Wikipedia:Reference desk/Archives/Science/2010 February 16
| Science desk | ||
|---|---|---|
| < February 15 | << Jan | February | Mar >> | February 17 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
February 16[edit]
Very large cube[edit]
What is the largest solid steel cube that could, floating in free space, sustain its shape against the force of its own internal gravity (i.e. avoid the tendency to become spherical)? What solid material would allow the largest such cube? 86.133.247.182 (talk) 00:44, 16 February 2010 (UTC)
- Any cube would deform to some extent under its own gravity, given that stress produces strain and thus some deformation. So. strictly speaking, no steel cube of any size whatever, even 1 cm, could maintain its form in space, with no deformation whatsoever. How much deformation is tolerable? Likewise, it is unlikely that a humongous cube of steel many lightyears across would become a perfect sphere under the deformation of its own gravity. Edison (talk) 01:17, 16 February 2010 (UTC)
- well, I suppose you could reframe this question to read 'how big does a steel cube have to be before its own gravity causes plastic (rather than elastic) deformation, but even given idealized assumptions I wouldn't know where to begin with the calculations. perhaps we should ask the Borg, since they seem to be into the large cube thing.
- Are roche limit and hydrostatic equilibrium relavent? ~AH1(TCU) 01:46, 16 February 2010 (UTC)
- I'm pretty sure that a steel cube many lightyears across would become a perfect sphere with radius zero Paul Stansifer 02:13, 16 February 2010 (UTC)
- My thought too! 86.133.247.182 (talk) 02:20, 16 February 2010 (UTC)
- At 7.8 g / cm^3, a steel sphere would exceed the Schwarzschild radius limit at only 2.7 AU (415 million km). Any cubic solid that is light years across is already doomed to be a black hole. Dragons flight (talk) 05:09, 16 February 2010 (UTC)
- Oops.My hyperbole collapsed under its own weight. I intended just to make the point that as steel cubes became larger and larger they would deform, but as they approached a sphere some small trace of the corners and edges might be seen. Edison (talk) 16:09, 16 February 2010 (UTC)
- @Edison: If you like, turn the question round and ask what would happen to a cube, say, 1,000 miles across, or 10,000 miles across, and so on. I'm only after ball-park ideas. For example, would a cube 1,000 miles across, or 10,000 miles across, or whatever, remain a cube as far as you could visually distinguish from a distance, or would it noticeably sag, or would it be completely mashed? 86.133.247.182 (talk) 02:20, 16 February 2010 (UTC)
- When discussing possible definitions of "planet" some 11 years ago, I came across the following passages in the junior-level The Universe and Planet Earth by Josip Kleczek and Petr Jakeš (Artia, Prague 1985; Translated by Stephen Finn, Octopus Books, London 1987) -
- Figure 48 on p35 shows a crystal, a rock, a 500 km-diameter irregular asteroid and a larger, rounded, internally molten body, with the caption: "Small bodies are kept together by electromagnetic force, large ones by self-gravitation."
- Discussing crystal structure, text on pp58-9 reads: " . . . The energy released by the crystal during its growth is also called binding energy. The greater it is, the more stable the system of molecules - the crystal - is, and the more resistant to external influences. It must be heated to a higher temperature before it melts. Or a greater pressure must be exerted on it to crush the crystal lattice. In the interior of solid bodies consisting of more than 1046 elementary particles (Figure 48) self-gravitation is strong enough to achieve this."
- Text on p61 discussing solar system bodies reads: "Small solid bodies made of rocks, such as meteoroids, the nuclei of comets, small satellites and the vast majority of minor planets, are held together by electrical force. Their mass is small, so their self gravitation is also low. [Various examples elided] Inside solid bodies more than 500 km (310 miles) in diameter the self-gravitation is so great that it breaks up the crystalline structure of the rocks. The solid rock thus becomes a pliable, dough-like material where the pressures in different directions are evened out. The irregularity of the body thus disappears: tall projections are heavy and sink downwards towards the middle while light, thin parts of the body (depressions) are pushed through the doughy material to the top. This is called isostatic equilibrium. The body tries to assume a round shape through its own gravitation (Figure 48)."
- The figures of 1046 elementary particles and 500 k (310 miles) diameter (which I've not come across elsewhere) may be of relevance - I suspect the difference between rock and steel at these dimensions may not be very significant. 87.81.230.195 (talk) 03:32, 16 February 2010 (UTC)
- I beg to differ, only because my exoplanet geology textbook classifies planetary formation geology into three types of material: "things that are like water," "things that are like silica," (rock), and "things that are like metal." (They use a little more technical terminology, but I left my copy in my office, so I'm glossing over the details). Iron is much denser and has a variety of different parameters than rock (melting point, compressability, bulk modulus, electromagnetic effects...). In other words, there is a major qualitative and quantitative difference between rock and steel at the dimensional scales we're talking about here. But, this is all just hand-wavey science anyway... In any case, one could solve for the hydrostatic equilibrium of a 100% metallic planet, as AH1 has linked above, and calculate the minimum mass needed to form a sphere under self-gravitation. Nimur (talk) 04:35, 16 February 2010 (UTC)
The yield stress for steel ranges from ~250 MPa to ~1650 MPa depending on composition. Steel has a density of order 7.8 g/cm3. Using that and the higher yield stress, a solid steel cube could grow to about 650 km in side length before it deforms plastically. As for the largest cube, I think diamond would be in the running (yield stress 35 GPa) and should get you up to about 7000 km in side length.
- Thanks! I'm impressed by the figure for diamond... never thought any solid material could grow that big! 86.134.30.55 (talk) 14:32, 16 February 2010 (UTC).
- Don't we already know those heavenly bodies that don't become spheres? And don't we have an idea of the smallest heavenly bodies that are spherical, and that are likely to have become that way under the stresses of their own gravitational forces? Bus stop (talk) 15:25, 16 February 2010 (UTC)
- Yes, but the OP asked specifically about a steel cube. We are partly arguing about (and avoiding actually calculating, because that would be too much like hard work) how much a steel cube would differ from comparable lumps of rock about which, as you say, we have observational as well as theoretical evidence. 87.81.230.195 (talk) 17:48, 16 February 2010 (UTC)
- Don't we already know those heavenly bodies that don't become spheres? And don't we have an idea of the smallest heavenly bodies that are spherical, and that are likely to have become that way under the stresses of their own gravitational forces? Bus stop (talk) 15:25, 16 February 2010 (UTC)
Effect of temperature change on skiing?[edit]
I'm watching the Olympic men's downhill right now, and the television commentators observed that there was a 5°C temperature difference from the top of the ski hill to the bottom — from 28°F to 36°F. What effect, if any, does this have on the conditions that the skier would notice? Nyttend (talk) 01:08, 16 February 2010 (UTC)
- Just for the record, the recent warm weather is caused by an El Nino and PNA pattern, and Canada is at a lack of snow while the US gets most of the snowstorms.[1] As for the ski hill, I'm guessing that there would be a difference in snow conditions in powder vs. packing. ~AH1(TCU) 01:38, 16 February 2010 (UTC)
- The article doesn't help me very much — I understand that powder is better from the skier's point of view, but is it enough that he'd notice it while going down the slope? However, I'm well aware of the weather at large; I'm in western Ohio, and I spent most of last week shovelling :-( Nyttend (talk) 02:50, 16 February 2010 (UTC)
- The skier likely wont notice a difference in the type of snow encountered (barring any ice that forms on the surface), as race courses are heavily groomed for a consistent surface. That said, the type of wax applied to the ski depends entirely on the snow temperature, and proper wax application is key to trimming those all important tenths of seconds off of times. Wired did a little write up that introduces the subject fairly well -here IMO, the most interesting thing is getting the layering of waxes just right so that the outer layer for colder snow is worn away by the time the skier gets to the warmer part of the course. 161.222.160.8 (talk) 05:10, 16 February 2010 (UTC)
- Powder snow may be great for snowboarding, but for downhill skiers it puts the brakes on damn quick! A downhill ski course must be mainly ice for the skiers to get any sort of speed. Given that, temperatures above freezing would create a film of water above the ice and make things much quicker - and possibly dangerous. For the later skiers, prolonged temperatures above freezing combined with the pressure of the wafer-thin ski blades would mean the piste would rapidly turn to mush, and that's no good for speed skiing at all. --TammyMoet (talk) 12:35, 16 February 2010 (UTC)
