask, do her a favour. Even if she does lose the water weight/fat, there is no guarantee that weight gain won't occur in the future, particularly with pregnancy being the way it is, and you're clearly not going to be happy with that. She's better off investing her time and emotions elsewhere, eh? Maedin\talk 13:29, 15 February 2010 (UTC)
Better make sure she doesn't see your browser history. I'm sure she wouldn't be thrilled that you trust random internet strangers more than her! APL (talk) 16:01, 15 February 2010 (UTC)
- However, if someone plans to gain "water weight" by drinking water, this is dangerous due to the likelihood for water intoxication. ~AH1(TCU) 03:18, 16 February 2010 (UTC)
Dude >.> you are so off....--Talk Shugoːː 21:17, 18 February 2010 (UTC)
Fujiwhara effect[edit]
The Fujiwhara effect says that "When cyclones approach each other, their centers will begin orbiting cyclonically about a point between the two systems." This is all fine except for one thing: How would it affect two storms in opposite hemispheres, say at 5°0′0″N 170°0′0″W / 5.00000°N 170.00000°W and 5°0′0″S 170°0′0″W / 5.00000°S 170.00000°W? If the two cyclones began to orbit, it would result in them crossing the equator, which I seem to recall tropical cyclones cannot do. Does this mean that the Fujiwhara effect would kill the two storms, or would it just not affect them at all? Ks0stm (T•C•G) 04:19, 15 February 2010 (UTC)
- I have fixed your coordinate template. Nimur (talk) 04:24, 15 February 2010 (UTC)
- Thanks. Ks0stm (T•C•G) 04:29, 15 February 2010 (UTC)
- There's not a hard rule about a tropical cyclone crossing the equator. The coriolis force tends to push storms away from the equator and towards the poles, but climate and storm dynamics are complicated things - if a local pressure perturbation was strong enough to suck a storm across the equator, it could happen. I suspect that since both the Fujiwhara effect and the internal cyclone spiral are both consequences of the coriolis effect, which is weaker near the equator, the tendency for this spiraling to impact storms that happen to be close the equator (which are rare to begin with) would be low. If two storms were on opposite sides of the equator simultaneously (which would be very rare), then the effect would still be weak-to-non-present - again, with the caveat that local conditions can behave counter to the global-scale trends. Nimur (talk) 04:39, 15 February 2010 (UTC)
- In thousands of examples, no cyclone has ever crossed the equator. [1] Dragons flight (talk) 06:39, 15 February 2010 (UTC)
- There's also the fact that if cyclones were on opposite sides of the equator they would spin in opposite directions, which would make the whole business break down. Looie496 (talk) 18:43, 15 February 2010 (UTC)
- For tornadoes (which are sometimes called cyclones around here) there are some which spin the wrong way around, but they are on the order of about 1:1000. Does the same hold true for hurricanes-typhoons? 65.121.141.34 (talk) 20:46, 15 February 2010 (UTC)
- There's also the fact that if cyclones were on opposite sides of the equator they would spin in opposite directions, which would make the whole business break down. Looie496 (talk) 18:43, 15 February 2010 (UTC)
Relative temperature of fabrics[edit]
Why do different fabrics feel colder than others? For instance, I have flannel sheets as well as some (probably) nylon/cotton blend sheets. The flannel "feels" warmer when getting into bed than the other sheets yet my room is the same temperature. Or another example would be when you slip on a coat or jacket that has a nylon lining in the sleeves. The nylon feels cooler than if you were to put on a flannel shirt. Why? Dismas|(talk) 05:03, 15 February 2010 (UTC)
- This has nothing to do with temperature, and everything to do with specific heat. The temperatures may be identical, but what is different is the rate at which different materials will conduct heat away from your skin. Your perception of the temperature of the fabiric really isn't a perception of temperature, its a perception of heat flow either into or out of your skin, which is dependent not only on the relative temperature difference between your skin and the fabric, but also on the relative difference in properties between the materials involved with regard to how heat energy affects them, aka specific heat. --Jayron32 05:09, 15 February 2010 (UTC)
about the wounds[edit]
why the wounds get,s dark blue coulour after ome time?? —Preceding unsigned comment added by 200.55.135.211 (talk) 05:16, 15 February 2010 (UTC)
- Wikipedia contributors cannot provide medical advice. The best we can do is direct you to articles like bruise which may contain information on the topic, but we can only tell you that you should not act on any information you find at Wikipedia, but should instead seek the direct advice of a qualified physician or other medical professional. --Jayron32 05:18, 15 February 2010 (UTC)
torro ant poison[edit]
i have carpenter ants. i got torro ant poison BAIT TRAPS there not eating it thou why —Preceding unsigned comment added by 67.246.254.35 (talk) 05:59, 15 February 2010 (UTC)
- If you are not having success using store-bought pest control products to control the ants, you should call in a professional. Carpenter ants, like termites and some other pests, can cause structural damage and should not be taken lightly. I would recommend that you look up "pest control" in your local phone book, and seek a qualified professional. --Jayron32 06:08, 15 February 2010 (UTC)
- Terro is a sweet bait. I suspect that carpenter ants are more interested in wood than fruit or carrion. -Craig Pemberton 06:44, 15 February 2010 (UTC)
do carpenter ants like sweet bait or greasy bait?--Thekiller35789 (talk) 07:10, 15 February 2010 (UTC)
hello?
- Doesn't Craig Pemberton's reply give you the answer to that? --TammyMoet (talk) 20:26, 15 February 2010 (UTC)
- Not really. I'm not an expert, so it is just an informed guess. -Craig Pemberton
- There is another possibility - I know that the ant bait that you buy here in Texas has a relatively short shelf life - if it's more than a few weeks old, it doesn't work. That's not to say that the stuff you are using is like that - I don't know - but it's definitely a possibility. SteveBaker (talk) 23:40, 15 February 2010 (UTC)
- Googling "carpenter ant bait" shows that the University of Minnesota disagrees with Craig [2], and says that carpenter ants don't eat wood, but rather like protein and sugar. That page says 1% boric acid in 10% sugar water (probably close to what Terro is) can be effective, but "is very slow acting and must be constantly replenished". It also says that "carpenter ants have complex food preferences, and some of the sugar-based baits will not be attractive to the ants long enough to be successful". It instead recommends (carefully) drilling holes into the nest and applying pesticides directly, or more effectively, calling a professional to do the job. The University of Nebraska-Lincoln also has a few things to say about ant baits [3], but mentions that baits don't work as well for carpenter ants as for other species. I'm sure there is more information in that Google search - those were just the first two pages I visited. -- 174.21.247.23 (talk) 15:46, 16 February 2010 (UTC)
Is clear a color?[edit]
??? 69.77.247.18 (talk) 06:03, 15 February 2010 (UTC)
- Not really. In computer graphics, opacity is measured on a separate channel from color - see alpha compositing. Whether this meets your definition for "color" is a matter of semantics. Outside of the realm of computers, most people use color to mean that quality which is the perceptual representation of the wavelength of light in the visible spectrum. Again, opacity is a separate quality from color in this context - it has to do with the transmission coefficient or transmittance of a material. Nimur (talk) 06:08, 15 February 2010 (UTC)
- It also depends on how you define "color". In some applications, and under some definitions, it may. Color is a nebulous quantity, and without knowing what context we are talking about, it is hard to discuss it in detail. Though Nimur's answer is pretty good. --Jayron32 06:11, 15 February 2010 (UTC)
- This is more of a linguistics question than a science question - what does the word "colo(u)r" mean - and, therefore, does "clear" count? Well, let's see how Wiktionary defines "color":
- The spectral composition of visible light.
- A particular set of visible spectral compositions, perceived or named as a class; blee.
- Hue as opposed to achromatic colors (black, white and greys).
- ...and some others that obviously don't apply here.
- Well, there is no "clear" in the spectral composition of light which rules out the first two definitions - and the third just serves to say that sometimes when people ask whether something has a color - they mean to exclude black/grey/white.
- So the answer is "No". Clear is not a color. Clear is a property of a material that alters the way we see it - but so is "shiny" and "rough" - but those are not colors.
- The tricky part (and we had this the other day) is to ask what color a clear object is. That's like asking what color a mirror is (the answer to which is "black"...IMHO).
