From Wikipedia, the free encyclopedia
Chemical compound
VU-0238429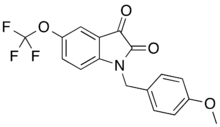 |
|
1-(4-methoxybenzyl)-5-(trifluoromethoxy)indole-2,3-dione
|
| CAS Number | |
|---|
| PubChem CID | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| ChEMBL | |
|---|
| CompTox Dashboard (EPA) | |
|---|
|
| Formula | C17H12F3NO4 |
|---|
| Molar mass | 351.281 g·mol−1 |
|---|
| 3D model (JSmol) | |
|---|
c2cc(OC)ccc2CN(C(=O)C1=O)c3ccc(cc13)OC(F)(F)F
COc1ccc(cc1)CN2c3ccc(cc3C(=O)C2=O)OC(F)(F)F
|
InChI=1S/C17H12F3NO4/c1-24-11-4-2-10(3-5-11)9-21-14-7-6-12(25-17(18,19)20)8-13(14)15(22)16(21)23/h2-8H,9H2,1H3  Y YKey:CKLGZXFOLMHCMC-UHFFFAOYSA-N  Y Y
|
| (verify) |
VU-0238429 is a drug which acts as a selective positive allosteric modulator for the muscarinic acetylcholine receptor M5. It was the first selective ligand developed for the M5 subtype,[1] and is structurally derived from older M1-selective positive allosteric modulators such as VU-0119498.[2][3] Replacing the O-methyl- by a phenyl group further improves the receptor subtype selectivity.[4]
- ^ Bridges TM, Marlo JE, Niswender CM, Jones CK, Jadhav SB, Gentry PR, et al. (June 2009). "Discovery of the first highly M5-preferring muscarinic acetylcholine receptor ligand, an M5 positive allosteric modulator derived from a series of 5-trifluoromethoxy N-benzyl isatins". Journal of Medicinal Chemistry. 52 (11): 3445–3448. doi:10.1021/jm900286j. PMC 3875304. PMID 19438238.
- ^ Conn PJ, Jones CK, Lindsley CW (March 2009). "Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders". Trends in Pharmacological Sciences. 30 (3): 148–155. doi:10.1016/j.tips.2008.12.002. PMC 2907736. PMID 19201489.
- ^ Bridges TM, Kennedy JP, Hopkins CR, Conn PJ, Lindsley CW (October 2010). "Heterobiaryl and heterobiaryl ether derived M5 positive allosteric modulators". Bioorganic & Medicinal Chemistry Letters. 20 (19): 5617–5622. doi:10.1016/j.bmcl.2010.08.042. PMC 3179183. PMID 20801651.
- ^ Bridges TM, Phillip Kennedy J, Noetzel MJ, Breininger ML, Gentry PR, Conn PJ, Lindsley CW (March 2010). "Chemical lead optimization of a pan Gq mAChR M1, M3, M5 positive allosteric modulator (PAM) lead. Part II: development of a potent and highly selective M1 PAM". Bioorganic & Medicinal Chemistry Letters. 20 (6): 1972–1975. doi:10.1016/j.bmcl.2010.01.109. PMC 2834874. PMID 20156687.