- For optimal traction and glide, Ski_wax is applied to the underside of skis. Which type of ski wax is applied is highly dependent on temperature. A single surprisingly bad performance by an athlete skier is often explained (rightly or not) by him or her having had the wrong type of ski wax. Amateur skiers will also be familiar with this problem, sometimes the snow stick to your skis because of the wrong wax. So yeah, the temperature could have a very big impact. EverGreg (talk) 13:05, 16 February 2010 (UTC)
The Planets[edit]
Why are the planets in the solar system so different in composition? Wouldnt the solar dust they were made of have been mixed up, so they should be made of similar material? 78.146.222.3 (talk) 01:44, 16 February 2010 (UTC)
- One would hope that Formation_of_the_solar_system#Formation_of_planets would have the answers you need. Vimescarrot (talk) 02:25, 16 February 2010 (UTC)
- Actually this is fairly similar to question which I've heard is one of the current difficulties in the theory of planetary formation. This is why the elements in the earth are often found in clumps. Like metal ores are generally found in big lumps of one type. No reason has been found yet why it isn't all arbitrarily mixed together. I wouldn't be at all surprised if there was at least some website out there claiming that just because we don't have a theory, must mean god did it. Vespine (talk) 04:10, 16 February 2010 (UTC)
- ...that seems incorrect. Ore Genesis —Preceding unsigned comment added by 24.137.114.204 (talk) 04:23, 16 February 2010 (UTC)
- That's extremely interesting! I only just heard that recently and I thought it was a fairly reliable source, maybe it's some related problem that I just misunderstood. I'll have to see if I can find where it was. Vespine (talk) 04:41, 16 February 2010 (UTC)
- Actually this is fairly similar to question which I've heard is one of the current difficulties in the theory of planetary formation. This is why the elements in the earth are often found in clumps. Like metal ores are generally found in big lumps of one type. No reason has been found yet why it isn't all arbitrarily mixed together. I wouldn't be at all surprised if there was at least some website out there claiming that just because we don't have a theory, must mean god did it. Vespine (talk) 04:10, 16 February 2010 (UTC)
- The short answer is that different elements and compounds are retained by planets of different temperatures and masses. The Earth, for example, is to small to hold on to hydrogen, so the only hydrogen we have is locked up in compounds (eg. water). Jupiter is plenty big enough to hold on to hydrogen, so its atmosphere is about 70% free hydrogen. --Tango (talk) 14:05, 16 February 2010 (UTC)
- Whereas Uranus is mostly filled with gases of various sorts. —Preceding unsigned comment added by 79.76.229.198 (talk) 00:41, 17 February 2010 (UTC)
- LOL 146.74.230.82 (talk) 00:59, 17 February 2010 (UTC)
- So if we could get close enough to Uranus, could we detect ammonia and methane expulsions? What about H2S? Is there any of that gas inside Uranus? —Preceding unsigned comment added by 79.76.229.198 (talk) 13:58, 17 February 2010 (UTC)
If Titan do not heat to earthlike temperatures[edit]
If Titan do not heat to earthlike temperatures and stay below 50 C would Titan keep some atmosphere or at least 3/4 of it would be gone. This forum agrees with us and even said if Titan gets to Earthlike atmoshpere it would just be gray like our moon, I wonder if Titan only gets to -60 C, then probably the atmoshpere would be thin like Mars?--69.229.36.56 (talk) 01:47, 16 February 2010 (UTC)
- I think the question is, if Titan's atmosphere is heated to Earth-like temperatures (possibly due to the expansion of the sun into a red giant), would its atmosphere evaporate from the heat and get thinner? ~AH1(TCU) 02:35, 17 February 2010 (UTC)
Hydrogen[edit]
I have three questions to ask you:
1. Are there any commercial power stations that produce electricity by burning hydrogen?
2. Are there any motorbikes, buses, trucks, trains, boats, ships, submarines, or airplanes, etc, that use hydrogen as a fuel?
3. Are there any cooking appliances, heaters, or water heaters, etc, that use hydrogen as a fuel?
Bowei Huang 2 (talk) 04:13, 16 February 2010 (UTC)
- The article Hydrogen vehicle has a few answers your second question. But mostly experimental stuff. APL (talk) 04:16, 16 February 2010 (UTC)
- BMW has sold an extremely limited number of BMW Hydrogen 7 vehicles. It would be a stretch to call them "commercially available." I'm not aware of any (commercial) power plants that use hydrogen combustion for electric production. A few spacecraft do use hydrogen as a fuel. The big orange part of the Space Shuttle (rather, the "STS", to use the terminology correctly), is mostly a large hydrogen tank. Nimur (talk) 04:24, 16 February 2010 (UTC)
- List of fuel cell vehicles Hydrogen fuel Hydrogen economy. BP had part of a power plant running on hydrogen but they pulled the plug. Italy has the 1st hydrogen power plant due to come online this year. strips hydrogen from methane. question 3, not sure. There is no readily available source of hydrogen yet, so most of those kind of things run on natural gas or electricity. I can't imagine they contribute enough to the problem to be considered a priority for conversion. Vespine (talk) 04:31, 16 February 2010 (UTC)
- (ec)Was the BP plant really burning hydrogen for energy, or was it reprocessing it as part of an enhanced hydrocarbon recovery/refinery project? I see some mention of directly using the hydrogen stream, but given that BP is an oil company, I'd believe it more if they were investing in other uses for hydrogen - both in cracking and desulfuring. Nimur (talk) 04:41, 16 February 2010 (UTC)
- (edit conflict)Hydrogen's potential as a fuel is better realized in its use in a device known as a fuel cell rather than in open combustion, such as in a standard internal combustion engine. Hydrogen has a rather low combustion potential; as actually burning it releases much less energy per gram than does burning most hydrocarbons, and the fact that it is a very light weight gas makes its use in open-combustion applications, like a standard car engine, quite impractical.
- The hydrogen fuel cell, however, is hardly new technology; the basic design concept had been demonstrated in the 1800's and has been in use commercially since the 1950's; hydrogen fuel cells powered all of the electronics on the Apollo missions, and continue to be used by NASA for all of their electricity generation on all of their Space Shuttle missions. Hydrogen fuel cells are perfectly fine ways to power just about anything, you can run a fully electric car on one, producing only water vapor as a by product, and they don't burn anything, so there is no combustion. They operate like any battery, but with hydrogen rather than a metal as the cathode. Electric cars powered by fuel cells have been made which operate at modern highway speeds, and which can run at distances comperable to the distances a gasoline or diesel powered car can run on a full tank. See this C-net article about a fuel cell vehicle developed by Honda, which has a top speed of 100 mph and can run 270 miles on a tank of hydrogen. Indeed, such technology to do so has been around for years. The biggest issue is not in the way the car runs. A fuel cell car isn't the problem in converting cars to fuel cells, its hydrogen delivery infrastructure. An efficient and safe means of a) producing enough hydrogen to power a national fleet of fuel-cell cars and b) distributing the hydrogen to people so they can run their cars on it are the two main reasons why hydrogen has not taken off.
- There are home generators which can make hydrogen for you using residential natural gas or just plain water and solar power; so hypothetically one possible solution is to allow everyone to make their own hydrogen at home rather than get it at fueling stations, see this description of both water and natural gas based systems, availible from Honda. --Jayron32 04:38, 16 February 2010 (UTC)
- The article Hydrogen vehicle has a few answers your second question. But mostly experimental stuff. APL (talk) 04:16, 16 February 2010 (UTC)
- Regarding commercial power stations, hydrogen gas doesn't occur naturally, so you'd be better off using whatever you where going to use to make the hydrogen. It's useful in cars and such because it can still produce power relatively efficiently at a small scale, and driving with your car plugged in isn't an option. — DanielLC 05:34, 17 February 2010 (UTC)
- That's a really key point: hydrogen is useful as an energy storage/transport medium, but not as a primary fuel itself. It's easy to make on large scale using some other energy source and then use to power other things elsewhere that are far from primary sources or somehow else can't use the primary source conveniently. But "generate hydrogen then use it" has an energetic cost, so you're better off not bothering with this intermediate form unless there is a reason. DMacks (talk) 05:43, 17 February 2010 (UTC)
I shouldn't have asked "by burning hydrogen". I should have asked if there were commercial power stations that use hydrogen to produce electricity.
Are there any commercial power stations that use hydrogen to produce electricity?