- SteveBaker (talk) 15:31, 15 February 2010 (UTC)
- "Silver" doesn't cut it as a color then? (Or isn't appropriate for mirrors?) -- 174.21.247.23 (talk) 15:35, 16 February 2010 (UTC)
- Silver is not a color. We talked about this extensively in the discussion of the color of mirrors last month. I recommend you read that discussion. SteveBaker (talk) 21:10, 16 February 2010 (UTC)
- "Silver" doesn't cut it as a color then? (Or isn't appropriate for mirrors?) -- 174.21.247.23 (talk) 15:35, 16 February 2010 (UTC)
Java loving roaches[edit]
Hi
I don't know if it's just me, but has anyone ever noticed (those of you who've had problems with cockroaches in your homes) that they seem to have a love for either coffee or something that's inside the coffee. A lot of times when someone has left half a cup of coffee or less overnight, we'd find the next morning that they're inside the cup floating on the coffee... dead and at times there would be more than one in a cup. Now I don't know if they like the coffee that much, but are unaware of something inside the coffee that can kill them or if they like the coffee so much that they drink themselves to death.
Thanks, NirocFX 41.193.16.234 (talk) 10:34, 15 February 2010 (UTC)
- I suspect they drown, rather than drink themselves to death or have adverse reactions. Sounds like you have a solution to your roach problem, too. --Tagishsimon (talk) 10:38, 15 February 2010 (UTC)
No, luckily we sorted them out (for good) in a different manner otherwise they would have probably opened their own Starbucks in our house sooner or later.
Thanks,
NirocFX
41.193.16.234 (talk) 11:23, 15 February 2010 (UTC)
- You might be interested to take a look at caffeine. It acts as a pretty effective pesticide on insects, and that appears to be its primary role in nature. – ClockworkSoul 17:05, 15 February 2010 (UTC)
Scientific experiments on brainwave activity when chanting nam myoho renge kyo[edit]
Have any scientific experiments on changes in brainwave activity been done while chanting nam myoho renge kyo? If so can I see the results please. Please indicate to me by my email <e-mail address removed to protect from spambots> where I can search for and find my question and answer (when it arrives) on wikipedia. Thanks.—Preceding unsigned comment added by 92.12.118.205 (talk) 12:09, 15 February 2010 (UTC)
- I can't lay my hands on any studies at the moment, but this organisation would be the one to contact for further research work: [4]--TammyMoet (talk) 12:33, 15 February 2010 (UTC)
Snowfall in Hawaii[edit]
Has there ever in record history been snow in state of hawaii? (Dr hursday (talk) 12:28, 15 February 2010 (UTC))
- Happens regularly at high elevations. Our article on Mauna Kea says "Snowfall often occurs at elevations above 11,000 feet (3,400 m) during the period from November through March". Gandalf61 (talk) 12:35, 15 February 2010 (UTC)
- I do not know how to make this question second section in question as have seen here but not want to make new question since subject is related] what is the farthest south snow has ever recorded to have fallen in earth? (Dr hursday (talk) 12:59, 15 February 2010 (UTC))
- At the South Pole, obviously! To answer the question I think you are asking, I'm sure it has snowed in the mountains of Ecuador, right on the Equator. anonymous6494 12:59, 15 February 2010 (UTC)
- Wouldn't be so sure of that. Extreme points of Antarctica claims "Antarctica has the world's lowest rainfall average (zero at the Geographic South Pole) and thus is the world's driest continent". That said, it's uncited. edit: I'm assuming snowfall and rainfall are treated the same, I don't know.Vimescarrot (talk) 14:56, 15 February 2010 (UTC)
- That's too big an assumption. They are not the same.--Shantavira|feed me 15:56, 15 February 2010 (UTC)
- The assumption is valid, though. While the article says "rainfall", it actually means "precipitation". See Climate of Antarctica#Precipitation for some details, although it doesn't confirm the zero precipitation at the South Pole, I have heard that before somewhere. --Tango (talk) 20:27, 15 February 2010 (UTC)
- That's too big an assumption. They are not the same.--Shantavira|feed me 15:56, 15 February 2010 (UTC)
- Wouldn't be so sure of that. Extreme points of Antarctica claims "Antarctica has the world's lowest rainfall average (zero at the Geographic South Pole) and thus is the world's driest continent". That said, it's uncited. edit: I'm assuming snowfall and rainfall are treated the same, I don't know.Vimescarrot (talk) 14:56, 15 February 2010 (UTC)
- At the South Pole, obviously! To answer the question I think you are asking, I'm sure it has snowed in the mountains of Ecuador, right on the Equator. anonymous6494 12:59, 15 February 2010 (UTC)
- There is a good photo in Andes that shows the reason for lack of snow in the Andes of Ecuador is the desert conditions. Being a desert doesn't mean that there is absolutely no precipitation. There is some. Above 10,000 feet, that precipitation will often become snow - snow on the equator. -- kainaw™ 15:05, 15 February 2010 (UTC)
- Mount Kilimanjaro is 3 degress from the equator, and is known for its snows. (C.f. The Snows of Kilimanjaro). --Jayron32 16:28, 15 February 2010 (UTC)
- Right now it might be - but give it a dozen more years and it won't be known for that anymore. Global warming... SteveBaker (talk) 23:31, 15 February 2010 (UTC)
- I thought it was "Climate Change", not "Global Warming" in order to hedge your bets. Googlemeister (talk) 14:43, 16 February 2010 (UTC)
- It's not about hedging bets, it's about a (failed) attempt to head off the folks who say "Global warming?!? But it is currently cold outside!! Haha! Stupid scientists!"
- "Global Warming" gives the (false) impression that one day the planet will be sweltering hot from pole to pole, when they're really just talking about a few degrees overall worldwide. APL (talk) 22:21, 17 February 2010 (UTC)
- Not to belabor the point, but certain scientists have recently found themselves in hot water over specific pseudoscientific claims about particular melt rates for particular mountains... In light of this, one might want to use caution when forecasting a particular glacial melt rate within the next dozen years for Kilimanjaro unless it's attributed to a realistic and scientific model. The last thing we need now is misinformation and reputation-damaging inaccuracy to cloud the very real and important science which does back up actual anthropogenic climate change. Not every glacier is melting, and not every mountain will be barren by 2030! Here is a report - Kilimanjaro Glaciers..., (Geophys. Research Letters, 2006), which discusses the retreat rates and implications therein. The glaciers are retreating, but predicting a rate is, as always, very difficult. "This strong imbalance can only be explained by a sudden shift in climate, which is not observed in the early 20th century. Results suggest glaciers on Kilimanjaro are merely remnants of a past climate rather than sensitive indicators of 20th century climate change." Nimur (talk) 01:23, 16 February 2010 (UTC)
- And here's an article from American Scientist that's a fun and easy read![5] Mac Davis (talk) 08:32, 18 February 2010 (UTC)
- I thought it was "Climate Change", not "Global Warming" in order to hedge your bets. Googlemeister (talk) 14:43, 16 February 2010 (UTC)
- The Andes cross the Equator too, and are snow-capped. BrainyBabe (talk) 17:40, 17 February 2010 (UTC)
- Right now it might be - but give it a dozen more years and it won't be known for that anymore. Global warming... SteveBaker (talk) 23:31, 15 February 2010 (UTC)
Chess[edit]
Hello i have always thought game of chess would be such that first player could win by analyzing possible moves and all possible responses to that move and so on is this possible to do if so could not a computer just be programed to analyze the possible paths and pick the move on the path where victory is certain and repeat until victory? also could this not also be true about other much more complex competion games even one like starcraft where evaluation of all possiblities of all avalible actions be allow to pick the path or branch that lead to definate victory? (Dr hursday (talk) 12:49, 15 February 2010 (UTC))
- The problem is that "all possible moves" is way too many. I'm pretty sure that if you stored each possible chess game on a single atom, you'd have enough to build several extra universes. Your intuition that chess is theoretically solvable is correct, in game theory, it's known that games like Chess and Go (though not games like Starcraft, because the players don't have complete information (and Starcraft might also have random events)) are solvable, and therefore either white or black can force a win every time. It's just utterly intractable to figure out which one, or how.