|
|---|
| mAChRsTooltip Muscarinic acetylcholine receptors | | Agonists | |
|---|
| Antagonists |
- 3-Quinuclidinyl benzilate
- 4-DAMP
- Aclidinium bromide (+formoterol)
- Abediterol
- AF-DX 250
- AF-DX 384
- Ambutonium bromide
- Anisodamine
- Anisodine
- Antihistamines (first-generation) (e.g., brompheniramine, buclizine, captodiame, chlorphenamine (chlorpheniramine), cinnarizine, clemastine, cyproheptadine, dimenhydrinate, dimetindene, diphenhydramine, doxylamine, meclizine, mequitazine, perlapine, phenindamine, pheniramine, phenyltoloxamine, promethazine, propiomazine, triprolidine)
- AQ-RA 741
- Atropine
- Atropine methonitrate
- Atypical antipsychotics (e.g., clozapine, fluperlapine, olanzapine (+fluoxetine), rilapine, quetiapine, tenilapine, zotepine)
- Benactyzine
- Benzatropine (benztropine)
- Benzilone
- Benzilylcholine mustard
- Benzydamine
- Bevonium
- BIBN 99
- Biperiden
- Bornaprine
- Camylofin
- CAR-226,086
- CAR-301,060
- CAR-302,196
- CAR-302,282
- CAR-302,368
- CAR-302,537
- CAR-302,668
- Caramiphen
- Cimetropium bromide
- Clidinium bromide
- Cloperastine
- CS-27349
- Cyclobenzaprine
- Cyclopentolate
- Darifenacin
- DAU-5884
- Desfesoterodine
- Dexetimide
- DIBD
- Dicycloverine (dicyclomine)
- Dihexyverine
- Difemerine
- Diphemanil metilsulfate
- Ditran
- Drofenine
- EA-3167
- EA-3443
- EA-3580
- EA-3834
- Emepronium bromide
- Etanautine
- Etybenzatropine (ethybenztropine)
- Fenpiverinium
- Fentonium bromide
- Fesoterodine
- Flavoxate
- Glycopyrronium bromide (+beclometasone/formoterol, +indacaterol, +neostigmine)
- Hexahydrodifenidol
- Hexahydrosiladifenidol
- Hexbutinol
- Hexocyclium
- Himbacine
- HL-031,120
- Homatropine
- Imidafenacin
- Ipratropium bromide (+salbutamol)
- Isopropamide
- J-104,129
- Hyoscyamine
- Mamba toxin 3
- Mamba toxin 7
- Mazaticol
- Mebeverine
- Meladrazine
- Mepenzolate
- Methantheline
- Methoctramine
- Methylatropine
- Methylhomatropine
- Methylscopolamine
- Metixene
- Muscarinic toxin 7
- N-Ethyl-3-piperidyl benzilate
- N-Methyl-3-piperidyl benzilate
- Nefopam
- Octatropine methylbromide (anisotropine methylbromide)
- Orphenadrine
- Otenzepad (AF-DX 116)
- Otilonium bromide
- Oxapium iodide
- Oxitropium bromide
- Oxybutynin
- Oxyphencyclimine
- Oxyphenonium bromide
- PBID
- PD-102,807
- PD-0298029
- Penthienate
- Pethidine
- pFHHSiD
- Phenglutarimide
- Phenyltoloxamine
- Pipenzolate bromide
- Piperidolate
- Pirenzepine
- Piroheptine
- Pizotifen
- Poldine
- Pridinol
- Prifinium bromide
- Procyclidine
- Profenamine (ethopropazine)
- Propantheline bromide
- Propiverine
- Quinidine
- 3-Quinuclidinyl thiochromane-4-carboxylate
- Revefenacin
- Rociverine
- RU-47,213
- SCH-57,790
- SCH-72,788
- SCH-217,443
- Scopolamine (hyoscine)
- Scopolamine butylbromide (hyoscine butylbromide)
- Silahexacyclium
- Sofpironium bromide
- Solifenacin
- SSRIsTooltip Selective serotonin reuptake inhibitors (e.g., femoxetine, paroxetine)
- Telenzepine
- Terodiline
- Tetracyclic antidepressants (e.g., amoxapine, maprotiline, mianserin, mirtazapine)
- Tiemonium iodide
- Timepidium bromide
- Tiotropium bromide
- Tiquizium bromide
- Tofenacin
- Tolterodine
- Tricyclic antidepressants (e.g., amitriptyline (+perphenazine), amitriptylinoxide, butriptyline, cidoxepin, clomipramine, desipramine, desmethyldesipramine, dibenzepin, dosulepin (dothiepin), doxepin, imipramine, lofepramine, nitroxazepine, northiaden (desmethyldosulepin), nortriptyline, protriptyline, quinupramine, trimipramine)
- Tridihexethyl
- Trihexyphenidyl
- Trimebutine
- Tripitamine (tripitramine)
- Tropacine
- Tropatepine
- Tropicamide
- Trospium chloride
- Typical antipsychotics (e.g., chlorpromazine, chlorprothixene, cyamemazine (cyamepromazine), loxapine, mesoridazine, thioridazine)
- Umeclidinium bromide (+vilanterol)
- WIN-2299
- Xanomeline
- Zamifenacin
|
|---|
|
|---|
Precursors
(and prodrugs) | |
|---|
|