An Unknown Person (talk) 03:55, 18 February 2010 (UTC)
- Solar power plants indirectly use the fusion of hydrogen into helium in the sun to produce electricity. Gandalf61 (talk) 15:57, 19 February 2010 (UTC)
Ground State Energy[edit]
What would be the potential real-world application(s) of knowing the ground state energy of one or more molecules? Truthforitsownsake (talk) 05:13, 16 February 2010 (UTC)
- Knowing the relationship between ground state energy and exicited energy states has lots of applications. Exciting the nuclei of atoms to an excited state and alowing them to relax to the ground state is a fundemental part of nuclear magnetic resonance and its medical analogue, magnetic resonance imaging. Understanding concepts like Phosphorescence and Fluorescence requires understanding how energy states work. If you don't know what the concept of a ground state is, the entire quantum model of the atom will not be understandable, and not knowing how atoms work means you won't know how to effectively analyze them and use them. The entire field of analytical chemistry basically requires understanding how electrons and other parts of the atom behave as they are excited and allowed to relax to the ground state. Also, you can't pull some fact out of an entire discipline like this and say "how does this have a real world application". Take a holistic view; understanding Chemistry is useful; and this is a core concept to understanding chemistry. --Jayron32 05:29, 16 February 2010 (UTC)
- It seems like your answer addressed the issue of knowing what the term "ground state energy" means. The question was in relation to knowing the actual specific energy value for a specific molecule. Truthforitsownsake (talk) 05:49, 16 February 2010 (UTC)
- it allows you to predict the energy and therefore the wavelength of a photon emitted by an electron falling from an excited state to the ground state, and thus it allows you to figure out which elements are in certain light-emitting objects (e.g. stars) by observing the emitted light. I'm sre there are hundreds of other applications too. —Preceding unsigned comment added by 83.134.159.68 (talk) 07:25, 16 February 2010 (UTC)
- The problem with that is that it would seem to require knowing the energy of one of or more of the excited states as well. Are there any you could think of using only the ground state energy? For example, is it possible to progress from the ground state energy of several molecules to a useful thermodynamic or kinetic property that could be used to predict the outcome of a reaction? I appreciate very much all the help being volunteered. Truthforitsownsake (talk) 16:31, 16 February 2010 (UTC)
KOH + CO2 → KHCO3[edit]
How much CO2 (in lbs or cu. ft.) will a gallon of reagent KOH convert to KHCO3 at STP and 100 deg. Celsius at standard pressure? (BTW - this is not a homework question but a question about how much KOH is needed to convert CO2 from IC engine exhaust to KHCO3. Also this is not a trick question since KOH is a solid or liquid and CO2 is a gas.) 71.100.8.16 (talk) 06:36, 16 February 2010 (UTC)
- First, take a look at stoichiometry, which explains the mathematics of conserving mass in chemistry reactions. As far as I know, this reaction will not occur at standard temperature and pressure. We have a brief mention at Potassium_carbonate#Production and potassium bicarbonate, which make reference to electrolysis (generating K+ ions) as a necessary precursor step. Maybe an expert chemist can fill in the details. Nimur (talk) 14:11, 16 February 2010 (UTC)
- I suppose that in a few hours, days or weeks I could calculate the output from an IC engine according to RPM x number of cylinder x cylinder bore and stroke, etc. and then how many lbs or gallons of KOH I would need to convert each cubic foot of CO2 to KHCO3 and at what temperature and pressure if I just knew how many lbs or grams of KOH I would need to convert just one cubic foot of CO2 to KHCO3. I was hoping this might have already been calculated somewhere and posted on the internet. 71.100.8.16 (talk) 14:37, 16 February 2010 (UTC)
- Two points: First, KOH is a solid, so you need to know either the mass of KOH or quantity in solution (e.g. molarity). Second, you probably shouldn't be mixing US standard measures or Imperial with SI (you have gallons and 100 °C in your problem above). -- Flyguy649 talk 14:43, 16 February 2010 (UTC)
- The issues you mention are also matters of conversion to the correct units, which should likewise be available somewhere on the Internet. So far I have only found this:
- Two points: First, KOH is a solid, so you need to know either the mass of KOH or quantity in solution (e.g. molarity). Second, you probably shouldn't be mixing US standard measures or Imperial with SI (you have gallons and 100 °C in your problem above). -- Flyguy649 talk 14:43, 16 February 2010 (UTC)
- I suppose that in a few hours, days or weeks I could calculate the output from an IC engine according to RPM x number of cylinder x cylinder bore and stroke, etc. and then how many lbs or gallons of KOH I would need to convert each cubic foot of CO2 to KHCO3 and at what temperature and pressure if I just knew how many lbs or grams of KOH I would need to convert just one cubic foot of CO2 to KHCO3. I was hoping this might have already been calculated somewhere and posted on the internet. 71.100.8.16 (talk) 14:37, 16 February 2010 (UTC)
1. CO2 + H2O --> H2CO3 2. H2CO3 + 2 NaOH (or KOH) --> Na2CO3 (or K2CO3) + 2 H2O + Energy 3. Na2CO3 (or K2CO3) + Ca(OH)2 --> CaCO3 + 2 NaOH (or KOH)
71.100.8.16 (talk) 16:00, 16 February 2010 (UTC)
- Good, now you have the balanaced reactions for getting what you want. These reactions are in terms of molecules ("1 CO2 molecule + 1 H2O molecule gives 1 H2CO3 molecule", etc.), so you can use the molecular weight of each compound to convert those molecule ratios to mass ratios. You can check our article about each chemical to find these conversion factors. You could even do that for the theoretical reaction you proposed in the initial question. The only other ingredient in your later equations is water, and you already heard you need a lot of water just to dissolve and mix everything. DMacks (talk) 17:43, 16 February 2010 (UTC)
- I'll assume that you want to use a temperature of 100 C, or 373.15 K, even though STP (Standard Temperature and Pressure) usually means 0 C or 273.15 K. First, lets assume we have a 1 molar solution of KOH. With a molar mass of 56.1 g/mol, that works out to be 56.1 g of KOH (1 mole) in 1 L of water. This means that the solution can theoretically absorb 1 mole of CO2, which weighs 44.1 g. Using the gas equation PV = nRT, we can solve for V using P = 0.986 atm (STP), n = 1 mole, T = 373.15 K and R = 0.08205746 atm*L/mol*K we get a volume of 31 L. This is all assuming that CO2 at these conditions can be treated as an ideal gas, but this should be a good estimate.