- One cute example is the game Hex, in which mathematicians were able to prove that the first player can force a win; but the proof was non-constructive, meaning that they don't know how. Paul Stansifer 13:11, 15 February 2010 (UTC)
- See computer chess, combinatorial explosion, game complexity and solved game. The most complex game that has been weakly solved (i.e. final result is known from starting position assuming perfect play on both sides) appears to be Nine Men's Morris with a game-tree complexity of 1050. Chess has a game-tree complexity of at least 10120 (some estimates are higher), so a complete solution is a long way out of reach. However, chess end-game positions with up to five or six pieces can be exhaustively analysed by a computer. Gandalf61 (talk) 13:29, 15 February 2010 (UTC)
- Note that Deep Blue worked, in part, by analyzing millions of possible results from its moves, picking the best ones. It was nowhere near the ability to analyze all possible moves, but it is along similar lines of thought. --Mr.98 (talk) 14:29, 15 February 2010 (UTC)
- Chess computers work by searching as many moves ahead as they have time for - then using a (typically crude) estimate of the "value" of each of the positions that they end up with. Typically they'll count things like the value of the pieces each side has, the number of pieces that are pinned - enable to move without another piece being vulnerable - and measurements like how much control of the center of the board they have. The best programs then search the most promising moves more deeply still. But they certainly can't search exhaustively more than a few moves ahead. Just think about it - there might be a dozen pieces you could move - and many of those could move in half a dozen ways - so there might easily be 50 possible moves at any given time. Each of those 50 moves might have 50 possible responses - so to search N moves ahead requires you to search 50N positions! To search just four moves ahead (you play, he plays, you play, he plays) requires you to evaluate 6 million possible outcomes! Even if the final evaluation function is rather simple, that's going to take a typical computer maybe a minute or so. Pushing it just one more move to evaluate 5 pushes the time out to an hour and going to six moves might easily take the computer a couple of days! SteveBaker (talk) 15:18, 15 February 2010 (UTC)
- Wikipedia has an article, of course : First-move_advantage_in_chess. It's pretty in depth, but basically, since Chess is not yet a solved game, we don't know for sure if there's an unbeatable solution for the white player. APL (talk) 15:51, 15 February 2010 (UTC)
- As far as I'm aware, no one has proved even that chess is not an outright win for Black. Whether we can be said to "know" that chess is not a win for black depends to some extent on what you mean by know. That happens a lot. I'm quite happy, for example, to say we know that Goldbach's conjecture is true, even though no one has a proof from any widely accepted axiom set.
- That's a bit of a digression, of course, as it's my understanding that the possibility that chess is a draw with perfect play is one that many experts believe; I have never heard of one who thinks it's a win for Black. --Trovatore (talk) 22:32, 15 February 2010 (UTC)
Playing a complex game at competition level demands exceptional mental resources (video). Cuddlyable3 (talk) 19:00, 15 February 2010 (UTC)
Malt Beverages and brewers yeast[edit]
Does Mike's Hard Cider (a malt beverage) contain brewers yeast? Is yeast used in the making of all malt beverages? —Preceding unsigned comment added by 75.38.232.192 (talk) 12:56, 15 February 2010 (UTC)
- Malt has the answers to your questions. Malting is about getting the grains to sprout, then drying them. It isn't about using yeast. 86.176.48.57 (talk) 13:05, 15 February 2010 (UTC)
- They probably do use brewer's yeast. Pretty much any fermented drink uses brewer's yeast, as bread yeast tends to give the drink a weird (bread-like, not surprisingly) taste. If you make your own hard cider or ginger beer, it is likewise best to use brewer's yeast. That said, I don't have any specific knowledge of Mike's Hard Cider. (For all I know, they could take cider, add a shot of grain alcohol and carbonate with seltzer). — Sam 76.24.222.22 (talk) 14:17, 15 February 2010 (UTC)
- Is cider a "malt beverage?" In the UK, cider is purely fermented apple juice - no malted grain involved. Scrumpy ciders tend to only use the natural wild yeasts found on the skins of the fruit for fermentation purposes. --TammyMoet (talk) 14:50, 15 February 2010 (UTC)
- I was confused about this too, but apparently Mike's whole line is "malt beverages." I don't really understand why you would have malt in hard cider, though, and even one of the home-brew recipes I saw purporting to be a clone of "Mike's Hard Cider" used just cider. In the US, Woodchuck cider is made with just cider. Anyway, whatever is being fermented (if it's actually being fermented, which I assume it is), they would probably be using brewer's yeast, although I guess naturally-occuring yeasts might be found on some of the more high-end stuff. — Sam 76.24.222.22 (talk) 15:05, 15 February 2010 (UTC)
- I can't find anything called "Mike's Hard Cider" online, except home-made recipes. There is a Mike's Hard Spiced Apple, but it isn't part of Mike's Hard malt line. Could you provide details of this drink? --Tango (talk) 15:53, 15 February 2010 (UTC)
- An expert on beer brewing told me that Champagne yeast is what I should use to brew hard cider. I have also had hard cider which fermented only from the wild yeast present on the apples before they were chopped and pressed. That was before all cider had to be sterilized. See Allexperts.com. Edison (talk) 19:37, 15 February 2010 (UTC)
INFORMATION ABOUT ENGINEERING VARIOUS EXPERIMENTS[edit]
I want know about applications and uses in industies and in day to day life of 1.Double purchase crab,2.Compound pendulum,3.Polygon law of forces,4.Worm of worm wheel —Preceding unsigned comment added by Ashlesh dahiwale (talk • contribs) 13:02, 15 February 2010 (UTC)
- Some of your terminology is a little archaic (or maybe it's region-specific). You might find more information if you search for winch instead of crab. We have an article on compound pendulum - the first "application" I think of is a robot manipulator arm like a Canadarm or a backhoe. I don't know what you mean by polygon law of forces. Finally, we have an article on worm drive. If you can ask a more specific question, we can give you more specific answers. Nimur (talk) 14:10, 15 February 2010 (UTC)
- Do you mean "Grab"? If so, then I think that this is normally called a "Clamshell grab". See Grab (tool)
- I can't imagine any uses for a free-swinging compound pendulum. (See Double pendulum). As Nimur says though, there are many double-linked mechanical structures that would be considered to be a compound pendulum if left to swing freely.
- I suspect you mean Parallelogram of forces - a method of adding together forces.
- Do you mean a Worm drive?
- SteveBaker (talk) 15:03, 15 February 2010 (UTC)
- 1. See [6]
- 2. See Earthquake_engineering#Tuned_mass_damper
- 3. See Pantograph
- 4. See Machine head
- Cuddlyable3 (talk) 18:53, 15 February 2010 (UTC)
- I think he really did mean double-purchase crab - one of these. It is a "crab" because the drive crank turns a large-diameter axle shaft which is actually doing the winding; and it is "double purchase" apparently because there are at least two gear reductions. This is some really arcane terminology. Nimur (talk) 19:00, 15 February 2010 (UTC)
Can soda cans rust?[edit]
Hi all,
I was thinking of using cut-open soda cans as a roof to my chicken coop, after seeing an instructible on it. However, my girlfriend says that it would eventually rust. I say that aluminum doesn't rust, and that soda cans have a plastic lining and then a hard layer of aluminum oxide. She says we've all seen rusted cans.