24.150.18.30 (talk) 02:26, 18 February 2010 (UTC)
sterno in racing[edit]
why did they use sterno in racing wouldent it make more sense to just use ethanol rather than hundreds of cans of sterno —Preceding unsigned comment added by 67.246.254.35 (talk) 08:49, 16 February 2010 (UTC)
- Are you referring to the allegation the Michael Waltrip used Sterno during 2007 Daytona qualifying, as mentioned at Sterno#Use in racing? Three things come to mind - it's unlikely that the "bluish gel" was Sterno brand canned fuel, since Sterno is dyed pink; "hundreds of cans" would not have been used for that incident, since it was during qualifying, which is only a limited number of laps, and whatever the substance was could conceivably have been purchased in bulk, rather than cans. --LarryMac | Talk 13:50, 16 February 2010 (UTC)
WHY NOT JUST USE ETHANOL THOU? —Preceding unsigned comment added by Thekiller35789 (talk • contribs) 23:55, 16 February 2010 (UTC)
- Possibly for timed release, or to avoid detection by inspectors looking for prohibited additives mixed directly into the fuel. --Smack (talk) 19:09, 17 February 2010 (UTC)
Wings and the human anatomy[edit]
I know this is impossible, but a human were to be born with feathered wings as pictured in typical angel pics, what kind of anatomy would he/she have to have? Meaning, will there be extra muscles, bones, etc? --Reticuli88 (talk) 13:51, 16 February 2010 (UTC)
- Are the wings just ornamental or are they supposed to actually make one fly? The latter would require a massive anatomical overhaul in terms of more muscles, lighter bones, etc., if it were even going to be a little tiny bit possible. If they are just ornamental, then it just depends on the weight of them. --Mr.98 (talk) 14:07, 16 February 2010 (UTC)
Not ornamental. I just want someone to detail to me how it will not look like the typical angel pictures. --Reticuli88 (talk) 14:09, 16 February 2010 (UTC)
- Angels are usually shown as having wings and arms. That means they have 6 limbs, which is a very different anatomy. If you replaced their arms with wings, it is much simpler. You can get an idea of how the arms and hands would need to change by looking at bats. The other major change in that the muscles in the chest would need to be much, much larger if you want them to be able to get off the ground. --Tango (talk) 14:12, 16 February 2010 (UTC)
(ec) We can't answer specific details about your hypothetical situation. You've already stepped outside the bounds of reality by suggesting the impossible - so requesting scientific analysis at this point is futile. There is no correct answer to this kind of "what-if" question. However, if you want to consider something scientific, you might notice that the closest winged relatives to humans are bats. Because they are mammals, like humans, they do not have feathers (their skin cells have adapted to form hair, not feathers). The wings are made of a skin membrane stretched across specially-adapted finger bones. Nimur (talk) 14:15, 16 February 2010 (UTC)
- In the case of wing muscles they might look more muscular rather than larger since insect wing muscles are the most powerful muscles known... (correct me if I'm wrong.) 71.100.8.16 (talk) 14:16, 16 February 2010 (UTC)
- The square-cube law means insects can fly extremely easily because they are so small, so using them as an example isn't useful. "More muscular" and "larger" are the same thing, anyway - muscles get stronger by getting bigger. --Tango (talk) 14:28, 16 February 2010 (UTC)
- That is for true flight. Gliding would be much more feasible with only minor changes. In fact, they have clothing that will let you do that. Googlemeister (talk) 14:29, 16 February 2010 (UTC)
- Obligatory link to Wingsuit flying. --Mr.98 (talk) 14:40, 16 February 2010 (UTC)
- Gliding in a wingsuit is lot like plummeting.APL (talk) 15:35, 16 February 2010 (UTC)
- errr... I'll point out that gliding usually preferences a non-feathered wing (feathers give control over lift, but introduce drag), but a human figure with huge bat-like wings starts to look decidedly non-angelic. --Ludwigs2 16:29, 16 February 2010 (UTC)
- And that calls for the obligatory link to Childhood's End... alteripse (talk) 21:09, 16 February 2010 (UTC)
- errr... I'll point out that gliding usually preferences a non-feathered wing (feathers give control over lift, but introduce drag), but a human figure with huge bat-like wings starts to look decidedly non-angelic. --Ludwigs2 16:29, 16 February 2010 (UTC)
- Gliding in a wingsuit is lot like plummeting.APL (talk) 15:35, 16 February 2010 (UTC)
- Obligatory link to Wingsuit flying. --Mr.98 (talk) 14:40, 16 February 2010 (UTC)
- That is for true flight. Gliding would be much more feasible with only minor changes. In fact, they have clothing that will let you do that. Googlemeister (talk) 14:29, 16 February 2010 (UTC)
- The square-cube law means insects can fly extremely easily because they are so small, so using them as an example isn't useful. "More muscular" and "larger" are the same thing, anyway - muscles get stronger by getting bigger. --Tango (talk) 14:28, 16 February 2010 (UTC)
- In the case of wing muscles they might look more muscular rather than larger since insect wing muscles are the most powerful muscles known... (correct me if I'm wrong.) 71.100.8.16 (talk) 14:16, 16 February 2010 (UTC)
- Also, angels are usually depicted with wingspans shorter than their armspans! Unless they can beat them faster than a hummingbird, that's not nearly long enough. Hangliders typically have wingspans of 30ft or more, and human-powered aircraft like the Gossamer Condor of nearly 100ft. It's difficult to imagine how that could fold comfortably onto the back of a humanoid when not in flight. APL (talk) 15:35, 16 February 2010 (UTC)
- I remember reading that they would requie a huge musculartor to fly - so tthey wouldnt look like they do in paintings. 89.242.101.230 (talk) 20:41, 16 February 2010 (UTC)
- Genetically - this is an essentially impossible mutation. It's conceivable that someone could be born with 6 limbs (this has probably happened more than once in human history)[citation needed] - but it's really hard to imagine how feathers could be there. The gene(s) for feathers came about as dinosaurs evolved into birds - but the mammal line had already branched off by then - so there isn't really a way for such a complex set of genes to appear in humans all in one go. Feathers are a modification of scales - and humans aren't scaley. So this would require truly massive amounts of incredibly lucky mutation. It's essentially impossible. SteveBaker (talk) 20:57, 16 February 2010 (UTC)
- If we go down the bat-like wings route, rather than the angel-like wings route, then it is a little more feasible (bats are quite closely related to humans). You swap some human genes for bat genes and see what happens. With current knowledge of genetics, I would expect it to require a significant amount of trial and error to get it even close. I expect the most difficult part would be turning human arms/hands into bat's wings while leaving the legs/feet unchanged - as I understand it, the same genes are used, in part, for both. You also need to do something about the muscles, but perhaps that could be done with some well placed hormones during gestation or shortly after birth. --Tango (talk) 03:12, 17 February 2010 (UTC)
- There are four levels we need to think about. First, could you make something the size and weight of an adult human fly under his/her own power? Theoretically, the answer is yes - there are birds and pterosaurs larger than humans who have flown under their own power. Second, could you make an animal with three sets of limbs? This is a hand-wavey theoretically "maybe" - I don't think it's completely and utterly impossible for there to be a second shoulder girdle at the bottom of the rib cage, for example. Third, could you make something the size of a human fly with wings as commonly depicted? No, not unless you seriously muck around with the mass involved. Of course, since an infinite number of angels can dance on the head of a pin without crushing it, perhaps this isn't really a problem! Finally, could you have wings in the place commonly depicted? No, there's just no extra space there for anything; your back muscles are a very complicated set of muscles that slide over and under one another; there's no room to just go adding extra bones and stuff there. And then you get into feathers... Matt Deres (talk) 22:05, 16 February 2010 (UTC)
- Bear in mind that many people think angels are spiritual beings, and as such are "transcendent and therefore metaphysical" in nature (ie. incorporeal and therefore without mass). In other words, angels don't need large wings with even larger muscles to enable them to fly. Astronaut (talk) 01:08, 17 February 2010 (UTC)
- There are flying birds the size of humans, but they are far lighter than humans due to different bone composition, etc. --Tango (talk) 01:12, 17 February 2010 (UTC)
- According to the article I linked to, Argentavis has been estimated to weigh between 60-110 kg. Close enough! Either way, it ain't gonna happen. Matt Deres (talk) 01:37, 17 February 2010 (UTC)
- According to the Bible, angels have neither wings nor a ring around their head. Maybe they look like humans but only "fly" vertically upward. ~AH1(TCU) 02:26, 17 February 2010 (UTC)
- We aren't being asked about supernatural angels - and we're not being asked whether such a creature could fly - we're being asked whether someone could be born looking that way - and for all practical purposes, the answer is a very clear "No". SteveBaker (talk) 02:57, 17 February 2010 (UTC)
- According to the Bible, angels have neither wings nor a ring around their head. Maybe they look like humans but only "fly" vertically upward. ~AH1(TCU) 02:26, 17 February 2010 (UTC)
- According to the article I linked to, Argentavis has been estimated to weigh between 60-110 kg. Close enough! Either way, it ain't gonna happen. Matt Deres (talk) 01:37, 17 February 2010 (UTC)
Thanks everyone for their input. I was trying to imagine if a human being had 6 limbs, two of those being wings (feathered or not) how much would it not look human. I mean, would the back look twice as huge? Or the chest? Will it have to have longer legs or a longer neck? --Reticuli88 (talk) 16:15, 17 February 2010 (UTC)
So I imagine that this human might have to look something like this. --Reticuli88 (talk) 19:28, 17 February 2010 (UTC)
- But that's just not possible given the way genetic change and developmental processes happen. If you have a strong stomach here are some photos of an actual six-limbed human. SteveBaker (talk) 02:51, 18 February 2010 (UTC)
- Even assuming you could genetically engineer (on purpose - highly unlikely - or by accident - infinitesimally unlikely) a human with wings, you have lots of additional problems to overcome before you could actually fly. As Matt Deres mentions, Quetzalcoatlus was more or less that same weight as a human, but it required a wing-span of around 10 metres. I don't know about you, but wings that size would seriously interfere with my social life.
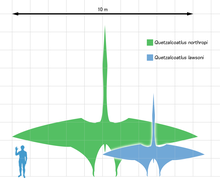
- We would likely need to revamp our metabolism and bone structure, to provide enough energy and tensile strength, while minimizing weight. Finally, we would need tendons, to lever the wings, and muscular physiology to power them, that are on a completely unhuman scale. I'm estimating here (you could probably do the math), but I would guess we would need pectoral muscles that were 10-20 times bigger than any human has. We would probably also need a tail to help steer, and would have to ditch our legs or develop some system to keep them horizontal during flight. In other words, generating wings is just the beginning: making them work is a whole other story. Rockpocket 03:44, 18 February 2010 (UTC)
- Why would you assume we'd develop an extra set of lims? As others here have alluded to, hexapods came on land separately from four-limbed animals. This shows any transitions between the two are extremely, extremely unlikely. Imagine Reason (talk) 04:24, 18 February 2010 (UTC)
Thanks RP. What does tensile strength mean? --Reticuli88 (talk) 15:02, 18 February 2010 (UTC)
got it --Reticuli88 (talk) 15:45, 18 February 2010 (UTC)
Signal White Now II[edit]
This question - [[3]] - let me thinking. If this dye absorbs light in the ultraviolet spectrum and emits light in the blue spectrum, it would yield a better or worse result depending on the amount of UV hitting the teeth. So, in a room with indirect natural light that comes in through a glass, its effect would possibly not be noticeable. However, under a black light in a night club the teeth could look whiter or perhaps simply blue (depending on how yellow they were), right?--ProteanEd (talk) 16:47, 16 February 2010 (UTC)
- Right, and under such lights, to my personal observations, teeth often do glow blue-white, just as do white clothes washed in detergents containing similar 'white-enhancing agents.' Note that although this particular product apparently claims to (and doubtless does) contain a newly formulated (or utilised) ingredient, toothpaste manufacturers have been using other such ingredients with similar properties for some time. 87.81.230.195 (talk) 17:29, 16 February 2010 (UTC)
Ferris/Chelsea wheel?[edit]
While researching the invention of the Ferris wheel I remebered years ago a story about a dispute over who had the invention first. The story was that a gentleman named Chelsea had first set out to build what is now the modern Ferris wheel. His invention was called "The Chelsea Roundabout" Has anyone heard this story or is it just some folk tale?Nijia2010 (talk) 19:09, 16 February 2010 (UTC)
- I couldn't find any reference to this story at all. However, it is known that after Ferris built his wheel for the 1893 World Fair in Chicago, many people copied the idea and he spent most of the rest of his life launching (and defending against) lawsuits. So it's very possible that other people claimed to have beaten him to the idea. It might help if you told us where you heard about this "Chelsea Roundabout". SteveBaker (talk) 02:54, 17 February 2010 (UTC)
Parts of brain responsible for street smartness and academic intelligence[edit]
Some indivdiuals, who are quite good academically are easily duped in real life. They lack what is called street smartness or tact . There are others with bad grades but are very clever in real life and go on to become businessman etc. There are a few who are strong in both areas. However, since one sees quite clear division of skills. One may conclude that different parts of brain are responsible for academic / real life intelligence. Could you please throw some light on that? —Preceding unsigned comment added by 131.220.46.25 (talk) 19:19, 16 February 2010 (UTC)
- Actually, terms like "street smarts" and "common sense" are probably most often used by the less-educated to claim some mental advantage over the more-educated. Your claims are quite dubious. A tangentially related article is Staircase wit, about people who are witty but slow in coming up with the response. Supposedly this described Rousseau. Comet Tuttle (talk) 19:49, 16 February 2010 (UTC)
- I don't think there is any scientific evidence for this phenomenon - and without that, we're not going to have any explanations. I suspect it's not true. SteveBaker (talk) 20:50, 16 February 2010 (UTC)
- In any case, I'm not sure how you would try to measure it. Compare someone's SAT scores with the number of times they've been mugged? "Street smarts" and "common sense" are necessarily fuzzy terms.
- That being said, I would hazard to guess that in the U.S., anyway, whatever we call "street smarts" (which is not the same thing as "common sense") does probably correlate with the economic conditions of one's upbringing, and so, probably, does advanced academic achievement. In my anecdotal experience, people who are very far along academically (e.g. Ph.D.s) at very prestigious East Coast institutions (e.g. Ivy League) are quite disproportionately from very wealthy personal backgrounds, and as a result have been, again from my experience, quite sheltered compared to the rest of the population (or, indeed, my own upbringing). As a result I have witnessed them be what I considered to be quite naive about the "facts of life" for the rest of the world out there, even if they are quite good in their own academic field of study. Again, totally anecdotal, not data. And I don't even know if my anecdotal examples are very representative. I also know a number of people who have achieved high academic status from remarkably low economic origins, even criminal backgrounds. None of it really tells us whether this is strong correlation, or just a stereotype. --Mr.98 (talk) 21:09, 16 February 2010 (UTC)
- In more general terms, there's the question about what you mean by street smarts. Sure these people from wealthy and sheltered backgrounds may not do well in the mean streets of LA (or whatever). How will the average person from the mean streets of LA, even with their 'street smarts' do in a wealthy neighbourhood or when interacting with these wealthy people? For that matter, people from uneducated backgrounds are prone to being ripped off by banks, money lenders, dubious service providers and other such things, while academics are far from immune to this they do tend to less commonly fall for some of the common practices and pitfalls (and not just because they don't have to or they have advisors). Nil Einne (talk) 22:43, 16 February 2010 (UTC)
It's a naive, not an unanswerable or meaningless question. First, read our intelligence article on the different kinds of intelligence. Second, contemplate all the types that can go into "street smarts": usually a combination of being good at reading strangers' behaviors and intentions and familiarity with local customs, resources, and hazards. Third, contemplate the types of intelligence that make for "book smart": good grades usually means school study skills, comfort in a school environment, and being good at giving teachers what they want, perhaps with unusual reading background. These are somewhat overlapping skills: people-reading and knowledgeability about social demands of a specific environment. Obviously there are other dimensions to both types of success: numeracy, risk taking, perseverance, ability to learn from mistakes, use of contacts, luck, charm, attractiveness, etc. I would not be so quick to deprecate the level of intelligence needed for success in the two areas. alteripse (talk) 21:41, 16 February 2010 (UTC)
- I think there's a reasonably good case for distinctions between spatial/mathematical intelligence, verbal intelligence, and social intelligence (ability to interact productively with other people), at the least. Street smarts, though, are largely a product of harsh experience rather than intelligence per se, I would guess. Looie496 (talk) 22:03, 16 February 2010 (UTC)
- My take on this would be that people who are used to a professional culture are inexperienced when they find themselves in a machismo culture. For example they may expect people to be reasonably honest or altruistic. Vice versa as well, but because professionalim is 'nice' then people from the dark side are not so harmed by it. People from a professional culture are also at a disadvantage by being ethically constrained from using the manipulation or coercion that the machismos use. 89.242.101.230 (talk) 23:51, 16 February 2010 (UTC)
- To the original poster: you might find the theory of multiple intelligences introduced by Howard Gardner in the 1980s of interest. It proposes different types of intelligence (usually eight), although none of them can be characterized as "street smarts". However, the "interpersonal" type is perhaps the closest to what you are talking about.--Eriastrum (talk) 00:00, 17 February 2010 (UTC)
- False dichotomy. The subprime loan applicants are one example. People can be smart or stupid in academic and non-academic areas. It all depends on what you're talking about. Imagine Reason (talk) 00:03, 17 February 2010 (UTC)
Concur with Alteripse: I think it's a reasonable observation, although it is not a rule by any means. The problem is that both perceived "academic intelligence" and perceived "street intelligence" are complex characteristics that include not only general "intelligence per se", which IQ tries to measure and which is a pretty problematic concept, but also various intellectual and social habits, acquired skills, experience and knowledge, as well as reaction speed (much more crucial in "street smartness"). Many of these things are such that you tend to specialize to varying degrees in each of them depending on your life history, and you are, naturally, weaker outside of your element. Others, such as reaction speed, probably have a genetic component which may influence what you choose to specialize in in the first place. --91.148.159.4 (talk) 04:16, 19 February 2010 (UTC)
- This is just a stereotype put about by the media to flatter dummies. It also appeals to people with the tough minded personality type. 89.242.89.218 (talk) 14:27, 19 February 2010 (UTC)
- So you think you're "smart" both academically and in real life, and the two are one and the same? I'm afraid this is a new stereotype put about by intellectuals and businessmen to flatter themselves. I'm an intellectual myself, but I don't buy it.--91.148.159.4 (talk) 14:32, 19 February 2010 (UTC)
Is the large blue part ocean or is it just iron mantle, since the tan-white part is the icy crust? I don't think Ganymede's surface ocean is as big as Europa's.--209.129.85.4 (talk) 21:43, 16 February 2010 (UTC)
- Neither of them have a surface ocean, they are far too cold. Europa is believed to have a liquid ocean underneath the ice and Ganymede might too, but the description of that image (which concurs with Ganymede (moon)#Internal structure) describes the blue layer as "a deep layer of warm soft ice" ("warm" is presumably a relative term). --Tango (talk) 23:15, 16 February 2010 (UTC)
Fuel Consumption Problem - (How Much Extra Fuel Would I Use by Carrying More Fuel to Begin With?)[edit]
Let's say I have a car that weighs w kilograms and it is driving in a straight line at a constant speed along a perfectly flat road of zero gradient and zero camber. Let's assume we want to travel a total distance of d kilometres, and we start with s litres of fuel. Now, let's assume that we used u litres of fuel to make that journey. Next time we start with m litres more fuel than last time and make the same journey, in the same conditions, along the same road.