So... will the soda cans rust? Will they corrode in some other way? Thanks! — Sam 76.24.222.22 (talk) 13:59, 15 February 2010 (UTC)
- "Rust" specifically refers to the formation of iron oxide. Used loosely, the terminology can also mean "the equivalent oxidation reaction as it applies to other metals." Aluminum forms aluminum oxide - which is known as a "self-limiting" oxide. In other words, the outermost layer of "rust" (oxidized aluminum) is impermeable and forms a protective coating which slows the rate of oxidation to the rest of the metal to almost negligible rates. (The introduction to our aluminum article discusses this formation of a passivation layer). In addition, any clear-coat or paint on the can will also slow the reaction. So, the simple answer is that the cans will not corrode for a very long time. Nimur (talk) 14:13, 15 February 2010 (UTC)
- Three sorts of cans: 1) all ferrous - tend not to be used for "soda"; 2) ferrous ends and aluminum sides - increasingly uncommon - will rust at the ends; 3) all aluminum - what Nimus said - essentially will not rust. Choose your can wisely. --Tagishsimon (talk) 14:18, 15 February 2010 (UTC)
- Although aluminum can predominate in the U.S. that is a recent deveolpment and not true worldwide. 75.41.110.200 (talk) 15:43, 15 February 2010 (UTC)
Remind me someone, why can't we have aluminium mufflers (UK: silencers) for cars? Cuddlyable3 (talk) 17:26, 15 February 2010 (UTC)
- Because aluminium is only stable in an oxygen environment. In a reducing environment (like car exhaust) the alumina coating gets reduced back to aluminum. And it crumbles. Put some aluminium foil in a flame and you can see it happen. (It also doesn't have a great melting point. Not sure how hot exhaust gets though.) [OK, so I made all that up, rather than learned it. I think I'm right though.] Ariel. (talk) 11:01, 16 February 2010 (UTC)
- I'll point out that (for aluminum cans) if a corrosion problem does develop, it will likely only affect one easily replaced can/tile. I'd make sure, however, to seal the cans first - don't want water to get inside the can and create small pockets of stagnant water. --Ludwigs2 17:53, 15 February 2010 (UTC)
- Soda cans won't rust. But regular cans will. Your girlfriend probably saw rusty cans. Ariel. (talk) 20:43, 15 February 2010 (UTC)
- In the US, at least, "regular" cans are often referred to as "tin cans". They're not actually made of tin, but rather they're tinplate or galvanized steel. The thin tin/zinc coating is a protective layer to prevent rusting. After a while, though, the coating wears through, and the steel underneath rusts. -- 174.21.247.23 (talk) 15:33, 16 February 2010 (UTC)
Radar silence[edit]
I heard or read somewhere that battleships sometimes go into "radar silence". If the location of various fixed radio transmitters on land was known, would it be possible to construct (using a lot of computing power I imagine) an image of the surroundings, due to those radio waves being distorted by objects? 89.240.201.172 (talk) 15:10, 15 February 2010 (UTC)
- Yes, but from the top of my head, I think you'd only get mountains and such, that you already know is there. The reason is the wavelength of radio transmissions, they'r too long. EverGreg (talk) 15:24, 15 February 2010 (UTC)
- It's not exactly battleships, but aircraft could achieve "radar silence" (I assume you mean not appear on radar) by using the cone of silence. Ks0stm (T•C•G) 15:54, 15 February 2010 (UTC)
- Few radars would use as low a frequency as 30 MHz (Wavelength=10 metres). See Radar#Frequency_bands. You would need rather large dishes for it to be practical at such a frequency. If a battleship goes 'quiet' it would be more to stop an enemy picking up their RF transmissions. It sounds a little like passive sonar, which works for submarines as all vessels give off some sound, but not everything gives off RF. Or reflects it in a predictable manner for analysis. Anything is possible, but I don't think it would be practical. —220.101.28.25 (talk) 16:17, 15 February 2010 (UTC)
- Indeed, "radar silence" is a matter of EMCON. Mind you, radar silence does not equal radio silence; radio transmissions and communication can be too crucial for the mission, and may continue. It is true that all emissions (both the typical radar and radio) can be picked up, and used by ELINT forces to triangulate and position the source. A notable difference is that radars are easily picked up by modern fighter aircraft. As such, the practise of "radar silence" is popular in scenarios where one does not have air superiority, and the enemy is likely to have control of the airspace. Standard radio transmissions, it should be noted, do not appear on any ordinary fighter jet. 77.18.11.24 (talk) 16:49, 15 February 2010 (UTC)
Some work has been done on obtaining an image of surroundings by analyzing reflection (not distortions) of existing fixed televison transmitters. Commercial television transmitters operate at VHF and UHF frequencies so resolutions of a few meters are expected, enough to distinguish a vehicle. For calculation one needs a database giving transmitter locations and frequencies. It needs information on the timing of the TV modulations. Only the horizontal blanking part of the TV waveform can be used. While the idea is workable it is unlikely to be of military use because of the long integration times needed to get usable signal/noise ratio, impenetrable shadow areas and the dependance on civilian TV. Cuddlyable3 (talk) 17:20, 15 February 2010 (UTC)
- Don't forget the utility of bistatic radar. A battleship can be totally passive, while a buoy, ELINT aircraft, ground-based target illuminator, satellite, or indeed any other source somewhere very far away, shines up the sky a little. The observers on the battleship can thus see everything while never broadcasting from their own location. As far as 30 MHz RADARs, they do exist, but they're of limited practical use for most purposes. I believe some civilian aircraft use such HF bands for electronically assisted "docking" (estimating closing-distance) with a jetway. Also worth pointing out the HAARP (a research RADAR), which operates at HF, and uses a variety of tricks to modulate up or down to a desired operating frequency that is usually out of band for most other RADARs. Nimur (talk) 18:15, 15 February 2010 (UTC)
- Yes, it's possible. You can use cell phone base towers (with known positions) to do that. They were able to detect airplanes. Here: [7] [8] [9] and of course Passive radar. Ariel. (talk) 20:40, 15 February 2010 (UTC)
Physics for launching rockets.[edit]
I am not actually against chemistry but can we use physics to launch the rockets. I mean due to chemical reactions the force is produced which helps the rocket to launch in its orbit but can we create such type of force by physics. I mean we can use a force something like which produces equal(''more than equal'' to launch)(''equal'' for hovering in space) and opposite to the gravitational force which may save the environment being polluted. —Preceding unsigned comment added by Itsrohit (talk • contribs) 17:24, 15 February 2010 (UTC)
- There are several ideas like that. The mass driver is a common one. Non-rocket spacelaunch has some others too. Staecker (talk) 17:39, 15 February 2010 (UTC)
- Not really, no. If we could launch rockets without burning fuel we'd already be doing it.
- Rockets can't be launched into orbit balistically (like, from a giant cannon or slingshot) because the starting speed would have to be so incredibly fast that they would burn up in the air.
- You could do other non-chemical rockets, like pressurized water or steam. But none of those options are currently practical. APL (talk) 17:44, 15 February 2010 (UTC)
- Personally I would take a little more care to differentiate between that which is practical and that which is possible. You seem to have blurred them together in your response. There are a number of methods that might prove possible, and research is being done into them, especially if nano-materials become more readily available and so forth. Whether they will be practical (or economical) is a different question altogether. Whether we can do them at the moment does not reflect on their possibility. To assume our current way of doing it is the only or best way to do it seems a little short-sighted to me, and puts a little bit too much faith in the giant bureaucracies and historical contingencies that have led us to our current situation. --Mr.98 (talk) 17:58, 15 February 2010 (UTC)
- Ultimately, a rocket works by throwing off mass at some velocity (and thus conserving momentum). The idea of using an inert gas blowing out the back of a rocket has been floated around many times. Reaction control systems often use an inert-gas pressure reservoir, or an electric heater to pressurize a tank of otherwise inert gas. When safety is an issue, this is a great way to ensure controlled release of energy. (Toy rockets often use a bicycle-pump to compress air; some even use a CO2 cartridge). But this approach limits the maximum exhaust velocity because the only source of energy is the Pressure-Volume product of your inert gas. A few off-the-wall ideas have suggested carrying an inert gas like nitrogen or argon and pressurizing it (adding energy) by heating it from another source (e.g. a nuclear fission reactor). The goal is of course to reach higher pressures, hence higher exhaust velocities, than you would by simply ejecting the stored gas. Similarly, ion engines use electric- or electromagnetic energy to get a very tiny mass of propellant flowing out at extraordinarily high velocities.