- How much extra fuel, say e litres, would I have used by starting off carrying more fuel?
- How far less, say l kilometres will the same u litres of fuel carry me given that I am starting off carrying more fuel?
To make things simple, assume that fuel consumption only depends on the weight of the car. Don't worry about the temperature of the fuel or of the tyres, etc. Add some assumptions if you like, and take some away if you like. I just want to know how much extra fuel I would use by carrying more fuel to begin with. I have a maths degree, and a strong theoretical grasp of differential equations, so don't worry about being too complicated. Thanks is advance. •• Fly by Night (talk) 22:13, 16 February 2010 (UTC)
- P.S. I tried the maths reference desk and I got a single, unhelpful, reply. •• Fly by Night (talk) 22:14, 16 February 2010 (UTC)
- The real question here is: How much does weight affect fuel efficiency for a typical car? Once you have that, the rest is algebra. This website says: "An extra 100 pounds in your vehicle could reduce your MPG by up to 2 percent. The reduction is based on the percentage of extra weight relative to the vehicle's weight and affects smaller vehicles more than larger ones." I'm not sure how reliable that is, but it will do for now. Petrol#Density says petrol ways about 6 lb per gallon. That means your MPG is going to reduce by about 0.12% per extra gallon of fuel. I suspect that is well within the margin of error of fuel efficiencies anyway, due to different driving styles, journey types, etc. --Tango (talk) 23:07, 16 February 2010 (UTC)
- Thank you. I thought that some differential equations might have been needed, but the amount of wasted fuel is so small that I would probably waste more brain power solving the equations than I would on the extra petrol. Thanks again. •• Fly by Night (talk) 23:32, 16 February 2010 (UTC)
- That single parameter will vary by type of vehicle. A truck which is already towing 25 tons will be largely unaffected by an extra 250 pounds of fuel. Meanwhile, a Prius (whose fuel efficiency is significantly improved by its extremely light weight may notice as much as a 10% fuel efficiency decrease when you add 250 pounds of fuel. Of course, a truck's fuel efficiency is already low - ~ 6.5 mpg, compared to a 45 mpg Prius. Nimur (talk) 23:12, 16 February 2010 (UTC)
- What Prius has a 40 gallon fuel tank??? Googlemeister (talk) 13:53, 17 February 2010 (UTC)
- The Prius HM - Hypothetical Model. The entire backseat was converted to a fuel tank. The connector hose goes out the window and connects to the main tank. Unfortunately, we forgot to account for the extra mass and aerodynamic effects of the tank, hose, and secondary fuel pump. Nimur (talk) 15:14, 17 February 2010 (UTC)
- I have to wonder: does weight really change the fuel consumption rate in a car going at a constant speed on a flat road? The only forces that need to be overcome by burning fuel are drag and mechanical friction, neither of which are affected by weight. Weight only increases fuel consumption in start-stop traffic where a larger force must be exerted to the larger mass in order achieve the same acceleration. That is irrelevant here as the car is going at a constant speed. Planes, on the other hand, are much more sensitive to weight as additional weight requires a higher angle of attack to generate the required lift, increasing the drag it faces. --antilivedT | C | G 02:00, 17 February 2010 (UTC)
- Wait... I'm pretty sure weight affects friction via the normal force. Weight is not an issue with a frictionless surface. John Riemann Soong (talk) 03:07, 17 February 2010 (UTC)
- For a car it is the rolling resistance that is of interest more than any sliding friction. 58.147.58.28 (talk) 05:53, 17 February 2010 (UTC)
- Wait... I'm pretty sure weight affects friction via the normal force. Weight is not an issue with a frictionless surface. John Riemann Soong (talk) 03:07, 17 February 2010 (UTC)
- What Prius has a 40 gallon fuel tank??? Googlemeister (talk) 13:53, 17 February 2010 (UTC)
- That single parameter will vary by type of vehicle. A truck which is already towing 25 tons will be largely unaffected by an extra 250 pounds of fuel. Meanwhile, a Prius (whose fuel efficiency is significantly improved by its extremely light weight may notice as much as a 10% fuel efficiency decrease when you add 250 pounds of fuel. Of course, a truck's fuel efficiency is already low - ~ 6.5 mpg, compared to a 45 mpg Prius. Nimur (talk) 23:12, 16 February 2010 (UTC)
- The problem is that it depends on how you drive. At a high constant speed on a level freeway, almost all of the energy goes into overcoming air resistance - the amount of power required to overcome air resistance goes up as the cube of the speed. Hence the weight hardly matters at all at freeway speeds. The amount of energy needed to go uphill or to accelerate depends largely on the weight and relatively little on everything else. At slow, constant speeds, the energy mostly goes into overcoming friction - some fraction of which depends on the weight of the payload and some fraction does not. This factor depends on the design and relative weight of the car itself. Consequently, we're not going to be able to come up with a definite formula. But at constant speed - the general answer is that the amount of weight in the car matters much less at high speed than it does at low speed because at high speed the energy consumption is dominated by drag and at low speed by friction. Because of the "cube of the speed" thing, you can get dramatically different fuel consumption driving into the wind versus with a tail wind because drag is determined largely by the speed relative to the air - not relative to the road. SteveBaker (talk) 02:33, 17 February 2010 (UTC)
- Nice answer, thanks. But doesn't momentum need to be taken into account when considering wind resistance? If I throw a table tennis ball into the wind then it won't travel very far. It has very little momentum and the wind overcomes it very quickly. If I fill the same table tennis ball with lead and then throw it with exactly the same initial conditions it will travel much, much further. I will use more energy getting the heavier ball up to speed and so it will travel further. The same is true for a light car and a heavier car. Let's say the two cars are exactly the same but one has a bigger engine (i.e. more weight and more power). We get the cars up to the same speed on the same road in the same conditions and then cut the power and let them glide. The lighter car will use less fuel to get to the starting speed but will also stop sooner. The heavier car will use more fuel to get up to speed but will travel further. Plus, a lot of this momentum is built up at low speeds when friction really does matter. •• Fly by Night (talk) 18:37, 17 February 2010 (UTC)
- P.S. The cube of the speed law needs some constants to make sense. A cube will grow faster than a square, that is true. But just because it grows faster it doesn't mean that it is larger. Consider x^3/1000 and x^2. We need x > 1000 for x^3/1000 > x^2. •• Fly by Night (talk) 18:47, 17 February 2010 (UTC)
- Even for the specified conditions straight, perfectly level road and constant speed, rarely to be found on this planet, more weight means more friction in bearings and more work done on the tires. Granted, at highway speeds wind resistance is the main factor limiting fuel mileage, and adding more weight of fuel need not increase wind resistance. A higher load should mean more tire heating and less mileage. On practical roads, there generally is some hill climbing, and accelerating and decelerating due to traffic conditions, so the extra weight would decrease mileage (except on a hybrid with ideal regenerative breaking). Real cars must accelerate up to speed, and inability to merge onto an expressway due to undersized engine for the weight is dangerous. A lighter car allows a smaller and more efficient engine and transmission. Some information on weight versus fuel economy in real cars driven on real roads: [4], [5].Edison (talk) 21:35, 17 February 2010 (UTC)
- P.S. The cube of the speed law needs some constants to make sense. A cube will grow faster than a square, that is true. But just because it grows faster it doesn't mean that it is larger. Consider x^3/1000 and x^2. We need x > 1000 for x^3/1000 > x^2. •• Fly by Night (talk) 18:47, 17 February 2010 (UTC)
- Nice answer, thanks. But doesn't momentum need to be taken into account when considering wind resistance? If I throw a table tennis ball into the wind then it won't travel very far. It has very little momentum and the wind overcomes it very quickly. If I fill the same table tennis ball with lead and then throw it with exactly the same initial conditions it will travel much, much further. I will use more energy getting the heavier ball up to speed and so it will travel further. The same is true for a light car and a heavier car. Let's say the two cars are exactly the same but one has a bigger engine (i.e. more weight and more power). We get the cars up to the same speed on the same road in the same conditions and then cut the power and let them glide. The lighter car will use less fuel to get to the starting speed but will also stop sooner. The heavier car will use more fuel to get up to speed but will travel further. Plus, a lot of this momentum is built up at low speeds when friction really does matter. •• Fly by Night (talk) 18:37, 17 February 2010 (UTC)
- This reminds me of similar considerations which lead to space rockets (nearly) always having three stages. If you objective was to go as far as possible on one filling of fuel, then you could tow a trailer full of fuel and ditch it when it when it was empty to save weight. 78.149.241.220 (talk) 16:20, 20 February 2010 (UTC)
Mars Colony[edit]
How much would it cost, in 2010 dollars with 2010 technology, to establish a self-sustainable colony of 50 men and 50 women on Mars? TheFutureAwaits (talk) 22:27, 16 February 2010 (UTC)