- Fundamentally, if you are using an impulse engine (e.g. propelling the rocket by conserving momentum), you want the product of (exhaust mass) x (exhaust velocity) to be very large. Typically, the best way to do this is to impart as much velocity (ergo, as much energy) as possible to the fixed mass of propellant that you're carrying. So far, the safest and most efficient way to energize that exhaust gas is via chemical reaction. This serves a double-whammy - you use the stored chemical energy AND mass stored in your propellant to improve your impulse product. Any other scheme you can think of to get momentum flowing out the back end of a rocket will work, but it might be dangerous, inefficient, or impractical with today's technology. Nimur (talk) 19:12, 15 February 2010 (UTC)
- Build a Space elevator. then you can use physics rather than chemistry to get rockets into space.--SPhilbrickT 22:00, 16 February 2010 (UTC)
- Personally I would take a little more care to differentiate between that which is practical and that which is possible. You seem to have blurred them together in your response. There are a number of methods that might prove possible, and research is being done into them, especially if nano-materials become more readily available and so forth. Whether they will be practical (or economical) is a different question altogether. Whether we can do them at the moment does not reflect on their possibility. To assume our current way of doing it is the only or best way to do it seems a little short-sighted to me, and puts a little bit too much faith in the giant bureaucracies and historical contingencies that have led us to our current situation. --Mr.98 (talk) 17:58, 15 February 2010 (UTC)
- We do use physics to get rockets into space. Rockets by definition, are things that take advantage of the fact that every action has an equal and opposite reaction. Rockets release mass from their nozzle and the equal and opposite reaction propels them forward. The faster the mass is expelled from the rocket, the more efficient the rocket is. Some simple rockets use only pressurized gas but this is far less efficient than burning a fuel and oxidizer in a combustion chamber and releasing the extremely hot and pressurized result.72.244.204.138 (talk) 00:11, 20 February 2010 (UTC)
Iron sulphide[edit]
Is iron sulphide a metal or a non-metal? —Preceding unsigned comment added by Tikasara (talk • contribs) 17:52, 15 February 2010 (UTC)
- I may not have this right (not a chemist), but I believe iron sulfide would be considered a salt. --Ludwigs2 18:06, 15 February 2010 (UTC)
- "metal" vs "non-metal" is usually a description of an element, not a compound. Please read your homework and textbook carefully to figure out what you really are dealing with. DMacks (talk) 18:07, 15 February 2010 (UTC)
- According to our metal article, there are metallic compounds as well as metallic elements. However, iron sulphide doesn't seem to be one. It also isn't a salt -- salts break up into their constituent ions when dissolved in water, and iron sulphide doesn't. Looie496 (talk) 18:25, 15 February 2010 (UTC)
- Yup, that's some good Nobel work in the metallic-compounds arena. Usually high-school-age science classes (and even into intro college) teach a dichotomy between "metal and nonmetal elements" as a way of categorizing them by certain properties and seeing patterns in the layout of the periodic table. Once they get into more advanced classes, the whole idea becomes fuzzed, as "metal" elements become anionic, compounds of non-metals have metallic properties, etc. I hate questions with no context:( DMacks (talk) 18:43, 15 February 2010 (UTC)
- According to our metal article, there are metallic compounds as well as metallic elements. However, iron sulphide doesn't seem to be one. It also isn't a salt -- salts break up into their constituent ions when dissolved in water, and iron sulphide doesn't. Looie496 (talk) 18:25, 15 February 2010 (UTC)
Broken glass[edit]
Hello! When someone goes into hospital with pieces of broken glass lodged in them, what methods are used to remove the glass? How do they know that they have got all the glass out of the person? Has it ever occured that someone has been walking around with a piece of broken glass in their foot that was overlooked by the surgeon, for example? What happens in this instance? Ice Pencil Made of Glass (talk) 18:02, 15 February 2010 (UTC)
- Tweezers. Cuddlyable3 (talk) 18:16, 15 February 2010 (UTC)
- Radiographic evaluation of some sort will usually be made prior to suturing of lacerations. Any glass that has entered a wound (including one it has produced) will be removed with forceps of some sort (unless it has entered and then rotated, in which case additional incision(s) may be necessary to properly remove it). DRosenbach (Talk | Contribs) 18:51, 15 February 2010 (UTC)
- This suggests that glass is reasonably visible in X-rays and this says they're also visible in ultrasound. -- Finlay McWalter • Talk 18:19, 15 February 2010 (UTC)
- I suspect that I still have a small piece of glass in my hand from an accident 2000. It's hard to tell because of the scar though. -Craig Pemberton 18:35, 15 February 2010 (UTC)
- very small pieces of glass (or any other material) will be enveloped by a cyst that will protect the surrounding tissue. the cyst may eventually work its way out (it it's close to the surface of the skin), or may remain in the body indefinitely. --Ludwigs2 18:49, 15 February 2010 (UTC)
Note: OP was indef'd for trolling. ←Baseball Bugs What's up, Doc? carrots→ 02:26, 16 February 2010 (UTC)
how?
Do non-spatial countable infinities occur in nature?[edit]
I've been trying to think an example of a countable infinity which occurs in nature. The only examples I have been able to find are rational subsets of irrational uncountably infinite sets occuring in space-time. For example, my meter-stick has a countably infinite number of points along its length such that they fall at the 1/n meters mark. The same sort of argument can be applied to time. That is assuming you don't eventually start violating quantum mechanics. I can't find any other examples. Can you? What is the deeper truth here? -Craig Pemberton 18:20, 15 February 2010 (UTC)
- Oh yeah, forgot to say this; things like pressure and force are derived from space-time dimensions so they don't really seem instructive. -Craig Pemberton 18:22, 15 February 2010 (UTC)
- There is a story about an alien who visited Earth to collect from libraries the total of human knowledge. To save space the alien digitised the data as a single binary number. To carry the number home the alien took a metal bar and scribed a mark at the point on its length where the ratio to the whole length equalled the number. Cuddlyable3 (talk) 18:32, 15 February 2010 (UTC)
- In exchange for such a nice story, I'll share one too.-Craig Pemberton 18:47, 15 February 2010 (UTC)
- I did some math on your alien story, using quartz as the bar (I think it would have to be a crystal), each molecule of quartz weighs 9.9772x10^-23 g, the linear density is 1.3809 g/cm, so you have 1.384x10^24 atoms in a meter of quartz, the log base 2 is 80.1, so you have about 80 bits of information in your rod. Not much. You would do much better to use the atoms as bits, and notch the rod multiple times. Hope I did the math right. Ariel. (talk) 20:24, 15 February 2010 (UTC)
- In exchange for such a nice story, I'll share one too.-Craig Pemberton 18:47, 15 February 2010 (UTC)
- There is a story about an alien who visited Earth to collect from libraries the total of human knowledge. To save space the alien digitised the data as a single binary number. To carry the number home the alien took a metal bar and scribed a mark at the point on its length where the ratio to the whole length equalled the number. Cuddlyable3 (talk) 18:32, 15 February 2010 (UTC)
- There are some countable infinities in our best current physical theories. For example, there's a countable infinity of galaxies/stars/atoms in ΛCDM, and bound quantum systems have a countable infinity of energy levels. But there's nothing in nature that we know for sure to be infinite, countably or uncountably. Just because we model spacetime as a continuum doesn't mean it is a continuum. Every other physical system that we model with calculus is known to actually be discrete at some level (individual molecules, individual members of a population, thousandths of a dollar). -- BenRG (talk) 19:47, 15 February 2010 (UTC)
- The inescapable reciprocal of that statement, though, is that "just because we model quantum mechanics as a discrete system doesn't mean it's actually discrete." It just so happens that our discrete model matches our observations very well. The universe simply is - its reality is independent of our attempt to apply abstract descriptions about continuity or discreteness to it. Nimur (talk) 15:09, 17 February 2010 (UTC)
- Is the universe definitely infinite in ΛCDM? I know it is flat, but our article doesn't say anything about whether it is infinite or not. Could you not have, say, a toroidal universe? --Tango (talk) 20:00, 15 February 2010 (UTC)
- I'd say that, technically, ΛCDM describes a spatially infinite universe. To describe a toroidal universe you'd need extra parameters governing the size and orientation of the torus, and ΛCDM doesn't have those parameters. But hardly anybody takes this "prediction" of ΛCDM seriously, which was my point. It's not a very good example, but I can't think of any others. -- BenRG (talk) 00:47, 16 February 2010 (UTC)
Antibiotic vs. antimicrobial[edit]
What is the source for the distinction given to anti-bacterial products, such that (at least in the US) would be strictly referred to in general as an antibiotic (such as penicillin) while others would be referred to as an antimicrobial (such as chlorhexidine). I've read the linked article (sections) but don't seem to grasp why chlorhexidine is rarely is ever called an antibiotic rinse, even though it is one. DRosenbach (Talk | Contribs) 18:39, 15 February 2010 (UTC)
- Is it, perhaps, that via their respective methods of activity, acquired bacterial immunity to antiseptics is impossible? DRosenbach (Talk | Contribs) 19:04, 15 February 2010 (UTC)
- Our article on antibiotics narrows the definition to include only compounds which have (specifically) antibacterial effect. Antimicrobials would include antibiotics as well as agents which kill other pathogenic organisms (fungi, protozoans) in addition to bacteria. TenOfAllTrades(talk) 19:15, 15 February 2010 (UTC)
- The first paragraph of antiseptic may provide the explanation:
- "Antiseptics are generally distinguished from antibiotics by the latter's ability to be transported through the lymphatic system to destroy bacteria within the body, and from disinfectants, which destroy microorganisms found on non-living objects. Some antiseptics are true germicides, capable of destroying microbes (bacteriocidal), whilst others are bacteriostatic and only prevent or inhibit their growth. Antibacterials are antiseptics that have the proven ability to act against bacteria."