- Well, it can't be done with 2010 technology. We don't currently have a spacecraft capable of taking people to Mars. Most of the cost is in developing the technology we need. How much that costs depends largely on how quickly you want to do it and what degree of safety you want. --Tango (talk) 22:55, 16 February 2010 (UTC)
- Conceivably, you could try to run up a sum of all the necessary costs to build and launch the space mission; estimate going-rates for all the needed technology, etc; and generate a cost-estimate that way. I think that would probably be an alright way to do a cost-estimate, but it's very sensitive to the details of your mission plan. This is the way I would approach the cost-estimation problem - without presupposing any particular mission scheme or technology design. It would be a reasonable, albeit loose, claim, to state that such an endeavor would require the entire force of the current space administration in order to drive the enormous project and sub-projects needed to make that voyage possible. If political bickering was not an issue and you could throw today's entire space program budget at that particular task, it might still take five or ten years to ramp up the technology. So, noting that NASA's annual budget is ~ 17 billion US dollars, and the project may take 5 to 10 years, I would estimate that it might cost on the order of 100 to 200 billion dollars. Since "$100 billion" is a hard-to-conceptualize quantity of money, here's some other ways to think of it. It'd order about 10 billion cheese pizza deliveries, if you could find enough cheese pizza shops to deliver them. Or, it would be about as expensive as 500 deep-water oil rigs, or about as expensive as one year of sustaining a large overseas military campaign, or about as expensive as five to ten years worth of nuclear deterrence. These numbers aren't meant to be particularly accurate - but they highlight another mode of thinking about the budgets for such enormous projects. When NASA embarked on the Apollo Program, it was not like they could hold a bake-sale and raise some money, and then waltz down to the nearest retail store and pick up a Moon Spaceship once they had the right change. These kinds of enormous projects are better viewed in the context of nation-scale economic prioritization - not total dollar cost. Take a look, for example, at the Space Shuttle program budget. You can boil down the price to a succinct number (as NASA has done here) - but that number's pretty meaningless without context. (Phrased differently - could a billionaire with disposable income purchase a Space Shuttle for $1.7 billion dollars? If not, then is that really how much they cost? Nimur (talk) 23:22, 16 February 2010 (UTC)
- 5-10 years sounds like a big underestimate to me. 10 years might be enough for a small scale manned mission to Mars, but not to establish a large colony. I think 20-30 years is more realistic, even if the whole of NASA is devoted to it. --Tango (talk) 01:28, 17 February 2010 (UTC)
- Well, when President Kennedy announced the plan to go to the moon in 1961, we had logged a total of 15 minutes of manned spaceflight - and had never even reached orbit. Yet, by devoting the entire national scientific establishment and a very significant chunk of our industrial base towards this simply-defined goal, we managed to make it happen. Mars missions bring a host of new technical challenges, but we have also advanced the state of the art very significantly since 1961. I think that we could do it in 5 to 10 years if we had the unfaltering, united will of the entire nation. Nimur (talk) 03:19, 17 February 2010 (UTC)
- I suppose if we were willing for the first manned mission to Mars to have 100 people on board then it could be done, but I expect people would want to do at least one test flight with a handful of people first. That adds several years. (Remember, just getting to Mars takes nearly a year.) --Tango (talk) 14:09, 17 February 2010 (UTC)
- Well, when President Kennedy announced the plan to go to the moon in 1961, we had logged a total of 15 minutes of manned spaceflight - and had never even reached orbit. Yet, by devoting the entire national scientific establishment and a very significant chunk of our industrial base towards this simply-defined goal, we managed to make it happen. Mars missions bring a host of new technical challenges, but we have also advanced the state of the art very significantly since 1961. I think that we could do it in 5 to 10 years if we had the unfaltering, united will of the entire nation. Nimur (talk) 03:19, 17 February 2010 (UTC)
- 5-10 years sounds like a big underestimate to me. 10 years might be enough for a small scale manned mission to Mars, but not to establish a large colony. I think 20-30 years is more realistic, even if the whole of NASA is devoted to it. --Tango (talk) 01:28, 17 February 2010 (UTC)
- Conceivably, you could try to run up a sum of all the necessary costs to build and launch the space mission; estimate going-rates for all the needed technology, etc; and generate a cost-estimate that way. I think that would probably be an alright way to do a cost-estimate, but it's very sensitive to the details of your mission plan. This is the way I would approach the cost-estimation problem - without presupposing any particular mission scheme or technology design. It would be a reasonable, albeit loose, claim, to state that such an endeavor would require the entire force of the current space administration in order to drive the enormous project and sub-projects needed to make that voyage possible. If political bickering was not an issue and you could throw today's entire space program budget at that particular task, it might still take five or ten years to ramp up the technology. So, noting that NASA's annual budget is ~ 17 billion US dollars, and the project may take 5 to 10 years, I would estimate that it might cost on the order of 100 to 200 billion dollars. Since "$100 billion" is a hard-to-conceptualize quantity of money, here's some other ways to think of it. It'd order about 10 billion cheese pizza deliveries, if you could find enough cheese pizza shops to deliver them. Or, it would be about as expensive as 500 deep-water oil rigs, or about as expensive as one year of sustaining a large overseas military campaign, or about as expensive as five to ten years worth of nuclear deterrence. These numbers aren't meant to be particularly accurate - but they highlight another mode of thinking about the budgets for such enormous projects. When NASA embarked on the Apollo Program, it was not like they could hold a bake-sale and raise some money, and then waltz down to the nearest retail store and pick up a Moon Spaceship once they had the right change. These kinds of enormous projects are better viewed in the context of nation-scale economic prioritization - not total dollar cost. Take a look, for example, at the Space Shuttle program budget. You can boil down the price to a succinct number (as NASA has done here) - but that number's pretty meaningless without context. (Phrased differently - could a billionaire with disposable income purchase a Space Shuttle for $1.7 billion dollars? If not, then is that really how much they cost? Nimur (talk) 23:22, 16 February 2010 (UTC)
- Whatever estimate you come up with, you need to at least double it. It's always the way. 86.138.42.82 (talk) 01:03, 17 February 2010 (UTC)
- How about this for a starting point: 100 people and their personal luggage have a mass of around 10 tonnes. All their food for a 4 year trip (18 months journey + something to get them started in their first martian year), all their water, and all their air would come to considerably more. Altogether a total payload of 100 tonnes or more. With launch costs of around $20,000 per kg that makes $2 billion just to get the payload into low earth orbit. You then have to develop the materials and tools to construct a martian habitat; and you have to develop, construct and fuel a spacecraft to get it all to Mars. And you have to get that lot into orbit as well. I wouldn't expect any change from Nimur's 100 - 200 billion dollars budget. Astronaut (talk) 01:50, 17 February 2010 (UTC)
- The Constellation Program had a budget of $230B through 2025 and that was supposed to get us to a small manned lunar outpost. I would suspect the Mars mission proposed above would be closer to a trillion dollars. Dragons flight (talk) 02:03, 17 February 2010 (UTC)
- Also, "self-sustaining" raises a lot of questions. It would be easier to make a "permanent" colony that requires regular rocket refills (which wouldn't need to be manned) than a truly "self-sustaining" one. --Mr.98 (talk) 02:31, 17 February 2010 (UTC)
- Agree with above. People have tried making an isolated self sustaining habitat on earth, Biosphere 2 and it did not work out all that great. It only involved 8 people and a habitat area of 3.2 acres. Googlemeister (talk) 13:52, 17 February 2010 (UTC)
- They tried to make an entirely biological system. That is useful for research purposes, but isn't required for an actual colony. For example, they had massive fluctuations in CO2 during each day due to photosynthesis dominating during the day and respiration at night. That is very difficult to solve with biology, but should be pretty easy with chemistry. There are plenty of ways of absorbing CO2 during the night and then releasing it during the day. --Tango (talk) 23:16, 17 February 2010 (UTC)
- Agree with above. People have tried making an isolated self sustaining habitat on earth, Biosphere 2 and it did not work out all that great. It only involved 8 people and a habitat area of 3.2 acres. Googlemeister (talk) 13:52, 17 February 2010 (UTC)
- You could estimate it by guessing how many more times more expensive than landing people on the moon it would be. I'd say ten times more expensive. So the cost would be ten times more expensive than the Apollo cost in real terms. 89.240.100.129 (talk) 15:03, 17 February 2010 (UTC)
- What kind of "Self sustaining" are we talking about? Is it acceptable to ship food from Earth? What about shipping supplies from Earth? What about shipping supplies from Earth, but they pay for them somehow by exporting martian goods? Or are you talking about a truly self sustaining colony where they can never get any help from Earth ever again?