- Essentially, the difference is that antibiotics are indicated for parenteral use rather than just topical use. Chlorhexidine may well have antibiotic properties in that it is a bacteriocide as a rinse, but it is contraindicated for use inside the body's tissues. I think. Someone correct me if I'm wrong, but I just had a huge, dull lecture on this. Regards, --—Cyclonenim | Chat 11:58, 18 February 2010 (UTC)
- Short answer to the original question: antibiotics are a subset of antimicrobials. -- Scray (talk) 02:20, 19 February 2010 (UTC)
Alpha Centauri[edit]
I am working on something at the moment and I really want to know a star which is near to the Alpha Centauri star system? Don't answer 'Our sun' or 'Proxima Centauri', I'm looking for something different. Thanks.--Editor510 drop us a line, mate 19:16, 15 February 2010 (UTC)
- So I guess you want a list like this but for alpha centauri rather than us... Maybe some astronomy software can calculate that? TastyCakes (talk) 19:22, 15 February 2010 (UTC)
- Centauri A, Centauri B, and Proxima are in the star system. Are you looking for what is the next closest system to this system? Beach drifter (talk) 19:23, 15 February 2010 (UTC)
- How about Beta Centauri? Beach drifter (talk) 19:25, 15 February 2010 (UTC)
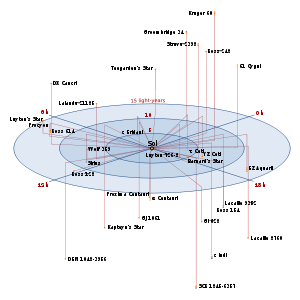
- This map may help. It is a little difficult to tell which star is nearest, because it's a 2D map of a 3D space, but Barnard's Star and GJ 628 look fairly close. --Tango (talk) 20:11, 15 February 2010 (UTC)
- Alpha Centauri also known as Rigel Kentaurus is a triple system (stars -A, -B and -C) of the closest stars to the Sun, about four light-years distant in the constellation Centaurus. I think Proxima Centauri, the closest star to the sun at 4.26 light-years, is a part of this star system. The visually closest stars marked in Google Earth Sky View are NGC5817, HD127724, HD131342 etc. all of which are closer than Beta Cantauri or Hadar. Cuddlyable3 (talk) 20:26, 15 February 2010 (UTC)
Map of very closest stars. Cuddlyable3 (talk) 20:32, 15 February 2010 (UTC)
- If my inexpert mucking around with Celestia is to be believed, the nearest stars to the Rigel Kentaurus system are the Sun (4.4 ly), Barnard's Star (6.5 ly), Ross 154 (8.1 ly), Wolf 359 (8.3 ly), SCR 1845-6357 (binary, 8.9 ly), and Sirius (binary, 9.5 ly) Paul Stansifer 00:24, 16 February 2010 (UTC) P.S. Celestia is free software and relatively easy-to-use, so if you like space, you might like it.
- I think the gravitational "spheres of influence" of the Sun and Alpha Cen overlap, so that a small object travelling through interstellar space could be gravitationally affected by both stars at the same time. ~AH1(TCU) 02:01, 16 February 2010 (UTC)
- Well, strictly speaking the gravitational "spheres of influence" of all bodies (within each others light cones, to be pedantic) are overlapping and objects will be influenced by all other objects. What is quite likely true, however, is that the regions of space where the sun's gravity is the dominant force and the region where Alpha Centauri's gravity is are directly adjacent. --Stephan Schulz (talk) 10:04, 16 February 2010 (UTC)
- "Sphere of influence" usually means the sphere where the influence is dominant. For a planet, that's the Hill sphere. The same concept applies to stars, but it's more complicated due to there not being a single massive object that the star is orbiting. --Tango (talk) 13:37, 16 February 2010 (UTC)
- Well, strictly speaking the gravitational "spheres of influence" of all bodies (within each others light cones, to be pedantic) are overlapping and objects will be influenced by all other objects. What is quite likely true, however, is that the regions of space where the sun's gravity is the dominant force and the region where Alpha Centauri's gravity is are directly adjacent. --Stephan Schulz (talk) 10:04, 16 February 2010 (UTC)
- Your results appear to largely agree with this source of stars within 10 light years except that lacks SCR 1845-6357 I guess because it not yet been fully included in their database [10] and includes Epsilon Indi which is 9.7 and I guess you didn't include because you stopped at Sirius. The database Celestia uses evidentally contains 120k stars, I'm not sure how complete it is for near earth stars particularly non visible ones but it doesn't seem there can be that many missing that we currently know of. For example GJ 1061 is not closer then 10 light years to alpha centauri [11]. Note sure about GJ 628 which Tango mentioned above but I doubt it's within 10 either (while lacking a page, it's not particularly recent so would probably be in the earlier list I guess). In any case anything that isn't with ~14.4 light years of sol, as [12] for example can be presumed to be further then 10 light years I think. Nil Einne (talk) 16:39, 16 February 2010 (UTC)
- I think the gravitational "spheres of influence" of the Sun and Alpha Cen overlap, so that a small object travelling through interstellar space could be gravitationally affected by both stars at the same time. ~AH1(TCU) 02:01, 16 February 2010 (UTC)
Humans to become extinct[edit]
Is this possible for humans to extinct? Ice Ages usually whites out alot of species, to FIW 5 million years from now we suppose to have another ice age, they will wipe out alot of species, or ultil 100 million years when earth is a hot house, posslbe for humans to extinct before next Pangaea even appears?--69.229.36.56 (talk) 20:55, 15 February 2010 (UTC)
- Of course it's possible. Humans themselves would certainly survive another ice age, though our current civilization might be wrecked by a severe one (or severe global warming). In the longer term, Earth itself is doomed; see Sun#Life cycle. If humans accumulate enough technology before our oceans boil away, then we could eventually create generation ships and continue the species. If you believe in the heat death of the universe, then that would be the final endpoint of our species. Comet Tuttle (talk) 21:02, 15 February 2010 (UTC)
- In long term fate 5 to 6 billion years from now, humans won't have any place in our solar system to escape fate. Outer planets would only achieve habitable surface temperature but it would not meet the basic enviornments. That is the problem, unless if humans find another solar system and stars that might work. Dinosaurs have been extinct 65 million years ago though.--69.229.36.56 (talk) 21:23, 15 February 2010 (UTC)
- Yes, of course it is possible. I'm not sure an ice age would wipe out the entire species - we survived the last one and we were just hunter-gatherers then. With modern technology, it would be easy for at least a few million people to survive near the equator. More likely to wipe us out are things like an asteroid hitting the Earth or nuclear war. I don't think either or those are very likely, but they are both possible. --Tango (talk) 21:06, 15 February 2010 (UTC)
- Would you really say that nuclear war would be the most likely of the least likely things that would wipe out humans? I would have guessed a fatal infectious disease. DRosenbach (Talk | Contribs) 21:13, 15 February 2010 (UTC)
- We have a Human extinction article that discusses some possibilities. CS Miller (talk) 21:26, 15 February 2010 (UTC)
- I think there are very few examples in nature of a disease alone wiping out an entire species. It is a risk for species like bananas that don't have enough genetic variation to develop immunity. Most every other disease I can think of doesn't kill 100% of a very large population, for humans the worst case on record was probably the black plague, and that was nowhere near 100% lethal (Ebola is apparently 90% fatal in some outbreaks). Rabbits in Australia were given a disease that killed 5/6 of them, but the remaining 1/6 had some degree of immunity and the population didn't die out, and indeed bounced back. So no, I don't think a fatal infection disease is likely to, alone, kill all humans, but one could be devastating for human civilization. TastyCakes (talk) 21:30, 15 February 2010 (UTC)
- Of course if an organization were to specifically create a series of diseases (say 10 different, unrelated diseases) with high lethality and virulence and intentionally release them, that would be much more likely to wipe out everyone then a single natural disease. Googlemeister (talk) 21:39, 15 February 2010 (UTC)
- That's true, I remember reading somewhere (I can't find it now) about attempts to create a disease for mosquitoes (something like this one) that isn't fatal in the first generation, it requires a certain number of generations to become "active" somehow, and then by the time it becomes fatal the genes have spread to all of the population and they all die. I don't remember the details, I think it may have been that they introduce a vulnerability to another disease that is not present in the wild, somehow release enough mosquitoes with a genetically designed vulnerability to that disease and then when they think that gene is present throughout the population, introduce the second disease. Bioweapons do seem like one of the more likely ways one would go about killing everyone, but like you say, the perpetrator would probably have to intentionally be trying to kill everyone; a case of one bioweapon being used on an enemy and getting out of control would likely not be enough. TastyCakes (talk) 21:57, 15 February 2010 (UTC)
- Of course if an organization were to specifically create a series of diseases (say 10 different, unrelated diseases) with high lethality and virulence and intentionally release them, that would be much more likely to wipe out everyone then a single natural disease. Googlemeister (talk) 21:39, 15 February 2010 (UTC)
- Would you really say that nuclear war would be the most likely of the least likely things that would wipe out humans? I would have guessed a fatal infectious disease. DRosenbach (Talk | Contribs) 21:13, 15 February 2010 (UTC)
- currently, pollution is the biggest threat to the human species. The one thing a species can never adapt to (almost by definition) is the accumulation of its own waste. --Ludwigs2 21:42, 15 February 2010 (UTC)
- The solution to pollution is dilution. Googlemeister (talk) 22:24, 15 February 2010 (UTC)
- Many species adapt to overpopulation - they do it by reproducing less. --Tango (talk) 23:01, 15 February 2010 (UTC)
- currently, pollution is the biggest threat to the human species. The one thing a species can never adapt to (almost by definition) is the accumulation of its own waste. --Ludwigs2 21:42, 15 February 2010 (UTC)
- I'm personally sceptical of any doomsday scenario to the banana, at least not without other human contributions. There are plenty of wild and different bananas (e.g. [13]) although many of these suffer some risks from human activity [14].