- That last might be very difficult. To do that, everything the martian colony depends on must be able to be manufactured locally. When a microchip fails, how will they replace it? When they start needing new spacesuits, how will they make them? If Mars had breathable air they could live a simple frontier life, but sadly, it doesn't. APL (talk) 15:57, 17 February 2010 (UTC)
- (EC) I have to agree this "self-sustaining" bit is likely to be very difficult to achieve in the near term and probably even in the medium term. Most of the cost and time estimates above appear to be largely ignoring it yet it is surely one of the most difficult and costly things to achieve. Having food for 4 years sounds great, but is it really realistic to become self-sustaining in 4 years? Highly doubtful. We're still not even sure how to get water. Nil Einne (talk) 16:03, 17 February 2010 (UTC)
- There's water ice underground in many places on Mars, that isn't a problem. --Tango (talk) 22:45, 17 February 2010 (UTC)
- Could you spot them from orbit? Most of the martian ice we know about is near the poles. Not great locals for setting up a first colony. APL (talk) 15:58, 18 February 2010 (UTC)
- You might be able to spot the ice from orbit, but if not you can just go somewhere that one of the landers we've already sent has found ice. --Tango (talk) 21:32, 18 February 2010 (UTC)
- I think you're missing my point. Yes there is water. Finding enough of it and using it is another matter. We're talking about a self-sustaining colony of 100 people with 2010 dollars and 2010 tech. And people above were describing a 4 year time frame. Coming up with estimates when you haven't even solved one of the most basic problems seems pointless to me. It's all very well it being 'there' but you still have to find it, and find enough of it, and find a way to use it (which means sustainably release it etc) for your colony. If you're going to need to continually move your colony, or go further and further from your colony or whatever you further add to your difficulty particularly given the lack of resources and construction ability someone mentioned above. Perhaps it's just me, but when you say self-sustaning, I'm thinking we're discussing something which can barring catostrophic events survive thousands and thousands of years at a minimum without any involvement from earth. To put it a different way, once you start thinking self-sustaining with 2010 tech in 2010 dollars, all these things which seem minor and basic issues suddenly become a very big deal. (Of course some may question the basic premise, that a colony of 100 can ever meaningfully be self-sustaning.) Nil Einne (talk) 22:54, 19 February 2010 (UTC)
- You might be able to spot the ice from orbit, but if not you can just go somewhere that one of the landers we've already sent has found ice. --Tango (talk) 21:32, 18 February 2010 (UTC)
- Could you spot them from orbit? Most of the martian ice we know about is near the poles. Not great locals for setting up a first colony. APL (talk) 15:58, 18 February 2010 (UTC)
- There's water ice underground in many places on Mars, that isn't a problem. --Tango (talk) 22:45, 17 February 2010 (UTC)
- (EC) I have to agree this "self-sustaining" bit is likely to be very difficult to achieve in the near term and probably even in the medium term. Most of the cost and time estimates above appear to be largely ignoring it yet it is surely one of the most difficult and costly things to achieve. Having food for 4 years sounds great, but is it really realistic to become self-sustaining in 4 years? Highly doubtful. We're still not even sure how to get water. Nil Einne (talk) 16:03, 17 February 2010 (UTC)
The details are not the same (far fewer than 100 people, not permanent, not self sustaining, and not necessarily relying on only 2010 technology) but estimates have varied widely. The first Bush administration called for a study and they came up with $400-$541 billion in 1989 dollars. Robert Zubrin and his Mars Direct plan say a mission is feasible for $55 billion (I think in 1990 dollars, possibly 1996 dollars when The Case for Mars was published). I think the real cost for a basic mission to mars would likely be somewhere in between - the 1989 estimate was way overboard and not a cost effective or sensible way to do things, Zubrin's estimate is optimistic and involves doing things on a shoe string budget that probably isn't practical (or is unacceptably risky). Based on even Zubrin's optimistic estimate, I don't think $100 to $200 billion is a realistic number for such a large colony (Zubrin's plan was for teams of 4). If I had to guess I'd say a 100 person colony would cost over a trillion dollars, possibly several trillion. TastyCakes (talk) 23:39, 17 February 2010 (UTC)
- Have we replicated any ecosystem that can support vegetable and meat production here on earth using only plots with no soil? Have we created large artificial atmosphere anywhere? Imagine Reason (talk) 04:21, 18 February 2010 (UTC)
- Well you don't really need to replicate an entire ecosystem to have a viable (or even mostly self-sufficient) colony. Nuclear submarines can stay underwater almost indefinitely as far as atmosphere goes, there is no reason to think similar technology wouldn't be possible on mars (see CO2 scrubber). And there's no reason I know of that you can't use martian soil as a plant medium. And even if farming is not possible, packing enough dehydrated food for years and years wouldn't be prohibitive. I think food and water are relatively small problems for such a mission, behind things like radiation, fuel, re-entries, debris/meteorites in transit and maybe even human personal issues after so long in a cramped space. TastyCakes (talk) 07:42, 18 February 2010 (UTC)
- It is a big problem because near-lightspeed travel is not feasible for the foreseeable future. 67.243.7.245 (talk) 14:10, 19 February 2010 (UTC)
- "packing enough dehydrated food for years and years " isn't self sustaining. Nil Einne (talk) 22:58, 19 February 2010 (UTC)
- Well you don't really need to replicate an entire ecosystem to have a viable (or even mostly self-sufficient) colony. Nuclear submarines can stay underwater almost indefinitely as far as atmosphere goes, there is no reason to think similar technology wouldn't be possible on mars (see CO2 scrubber). And there's no reason I know of that you can't use martian soil as a plant medium. And even if farming is not possible, packing enough dehydrated food for years and years wouldn't be prohibitive. I think food and water are relatively small problems for such a mission, behind things like radiation, fuel, re-entries, debris/meteorites in transit and maybe even human personal issues after so long in a cramped space. TastyCakes (talk) 07:42, 18 February 2010 (UTC)
- That reminds me of a project in the US where several people were put in a large hermetically sealed greenhouse-like building. It was on tv a few years ago. Unexpectedly, they had problems getting enough oxygen, even though they had been helped by the new concrete giving off oxygen. I think they were also short of food. 92.24.96.55 (talk) 21:18, 18 February 2010 (UTC)
- That would be Biosphere 2, as mentioned above. --Tango (talk) 21:32, 18 February 2010 (UTC)
- Or possibly Bio-Dome. TastyCakes (talk) 23:28, 18 February 2010 (UTC)
- That would be Biosphere 2, as mentioned above. --Tango (talk) 21:32, 18 February 2010 (UTC)
- That reminds me of a project in the US where several people were put in a large hermetically sealed greenhouse-like building. It was on tv a few years ago. Unexpectedly, they had problems getting enough oxygen, even though they had been helped by the new concrete giving off oxygen. I think they were also short of food. 92.24.96.55 (talk) 21:18, 18 February 2010 (UTC)
- It would be a lot cheaper and perhaps more beneficial to mankind to spend money on developing some really clever robots. Then you could get them there with existing rocketry, and they would not need to come back. 92.24.96.55 (talk) 22:01, 18 February 2010 (UTC)
- This somewhat glum editorial argues that maybe we shouldn't worry so much about bringing astronauts back either. TastyCakes (talk) 23:30, 18 February 2010 (UTC)
- Again I think people are missing the point of the question which was a self-sustaining colony. Bringing them back isn't a big issue here. Nil Einne (talk) 22:55, 19 February 2010 (UTC)
- God forbid a response to a ref desk question wander off topic... TastyCakes (talk) 20:48, 20 February 2010 (UTC)
- Again I think people are missing the point of the question which was a self-sustaining colony. Bringing them back isn't a big issue here. Nil Einne (talk) 22:55, 19 February 2010 (UTC)
- This somewhat glum editorial argues that maybe we shouldn't worry so much about bringing astronauts back either. TastyCakes (talk) 23:30, 18 February 2010 (UTC)