- I know there's been a lot of discussion about the risks to banana cultivation [15] [16] [17] [18] [19] and I'm not denying that given the reliance on the Cavendish for export by most banana growers there's a risk of banana cultivation collapsing unless a suitable replacement is found with good yields and which exports well and meets the expectations (taste, texture etc) of consumers and this replacement starts to be used fairly soon; and such a collapse which would have a corresponding catastrophic effect on the economies of countries heavily depend on bananas and may mean the almost disappearance (at least for a time) of the banana from shelves of supermarkets and fruit markets in most non-tropical countries. And panama's disease (and other diseases) is and is likely to continue to spread to other non-resistant varieties (one of the big issues appears to be that it stays in the soil for a long time so cultivating disease free but non resistant bananas in that soil becomes impossible) having a bad effect on both cultivated and probably some wild populations and a corresponding bad effect on people who rely on the banana for reasons other then export (note that according to our article "Almost all export bananas are of the dessert types; however, only about 10–15% of production is for export").
- In other words it's of course possible perhaps even likely that the population of banana trees may drop drastically and this will have a terrible effect on those that rely on the banana for a variety of purposes.
- However this doesn't mean the banana itself will die out. Worst case scenario, the banana may die out as a food crop but some varieties may still survive (particularly in SEA and South Asia) provided deforestation etc doesn't kill these. More likely some cultivation will continue. Particularly since we already have some semi-resistant or resistant cultivars even if they lack the consumer acceptance of Cavendish in many countries.
- IMHO the Cavendish isn't even that good a tasting banana anyway (I like the ms:Pisang Mas). This has some interesting info on Panama's Disease BTW [20]
- Nil Einne (talk) 09:46, 16 February 2010 (UTC)
- There is no scientific reason to think that humanity has anything but extinction in its long-term future, like all species. There is really no shame in that—such is how the universe works. At some point it will run out of energy. I suspect humans will be extinct long before that—we apparently lack the ability to tackle long-term problems in a truly effective way, which will effectively preclude us getting off this rock, and will doom us when the resources run out, the heat turns up, and we go with our usual tendencies of tearing each other apart. Peaceful civilization is, so far, just a blip in the history of our universe, and even it hasn't really ever been very peaceful. --Mr.98 (talk) 22:50, 15 February 2010 (UTC)
- There are plenty of species that have survived for a very long time and show no signs of dying off. You also need to be careful to distinguish species that went extinct due to being wiped out and those than went extinct due to evolving into a new species. --Tango (talk) 23:01, 15 February 2010 (UTC)
- I think I've read an estimate that humans will most likely become extinct about 2 to 5 million years from now. See human extinction and exitmundi. Also the recent release of clathrates may help to explain the Fermi paradox as suggested on the RefDesk more than a year ago. ~AH1(TCU) 01:51, 16 February 2010 (UTC)
- There are plenty of species that have survived for a very long time and show no signs of dying off. You also need to be careful to distinguish species that went extinct due to being wiped out and those than went extinct due to evolving into a new species. --Tango (talk) 23:01, 15 February 2010 (UTC)
- I've wondered why I'm here now instead of being whatever humans evolve into in say another 100 million years. The chances just seem against my being alive at the start of it all rather than later. I see no reason that descendants of humans won't be around then and hopefully they'll be a bit more intelligent than the vandals that beset wikipedia. Dmcq (talk) 04:46, 16 February 2010 (UTC)
- read "The Time Machine". most of the systems administrators I know are already halfway down the path to Morlock-hood, and I run into people every day who could pass for Eloi. it's just a matter of degree at this point, and a bit of a shift down the Soylent Green path... --Ludwigs2 09:40, 16 February 2010 (UTC)
- "extinct due to being wiped out and those than went extinct due to evolving into a new species"—Do I? Why? Extinction is as extinction does, from the point of view of the extinct species. The birds are not the dinosaurs. --Mr.98 (talk) 21:12, 16 February 2010 (UTC)
- The problem then becomes how you decide when and whether a species became 'extinct' due to the the complicated issues we've discussed before of what a species is and how you determine when a species has diverged to become a new species. Humans and chimpanzees (of both kinds) may seem to be clearly different species from our last common ancestor but we obviously never co-existed with said ancestor and the question of when humans became humans, the chimps became their individual species and our ancestor went extinct some may consider meaningless.
- Even under a Punctuated equilibrium model and regardless of which definition of a species you use, it's probably fairly common you can't give a fixed point in time when one species evolved into another, that's not how evolution works. Species that underwent some polyploidy event may seem one obvious general case but even that's not always clear cut Speciation#Speciation via polyploidization and things like chromosomal fusion such as occured with human chromosome 2 even less so [21] [22] [23]).
- In fact the speciation event as it were may simply be some non biological change, such as a seperation of two different populations but it seems rather odd to consider these two different populations different 'species' as soon as they seperate. If some sort of catastrophic event destroys most human populations leaving only a few fragments in different geographically seperated places and no way to regain contact between the two in at least several thousands of years and given the likely major problems each population will have to face in a world which underwent this event, it's easy to imagine speciation could occur. Does this mean you and I are different species now (as I don't believe you live in NZ)? Or the moment after this event if we both survive?
- Some taxonomists prefer to avoid giving species names for extinct (particularly ancienct) species (or at least treat them specially) given these reasons and more. It's worth remembering that unlike what some creationists like to believe, evolution doesn't 'stop'. Even living fossils like the tuatara have changed significantly (whether enough to be meaningfully considered a different species I don't know although I would guess yes and as I mentioned some may consider that a meaningless question anyway).
- P.S. I came across [24] which I didn't read but may be of some interest to this issue.
- Nil Einne (talk) 21:52, 16 February 2010 (UTC)
- Actually, cladists do consider birds to be dinosaurs and say "non-avian dinosaur" when they want to refer to what laymen call dinosaurs. I think the distinction is important because I wouldn't consider the human race evolving to a point where they can't reproduce with current humans to be a bad thing, while all humans dying out would be a bad thing. --Tango (talk) 03:19, 17 February 2010 (UTC)
- "extinct due to being wiped out and those than went extinct due to evolving into a new species"—Do I? Why? Extinction is as extinction does, from the point of view of the extinct species. The birds are not the dinosaurs. --Mr.98 (talk) 21:12, 16 February 2010 (UTC)
Stupid question about molecular symmetry[edit]
Is anthraquinone in the D2h point group? I'm having a real blonde moment and confusing myself with normally comprehensible textbooks......I normally can just work them out fairly quickly in my head, but I've got myself in a tizz. 188.221.55.165 (talk) 21:20, 15 February 2010 (UTC)
- I wouldn't call that a stupid question; crystallography is important. 99.22.95.61 (talk) 01:08, 16 February 2010 (UTC)
- D2h sounds right (assuming no deviations from idealized atomic geometries heh:). The ext-links at Point_group and Crystallographic point group are pretty good examples of all of the different possibilities. Doi:10.1063/1.1841008 says that D2h is either correct experimentally or at least a useful approximation for analyzing experimental structural parameters. DMacks (talk) 22:57, 15 February 2010 (UTC)
Emergency, normal care, etc[edit]
Want to know this because forgot the answer to this and way too lazy to do a search for this. At the same time results for the search are complicated to follow.
Want to know how long does a midwife (all types of midwifes) spend with her patients compared to doctors?
Extended content
|
|---|
|
Had my yearly GYN exam, today with my OB/GYN. Time spent with her was less then 5 minutes and she hardly looked into my chart. Especially after getting BRAC Test done (8/09) and getting positive result back. That means I have a chance of getting breast cancer because of that positive result. By the way my fraternal twin sister got that test done (around the same time as me) because our mother was diagnosed with stage 4 ovarian cancer (almost 2 yr ago, 6/08). Thats a long story and prefer not to get into on her. My mom gone though several rounds of chemo, surgery to remove it, and maintence rounds of chemo as well. In my opinion she gone though more chemo then she should had to and etc. Then, my sister and my friend's mom was diagnosed with breast cancer (9/09 or 10/09), but forgot what stage it was in. After founding a lump in one of her breasts and she already had the surgery to remove it. Shes also undergoing rounds of chemo and radiation theraphy as well. At the same time left parts of our friends mom story out as well. My OB/GYN a new mother (shes was pregnant with it when I 1st saw her, last yr). She worked though the pregnancy, had the baby, and was back at work after being on maternity leave for the minium amount of time. As know of she didn't didn't or intend bf it because of her work and etc. By the way her father is also is OB/GYN as well, but mainly GYN now. Unsure when he stopped with OB part of the practice.
|
—Preceding unsigned comment added by Mybodymyself (talk • contribs) 22:05, 15 February 2010 (UTC)
- I collapsed your medical details because the Reference Desk doesn't give any medical advice, for ethical reasons — suppose we were wrong and you took our advice anyway? If you are uncomfortable with the medical care that you received from the midwife, one option is to get a second opinion from a physician. If you happen to live in the US, I believe most insurance plans cover a second opinion. If it were me, I'd get a second opinion anyway if I was not comfortable with the care received. Comet Tuttle (talk) 22:37, 15 February 2010 (UTC)
- In general midwifes spend longer, but they also deal with less complicated cases than doctors, and their job includes a lot more support, and less "action". In any case, I think you went to the wrong type of doctor for the genetic test. Go to a cancer doctor or a geneticist, not an OB/GYN. Ariel. (talk) 23:14, 15 February 2010 (UTC)
Thank you for your answers and editing the details part of this. Both were interesting. As for where I'm in the US (Metro New York on New Jersey Side of it) its mostly OBs/GYNs, unsure if theres any FP (Family Practioners), some midwifes. Mostly the midwifes are CNMs (certified nurse midwifes) then CPMs (certified proferrisonal midwifes) or CMs (certified midwifes) or other types (which I have no clue what they are).--Jessica A Bruno (talk) 00:17, 16 February 2010 (UTC)
Why does Energy Information Administration predict a fall-off in wind power growth?[edit]
Why is there an inflection point on the wind power projections at about year 2013 on page 21 of this presentation? Given some regions' projections that 30% renewable will be possible in 2020 "with or without legislation" is there any reason to suspect that wind, along with pumped storage hydroelectricity (heavily funded in this year's U.S. Army Corps of Engineers budget, where it is also referred to as flood mitigation capability) and thermal storage will not continue to dominate energy growth world wide? 99.22.95.61 (talk) 22:38, 15 February 2010 (UTC)
- This point was brought up in a discussion last month. Some suggestions were provided, but ultimately, we don't make speculations on the reference desk. If you really want to know, you might want to contact the author of the presentation, who is listed on every page; or follow up with the various sources that are cited throughout that presentation. Nimur (talk) 14:51, 16 February 2010 (UTC)
- After being transferred four times, I spoke with Chris Namovicz in the EIA who said that the National Energy Modeling System, which takes 8-12 hours to run on a fast CPU and produces these projections based on assumptions about the state of the laws in September of each year, always assumes that tax credits for wind will not be renewed (even though they always have been after no more than a year, and when they have expired temporarily, they did not substantially impact the growth rates for new wind) and assumes that fossil fuel subsidies will never be repealed. Chris agreed that there was no effective way to measure the accuracy of the Modeling System's projections, because it doesn't produce projection confidence intervals or expected errors of prediction in its output. Chris referred me to John Conti on that point, who called me back after I emailed, confirming my misgivings about false and unfounded assumptions skewed in the direction of fossil fuel producers on technicality judgements. Conti claimed that discrete economic simulations are incapable of producing projection confidence intervals or expected errors of measurements, and gave no reasons that leaving those out does not destroy the ability to correct the Model in the face of historical outcomes compared against earlier projections. When confronted with my feelings on the matter, Conti asked for the name of my employer. I told him I wanted our future communications to be in writing, and he said he would respond in writing. 99.22.95.61 (talk) 22:09, 16 February 2010 (UTC)
- Huh? flood mitigation capability is not the same as pumped storage hydro. 75.41.110.200 (talk) 17:15, 16 February 2010 (UTC)
- The capabilities overlap. 99.22.95.61 (talk) 20:25, 16 February 2010 (UTC)
Brain damage and pacing in animals[edit]
What is the neuropathology of pacing / walking in circles after some sort of brain injuries in animals (most obviously in dogs and cats)? Is tere a similar phenomenon in humans? What is the clinical term? —Preceding unsigned comment added by 166.137.137.154 (talk) 23:14, 15 February 2010 (UTC)
- Causes of a dog walking in circles. Cuddlyable3 (talk) 23:29, 15 February 2010 (UTC)
- Thanks. I've seen those kinds of articles as well. I'm specifically interested in the details of what brain structures are involved. Those kinds of pages focus on broad explainations like "brain damage". I would like an idea of the clinical terminology amd context so I can search indepth 166.137.137.154 (talk) 23:51, 15 February 2010 (UTC)
- There are a number of interventions that can produce circling when delivered to one side of the brain -- search for "ipsilateral circling" and "contralateral circling" and you'll find quite a number of publications. Structures belonging to the basal ganglia are the best-known, but damage to the vestibular system can also produce circling by altering balance. In humans I believe that circling is rare, but shows up occasionally in cases of hemispatial neglect. Looie496 (talk) 00:02, 16 February 2010 (UTC)
- An unrelated illness, scrapie, causes hopping gait syndrome in sheep and other livestock. Scrapie is a type of spongiform encephalopathy, commonly known as "mad cow disease." Nimur (talk) 14:55, 16 February 2010 (UTC)
- To be clear, scrapie is used to refer to the PrP-caused transmissible spongiform encephalopathy in sheep and goats. Mad cow disease (properly bovine spongiform encephalopathy or BSE) is only used to describe the disease in cows. TenOfAllTrades(talk) 15:12, 16 February 2010 (UTC)
- The term, by the way, for this sort of repetitive behavior is Stereotypy, perusing that article may help the OP and others interested in learning a bit more. --Jayron32 03:13, 17 February 2010 (UTC)
